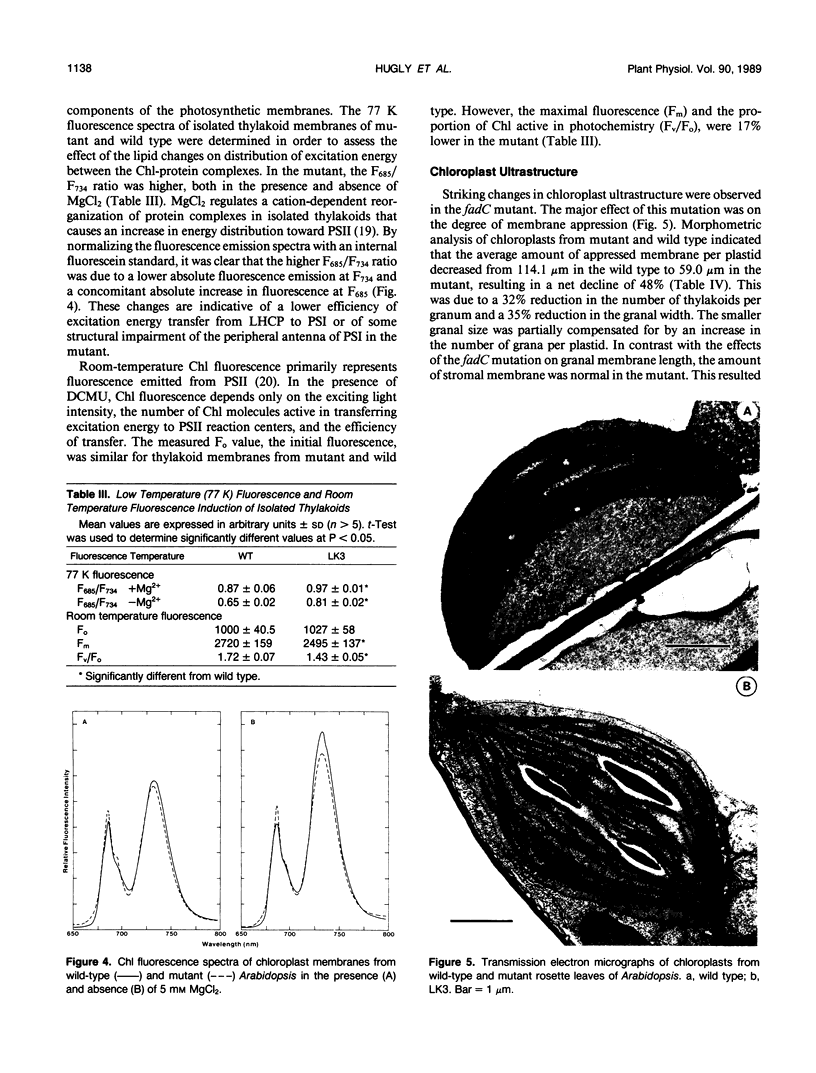
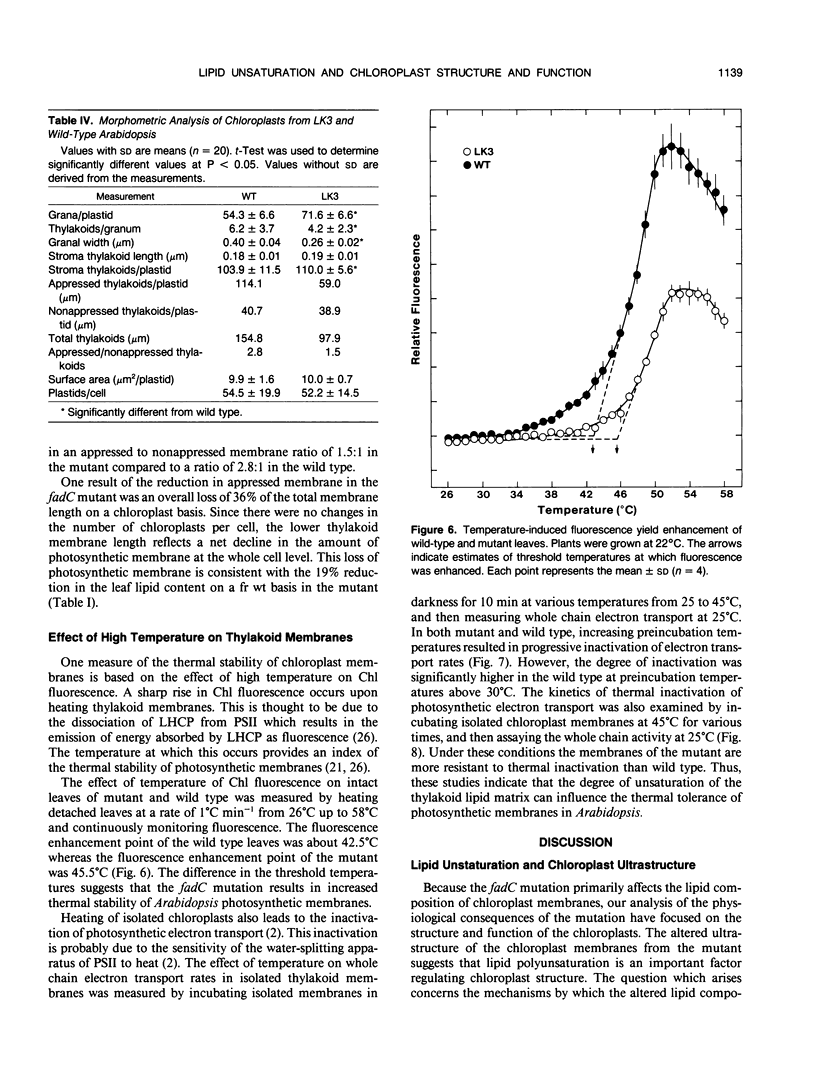
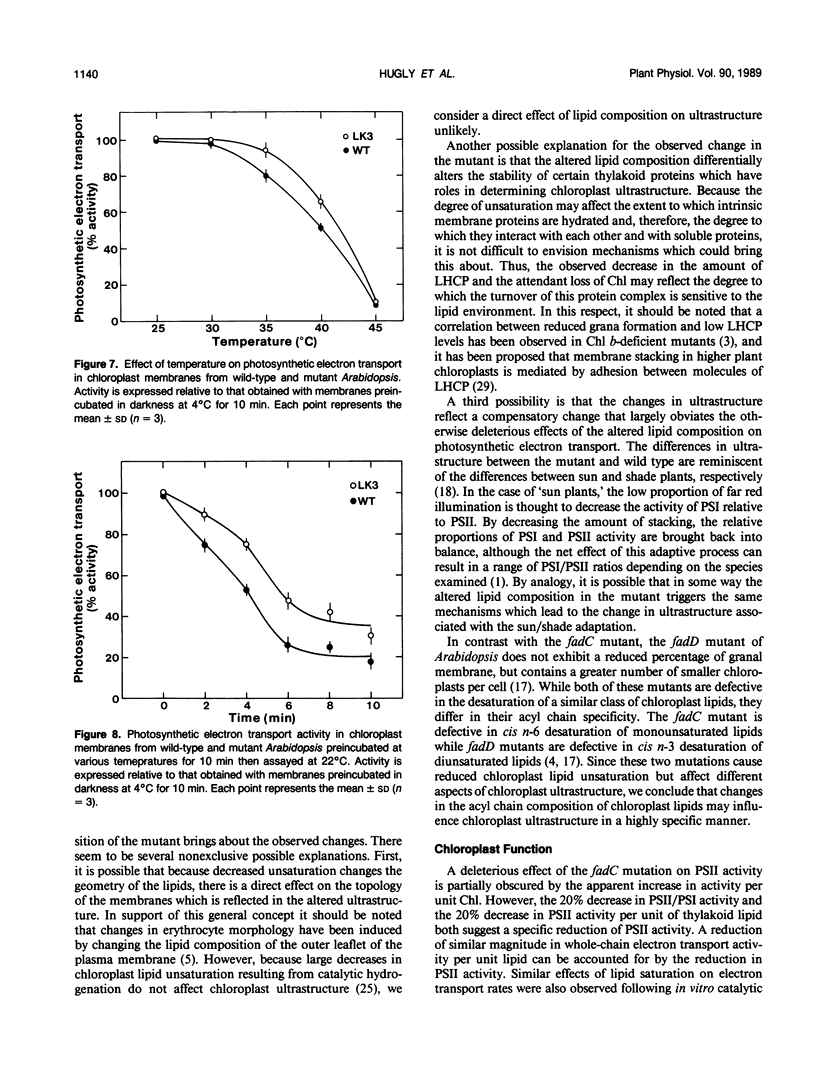
Abstract
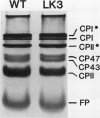
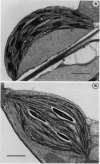
A mutant of Arabidopsis thaliana, deficient in activity of the chloroplast n-6 desaturase, accumulated high levels of C16:1 and C18:1 lipids and had correspondingly reduced levels of polyunsaturated lipids. The altered lipid composition of the mutant had pronounced effects on chloroplast ultrastructure, thylakoid membrane protein and chlorophyll content, electron transport rates, and the thermal stability of the photosynthetic membranes. The change in chloroplast ultrastructure was due to a 48% decrease in the amount of appressed membranes that was not compensated for by an increased amount of nonappressed membrane. This resulted in a net loss of 36% of the thylakoid membrane per chloroplast and a corresponding reduction in chlorophyll and protein content. Electrophoretic analysis of the chlorophyll-protein complexes further revealed a small decrease in the amount of light-harvesting complex. Relative levels of whole chain and protosystem II electron transport rates were also reduced in the mutant. In addition, the mutation resulted in enhanced thermal stability of photosynthetic electron transport. These observations suggest a central role of polyunsaturated lipids in determining chloroplast structure and maintaining normal photosynthetic function and demonstrate that lipid unsaturation directly affects the thermal stability of photosynthetic membranes.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bolton P., Wharfe J., Harwood J. L. The lipid composition of a barley mutant lacking chlorophyll b. Biochem J. 1978 Jul 15;174(1):67–72. doi: 10.1042/bj1740067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Browse J., Kunst L., Anderson S., Hugly S., Somerville C. A mutant of Arabidopsis deficient in the chloroplast 16:1/18:1 desaturase. Plant Physiol. 1989 Jun;90(2):522–529. doi: 10.1104/pp.90.2.522. