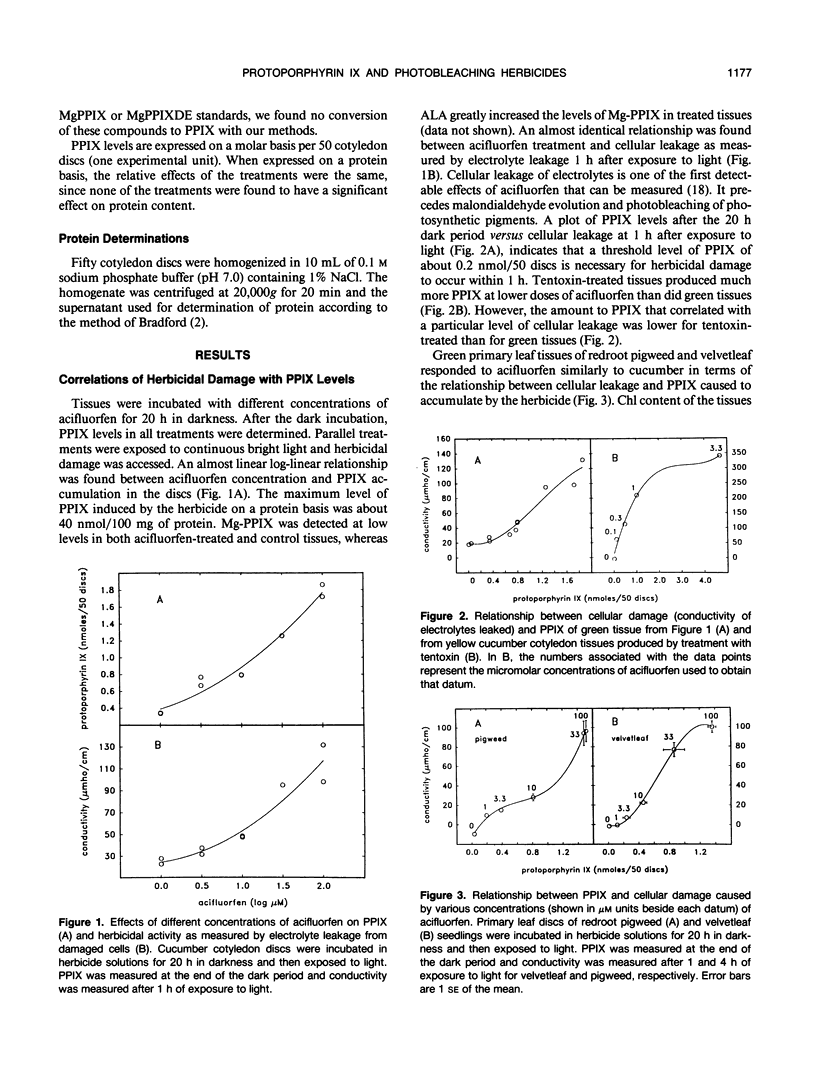
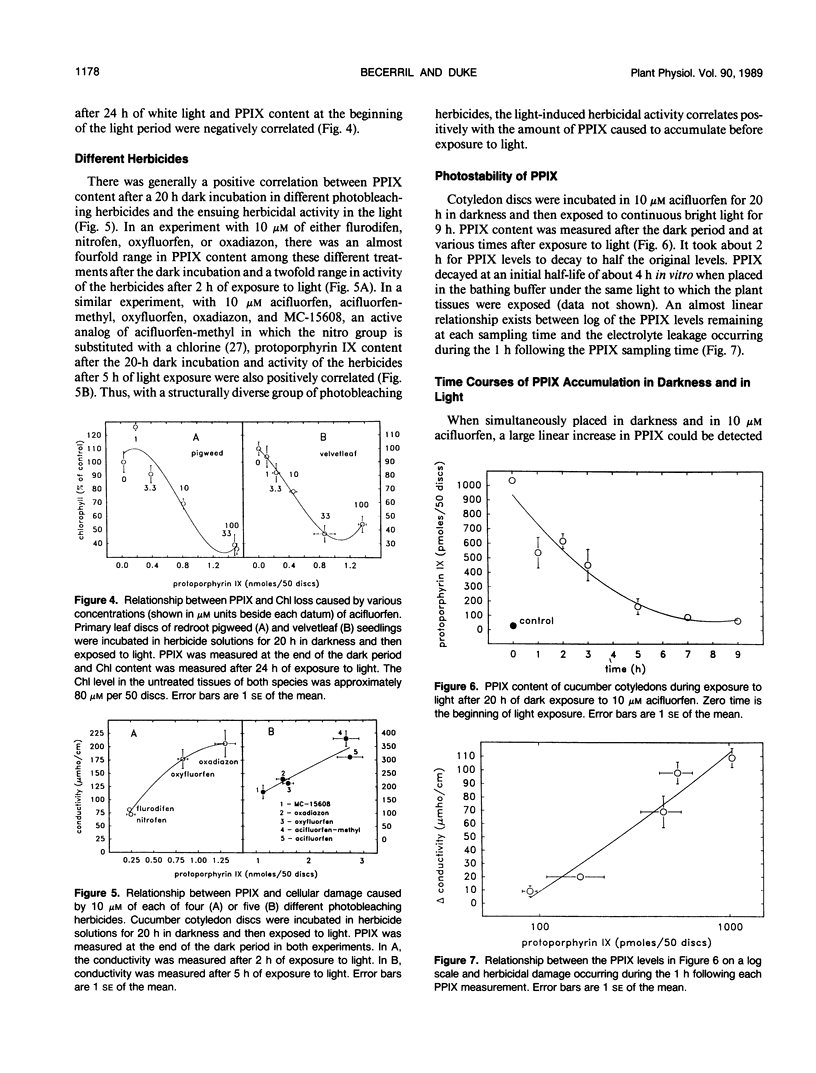
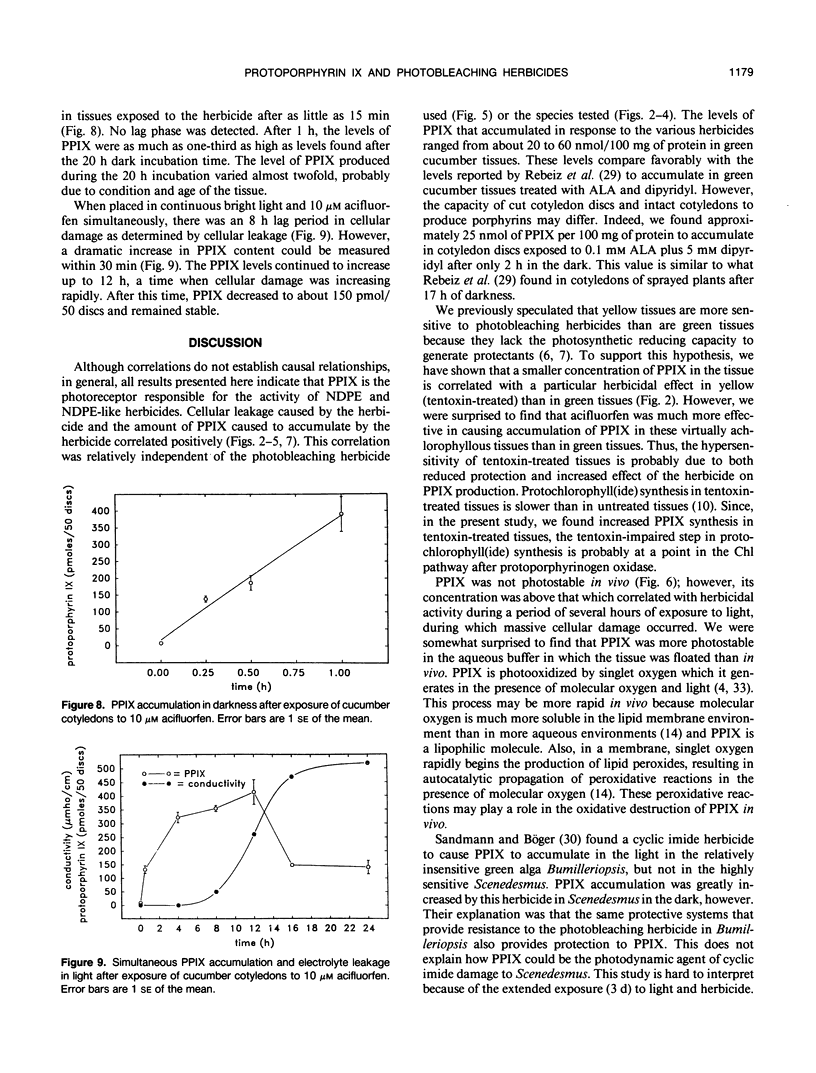
Abstract
Several laboratories have demonstrated recently that photobleaching herbicides such as acifluorfen and oxadiazon cause accumulation of protoporphyrin IX (PPIX), a photodynamic pigment capable of herbicidal activity. We investigated, in acifluorfen-treated tissues, the in vivo stability of PPIX, the kinetics of accumulation, and the correlation between concentration of PPIX and herbicidal damage. During a 20 hour dark period, PPIX levels rose from barely detectable concentrations to 1 to 2 nanomoles per 50 cucumber (Cucumis sativus L.) cotyledon discs treated with 10 micromolar acifluorfen. When placed in 500 micromoles per square meter per second PAR, PPIX levels decayed logarithmically, with an initial half-life of about 2.5 hours. PPIX levels at each time after exposure to light correlated positively with the cellular damage that occurred during the following 1 hour in both green and yellow (tentoxin-treated) cucumber cotyledon tissues. PPIX levels in discs incubated for 20 hours in darkness correlated positively with the acifluorfen concentration in which they were incubated. In cucumber, the level of herbicidal damage caused by several p-nitrodiphenyl other herbicides, a p-chlorodiphenylether herbicide, and oxadiazon correlated positively with the amount of PPIX induced to accumulate by each of the herbicide treatments. Similar results were obtained with acifluorfen-treated pigweed and