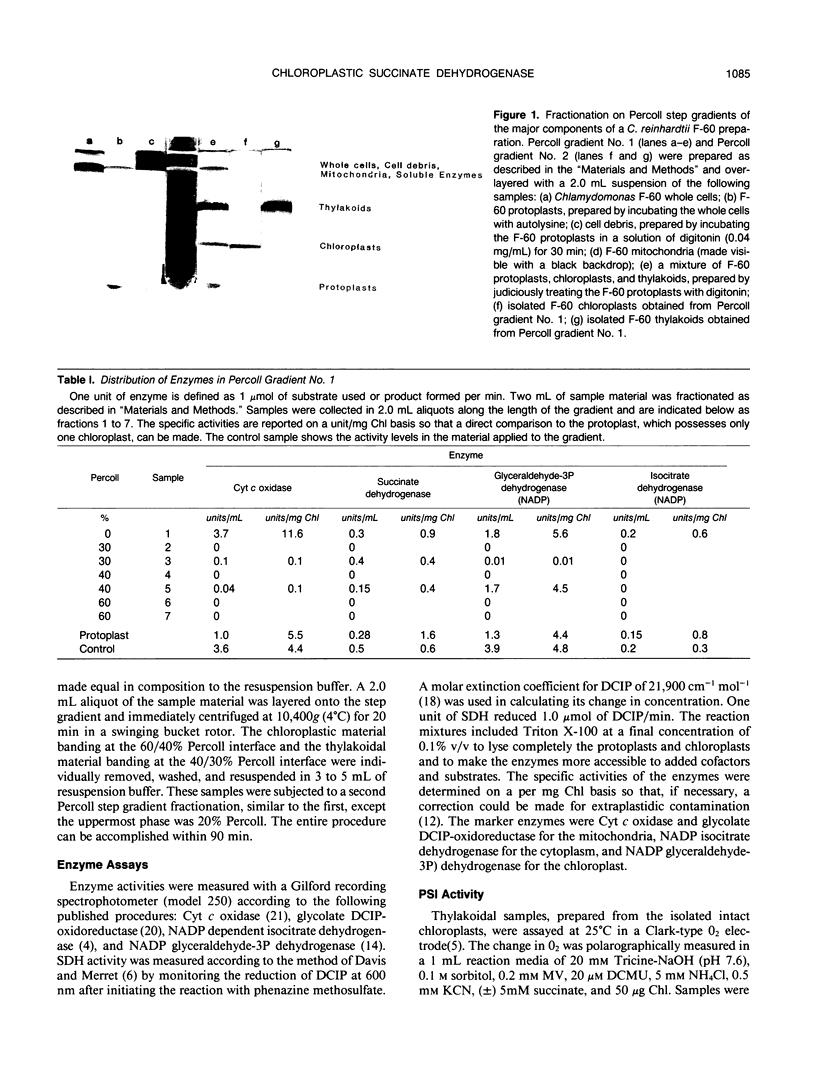
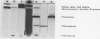Abstract
A method for isolating intact chloroplasts from Chlamydomonas reinhardtii F-60 was developed from the Klein, Chen, Gibbs, Platt-Aloia procedure ([1983] Plant Physiol 72: 481-487). Protoplasts, generated by treatment with autolysine, were lysed with a solution of digitonin and fractionated on Percoll step gradients. The chloroplasts were assessed to be 90% intact (ferricyanide assay) and free from cytoplasmic contamination (NADP isocitrate dehydrogenase activity) and to range from 2 to 5% in mitochondrial contamination (cytochrome c oxidase activity). About 25% of the cellular succinate dehydrogenase activity (21.6 micromoles per milligram chlorophyll per hour, as determined enzymically) was placed within the chloroplast. Chloroplastic succinate dehydrogenase had a Km for succinate of 0.55 millimolar and was associated with the thylakoidal material derived from the intact chloroplasts. This same thylakoidal material, with an enzymic assay of 21.6 micromoles per milligram chlorophyll per hour was able to initiate a light-dependent uptake of oxygen at a rate of 16.4 micromoles per milligram chlorophyll per hour when supplied with succinate and methyl viologen. Malonate was an apparent competitive inhibitor of this reaction. The succinate dehydrogenase activity present in the chloroplast was sufficient to account for the photoanaerobic rate of acetate dissimilation in H2 adapted Chlamydomonas (M Gibbs, RP Gfeller, C Chen [1986] Plant Physiol 82: 160-166).
