Abstract
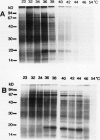
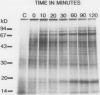
Effect of heat shock on the growth of cultured sugarcane cells (Saccharum officinarum L.) was measured. Heat shock (HS) treatment at 36 to 38°C (2 hours) induced the development of maximum thermotolerance to otherwise nonpermissive heat stress at 54°C (7 minutes). Optimum thermotolerance was observed 8 hours after heat shock. Development of thermotolerance was initiated by treatments as short as 30 minutes at 36°C. Temperatures below 36°C or above 40°C failed to induce maximum thermotolerance. In vivo labeling revealed that HS at 32 to 34°C induced several high molecular mass heat shock proteins (HSPs). A complex of 18 kilodalton HSPs required at least 36°C treatment for induction. The majority of the HSPs began to accumulate within 10 minutes, whereas the synthesis of low molecular mass peptides in the 18 kilodalton range became evident 30 minutes after initiation of HS. HS above 38°C resulted in progressively decreased HSP synthesis with inhibition first observed for HSPs larger than 50 kilodaltons. Analysis of two-dimensional gels revealed a complex pattern of label incorporation including the synthesis of four major HSPs in the 18 kilodalton range and continued synthesis of constitutive proteins during HS.
Full text
PDF






Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Ashburner M., Bonner J. J. The induction of gene activity in drosophilia by heat shock. Cell. 1979 Jun;17(2):241–254. doi: 10.1016/0092-8674(79)90150-8. [DOI] [PubMed] [Google Scholar]
- Aujame L., Firko H. The major inducible heat shock protein hsp68 is not required for acquisition of thermal resistance in mouse plasmacytoma cell lines. Mol Cell Biol. 1988 Dec;8(12):5486–5494. doi: 10.1128/mcb.8.12.5486. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ballinger D. G., Pardue M. L. The control of protein synthesis during heat shock in Drosophila cells involves altered polypeptide elongation rates. Cell. 1983 May;33(1):103–113. doi: 10.1016/0092-8674(83)90339-2. [DOI] [PubMed] [Google Scholar]
- Bonham-Smith P. C., Kapoor M., Bewley J. D. Establishment of thermotolerance in maize by exposure to stresses other than a heat shock does not require heat shock protein synthesis. Plant Physiol. 1987 Oct;85(2):575–580. doi: 10.1104/pp.85.2.575. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bosch T. C., Krylow S. M., Bode H. R., Steele R. E. Thermotolerance and synthesis of heat shock proteins: these responses are present in Hydra attenuata but absent in Hydra oligactis. Proc Natl Acad Sci U S A. 1988 Nov;85(21):7927–7931. doi: 10.1073/pnas.85.21.7927. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Callis J., Fromm M., Walbot V. Heat Inducible Expression of a Chimeric Maize hsp70CAT Gene in Maize Protoplasts. Plant Physiol. 1988 Dec;88(4):965–968. doi: 10.1104/pp.88.4.965. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chrétien P., Landry J. Enhanced constitutive expression of the 27-kDa heat shock proteins in heat-resistant variants from Chinese hamster cells. J Cell Physiol. 1988 Oct;137(1):157–166. doi: 10.1002/jcp.1041370119. [DOI] [PubMed] [Google Scholar]
- Cooper P., Ho T. H. Heat shock proteins in maize. Plant Physiol. 1983 Feb;71(2):215–222. doi: 10.1104/pp.71.2.215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harrington H. M., Alm D. M. Interaction of heat and salt shock in cultured tobacco cells. Plant Physiol. 1988 Nov;88(3):618–625. doi: 10.1104/pp.88.3.618. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harrington H. M., Smith I. K. Cysteine metabolism in cultured tobacco cells. Plant Physiol. 1980 Jan;65(1):151–155. doi: 10.1104/pp.65.1.151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jackson R. J. The heat-shock response in Drosophila KC 161 cells. mRNA competition is the main explanation for reduction of normal protein synthesis. Eur J Biochem. 1986 Aug 1;158(3):623–634. doi: 10.1111/j.1432-1033.1986.tb09800.x. [DOI] [PubMed] [Google Scholar]
- Johnston R. N., Kucey B. L. Competitive inhibition of hsp70 gene expression causes thermosensitivity. Science. 1988 Dec 16;242(4885):1551–1554. doi: 10.1126/science.3201244. [DOI] [PubMed] [Google Scholar]
- Kanabus J., Pikaard C. S., Cherry J. H. Heat Shock Proteins in Tobacco Cell Suspension during Growth Cycle. Plant Physiol. 1984 Jul;75(3):639–644. doi: 10.1104/pp.75.3.639. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Key J. L., Lin C. Y., Chen Y. M. Heat shock proteins of higher plants. Proc Natl Acad Sci U S A. 1981 Jun;78(6):3526–3530. doi: 10.1073/pnas.78.6.3526. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Lin C. Y., Roberts J. K., Key J. L. Acquisition of Thermotolerance in Soybean Seedlings : Synthesis and Accumulation of Heat Shock Proteins and their Cellular Localization. Plant Physiol. 1984 Jan;74(1):152–160. doi: 10.1104/pp.74.1.152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lindquist S. The heat-shock response. Annu Rev Biochem. 1986;55:1151–1191. doi: 10.1146/annurev.bi.55.070186.005443. [DOI] [PubMed] [Google Scholar]
- Loomis W. F., Wheeler S. A. Chromatin-associated heat shock proteins of Dictyostelium. Dev Biol. 1982 Apr;90(2):412–418. doi: 10.1016/0012-1606(82)90390-6. [DOI] [PubMed] [Google Scholar]
- Mansfield M. A., Key J. L. Synthesis of the low molecular weight heat shock proteins in plants. Plant Physiol. 1987 Aug;84(4):1007–1017. doi: 10.1104/pp.84.4.1007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nover L., Scharf K. D. Synthesis, modification and structural binding of heat-shock proteins in tomato cell cultures. Eur J Biochem. 1984 Mar 1;139(2):303–313. doi: 10.1111/j.1432-1033.1984.tb08008.x. [DOI] [PubMed] [Google Scholar]
- O'Farrell P. Z., Goodman H. M., O'Farrell P. H. High resolution two-dimensional electrophoresis of basic as well as acidic proteins. Cell. 1977 Dec;12(4):1133–1141. doi: 10.1016/0092-8674(77)90176-3. [DOI] [PubMed] [Google Scholar]
- Riabowol K. T., Mizzen L. A., Welch W. J. Heat shock is lethal to fibroblasts microinjected with antibodies against hsp70. Science. 1988 Oct 21;242(4877):433–436. doi: 10.1126/science.3175665. [DOI] [PubMed] [Google Scholar]