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Christiansson A., Kuypers F. A., Roelofsen B., Op den Kamp J. A., van Deenen L. L. Lipid molecular shape affects erythrocyte morphology: a study involving replacement of native phosphatidylcholine with different species followed by treatment of cells with sphingomyelinase C or phospholipase A2. J Cell Biol. 1985 Oct;101(4):1455–1462. doi: 10.1083/jcb.101.4.1455. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Downton W. J., Berry J. A., Seemann J. R. Tolerance of photosynthesis to high temperature in desert plants. Plant Physiol. 1984 Apr;74(4):786–790. doi: 10.1104/pp.74.4.786. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dunahay T. G., Staehelin L. A. Isolation of photosystem I complexes from octyl glucoside/sodium dodecyl sulfate solubilized spinach thylakoids : characterization and reconstitution into liposomes. Plant Physiol. 1985 Jul;78(3):606–613. doi: 10.1104/pp.78.3.606. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hurkman W. J., Tanaka C. K. Solubilization of plant membrane proteins for analysis by two-dimensional gel electrophoresis. Plant Physiol. 1986 Jul;81(3):802–806. doi: 10.1104/pp.81.3.802. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kunst L., Browse J., Somerville C. Altered chloroplast structure and function in a mutant of Arabidopsis deficient in plastid glycerol-3-phosphate acyltransferase activity. Plant Physiol. 1989 Jul;90(3):846–853. doi: 10.1104/pp.90.3.846. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Markwell J. P., Thornber J. P., Boggs R. T. Higher plant chloroplasts: Evidence that all the chlorophyll exists as chlorophyll-protein complexes. Proc Natl Acad Sci U S A. 1979 Mar;76(3):1233–1235. doi: 10.1073/pnas.76.3.1233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Markwell M. A., Haas S. M., Bieber L. L., Tolbert N. E. A modification of the Lowry procedure to simplify protein determination in membrane and lipoprotein samples. Anal Biochem. 1978 Jun 15;87(1):206–210. doi: 10.1016/0003-2697(78)90586-9. [DOI] [PubMed] [Google Scholar]