Full text
PDF

Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Arnon D. I. COPPER ENZYMES IN ISOLATED CHLOROPLASTS. POLYPHENOLOXIDASE IN BETA VULGARIS. Plant Physiol. 1949 Jan;24(1):1–15. doi: 10.1104/pp.24.1.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Belknap W. R. Partial Purification of Intact Chloroplasts from Chlamydomonas reinhardtii. Plant Physiol. 1983 Aug;72(4):1130–1132. doi: 10.1104/pp.72.4.1130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Belknap W. R., Togasaki R. K. Chlamydomonas reinhardtii cell preparation with altered permeability toward substrates of organellar reactions. Proc Natl Acad Sci U S A. 1981 Apr;78(4):2310–2314. doi: 10.1073/pnas.78.4.2310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berkowitz G. A., Gibbs M. Effect of osmotic stress on photosynthesis studied with the isolated spinach chloroplast : generation and use of reducing power. Plant Physiol. 1982 Oct;70(4):1143–1148. doi: 10.1104/pp.70.4.1143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davis B., Merrett M. J. The Effect of Light on the Synthesis of Mitochondrial Enzymes in Division-synchronized Euglena Cultures. Plant Physiol. 1974 Apr;53(4):575–580. doi: 10.1104/pp.53.4.575. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gibbs M., Gfeller R. P., Chen C. Fermentative Metabolism of Chlamydomonas reinhardii: III. Photoassimilation of Acetate. Plant Physiol. 1986 Sep;82(1):160–166. doi: 10.1104/pp.82.1.160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gorman D. S., Levine R. P. Cytochrome f and plastocyanin: their sequence in the photosynthetic electron transport chain of Chlamydomonas reinhardi. Proc Natl Acad Sci U S A. 1965 Dec;54(6):1665–1669. doi: 10.1073/pnas.54.6.1665. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hiatt A. J. Preparation & some properties of soluble succinic dehydrogenase from higher plants. Plant Physiol. 1961 Sep;36(5):552–557. doi: 10.1104/pp.36.5.552. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klein U., Chen C., Gibbs M., Platt-Aloia K. A. Cellular Fractionation of Chlamydomonas reinhardii with Emphasis on the Isolation of the Chloroplast. Plant Physiol. 1983 Jun;72(2):481–487. doi: 10.1104/pp.72.2.481. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Latzko E., Gibbs M. Enzyme activities of the carbon reduction cycle in some photosynthetic organisms. Plant Physiol. 1969 Feb;44(2):295–300. doi: 10.1104/pp.44.2.295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moll B., Levine R. P. Characterization of a Photosynthetic Mutant Strain of Chlamydomonas reinhardi Deficient in Phosphoribulokinase Activity. Plant Physiol. 1970 Oct;46(4):576–580. doi: 10.1104/pp.46.4.576. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nelson E. B., Tolbert N. E. Glycolate dehydrogenase in green algae. Arch Biochem Biophys. 1970 Nov;141(1):102–110. doi: 10.1016/0003-9861(70)90112-8. [DOI] [PubMed] [Google Scholar]
- Tolbert N. E., Oeser A., Kisaki T., Hageman R. H., Yamazaki R. K. Peroxisomes from spinach leaves containing enzymes related to glycolate metabolism. J Biol Chem. 1968 Oct 10;243(19):5179–5184. [PubMed] [Google Scholar]