velvetleaf primary leaf tissues. In cucumber, PPIX levels increased within 15 and 30 minutes after exposure of discs to 10 micromolar acifluorfen in the dark and light, respectively. These data strengthen the view that PPIX is responsible for all or a major part of the photobleaching activity of acifluorfen and related herbicides.
Full text
PDF



Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bradford M. M. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem. 1976 May 7;72:248–254. doi: 10.1016/0003-2697(76)90527-3. [DOI] [PubMed] [Google Scholar]
- Brenner D. A., Bloomer J. R. The enzymatic defect in variegate prophyria. Studies with human cultured skin fibroblasts. N Engl J Med. 1980 Apr 3;302(14):765–769. doi: 10.1056/NEJM198004033021401. [DOI] [PubMed] [Google Scholar]
- Duggan J., Gassman M. Induction of porphyrin synthesis in etiolated bean leaves by chelators of iron. Plant Physiol. 1974 Feb;53(2):206–215. doi: 10.1104/pp.53.2.206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duke S. O., Kenyon W. H. Photosynthesis Is Not Involved in the Mechanism of Action of Acifluorfen in Cucumber (Cucumis sativus L.). Plant Physiol. 1986 Jul;81(3):882–888. doi: 10.1104/pp.81.3.882. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ensminger M. P., Hess F. D. Action Spectrum of the Activity of Acifluorfen-methyl, a Diphenyl Ether Herbicide, in Chlamydomonas eugametos. Plant Physiol. 1985 Feb;77(2):503–505. doi: 10.1104/pp.77.2.503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Halling B. P., Peters G. R. Influence of chloroplast development on the activation of the diphenyl ether herbicide acifluorfen-methyl. Plant Physiol. 