- McCourt P., Kunst L., Browse J., Somerville C. R. The effects of reduced amounts of lipid unsaturation on chloroplast ultrastructure and photosynthesis in a mutant of Arabidopsis. Plant Physiol. 1987 Jun;84(2):353–360. doi: 10.1104/pp.84.2.353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Melis A. Light regulation of photosynthetic membrane structure, organization, and function. J Cell Biochem. 1984;24(3):271–285. doi: 10.1002/jcb.240240308. [DOI] [PubMed] [Google Scholar]
- Pearcy R. W. Effect of Growth Temperature on the Fatty Acid Composition of the Leaf Lipids in Atriplex lentiformis (Torr.) Wats. Plant Physiol. 1978 Apr;61(4):484–486. doi: 10.1104/pp.61.4.484. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Quinn P. J. Lipid phase behaviour and lipid-protein interactions in the chloroplast photosynthetic membrane. Biochem Soc Trans. 1987 Feb;15(1):86–91. doi: 10.1042/bst0150086. [DOI] [PubMed] [Google Scholar]
- Restall C. J., Williams P., Percival M. P., Quinn P. J., Chapman D. The modulation of membrane fluidity by hydrogenation processes. III. The hydrogenation of biomembranes of spinach chloroplasts and a study of the effect of this on photosynthetic electron transport. Biochim Biophys Acta. 1979 Jul 19;555(1):119–130. doi: 10.1016/0005-2736(79)90077-4. [DOI] [PubMed] [Google Scholar]
- Staehelin L. A., Arntzen C. J. Regulation of chloroplast membrane function: protein phosphorylation changes the spatial organization of membrane components. J Cell Biol. 1983 Nov;97(5 Pt 1):1327–1337. doi: 10.1083/jcb.97.5.1327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vigh L., Joó F., Droppa M., Horváth L. I., Horváth G. Modulation of chloroplast membrane lipids by homogeneous catalytic hydrogenation. Eur J Biochem. 1985 Mar 15;147(3):477–481. doi: 10.1111/j.0014-2956.1985.00477.x. [DOI] [PubMed] [Google Scholar]
- Yamamoto M., Kosuda T., Yanagawa H., Tachibana T., Shima K., Hosoda Y., Mikami R., Homma H. Long-term follow-up in sarcoidosis in Japan. Z Erkr Atmungsorgane. 1977 Aug;149(2):191–196. [PubMed] [Google Scholar]