1987 Aug;84(4):1114–1120. doi: 10.1104/pp.84.4.1114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haworth P., Hess F. D. The generation of singlet oxygen (o(2)) by the nitrodiphenyl ether herbicide oxyfluorfen is independent of photosynthesis. Plant Physiol. 1988 Mar;86(3):672–676. doi: 10.1104/pp.86.3.672. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matringe M., Scalla R. Studies on the mode of action of acifluorfen-methyl in nonchlorophyllous soybean cells : accumulation of tetrapyrroles. Plant Physiol. 1988 Feb;86(2):619–622. doi: 10.1104/pp.86.2.619. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Orr G. L., Elliott C. M., Hogan M. E. Activity in vivo and redox States in vitro of nitro- and chlorodiphenyl ether herbicide analogs. Plant Physiol. 1983 Dec;73(4):939–944. doi: 10.1104/pp.73.4.939. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sato R., Nagano E., Oshio H., Kamoshita K., Furuya M. Wavelength Effect on the Action of a N-Phenylimide S-23142 and a Diphenylether Acifluorfen-Ethyl in Cotyledons of Cucumber (Cucumis sativus L.) Seedlings. Plant Physiol. 1987 Dec;85(4):1146–1150. doi: 10.1104/pp.85.4.1146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Witkowski D. A., Halling B. P. Accumulation of photodynamic tetrapyrroles induced by acifluorfen-methyl. Plant Physiol. 1988 Jul;87(3):632–637. doi: 10.1104/pp.87.3.632. [DOI] [PMC free article] [PubMed] [Google Scholar]


