Abstract
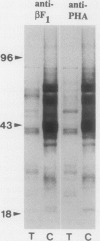
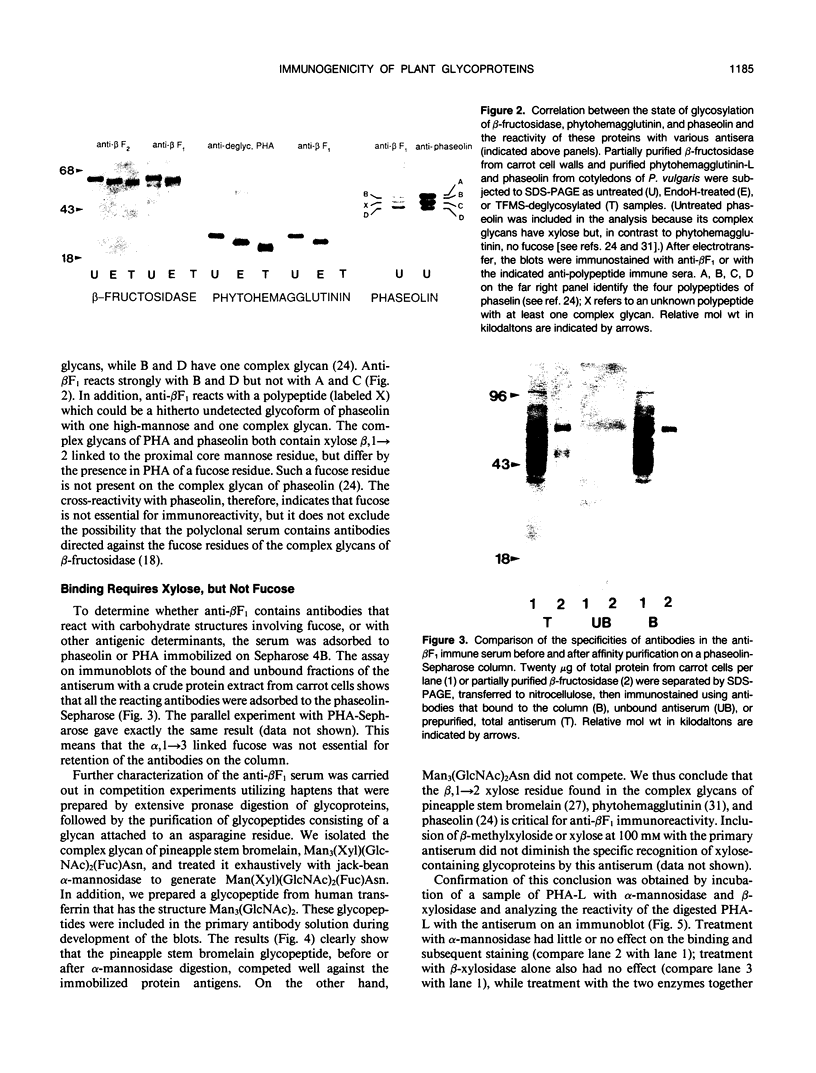
Antibodies were raised against carrot (Daucus carota) cell wall β-fructosidase that was either in a native configuration (this serum is called anti-βF1) or chemically deglycosylated (anti-βF2). The two antisera had completely different specificities when tested by immunoblotting. The anti-βF1 serum reacted with β-fructosidase and many other carrot cell wall proteins as well as with many proteins in extracts of bean (Phaseolus vulgaris) cotyledons and tobacco (Nicotiana tabacum) seeds. It did not react with chemically deglycosylated β-fructosidase. The anti-βF1 serum also reacted with the bean vacuolar protein, phytohemagglutinin, but not with deglycosylated phytohemagglutinin. The anti-βF2 serum reacted with both normal and deglycosylated β-fructosidase but not with other proteins. These results indicate that the βF2 antibodies recognize the β-fructosidase polypeptide, while the βF1 antibodies recognize glycan sidechains common to many glycoproteins. We used immunoadsorption on glycoprotein-Sepharose columns and hapten inhibition of immunoblot reactions to characterize the nature of the antigenic site. Antibody binding activity was found to be associated with Man3(Xyl)(GIcNAc)2Fuc, Man3(Xyl)(GIcNAc)2, and Man(Xyl) (GIcNAc)2 glycans, but not with Man3(GIcNAc)2. Treatment of phytohemagglutinin, a glycoprotein with a Man3(Xyl)(GIcNAc)2Fuc glycan, with Charonia lampas β-xylosidase (after treatment with jack-bean α-mannosidase) greatly diminished the binding between the antibodies and phytohemagglutinin. We conclude, therefore, that the antibodies bind primarily to the xyloseβ, 1→ 2mannose structure commonly found in the complex glycans of plant glycoproteins.
Full text
PDF




Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Ashford D., Dwek R. A., Welply J. K., Amatayakul S., Homans S. W., Lis H., Taylor G. N., Sharon N., Rademacher T. W. The beta 1----2-D-xylose and alpha 1----3-L-fucose substituted N-linked oligosaccharides from Erythrina cristagalli lectin. Isolation, characterisation and comparison with other legume lectins. Eur J Biochem. 1987 Jul 15;166(2):311–320. doi: 10.1111/j.1432-1033.1987.tb13516.x. [DOI] [PubMed] [Google Scholar]
- Bollini R., Vitale A., Chrispeels M. J. In vivo and in vitro processing of seed reserve protein in the endoplasmic reticulum: evidence for two glycosylation steps. J Cell Biol. 1983 Apr;96(4):999–1007. doi: 10.1083/jcb.96.4.999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Conrad T. A., Lamport D. T., Hammerschmidt R. Detection of glycosylated and deglycosylated extensin precursors by indirect competitive ELISA. Plant Physiol. 1987 Jan;83(1):1–3. doi: 10.1104/pp.83.1.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Edge A. S., Faltynek C. R., Hof L., Reichert L. E., Jr, Weber P. Deglycosylation of glycoproteins by trifluoromethanesulfonic acid. Anal Biochem. 1981 Nov 15;118(1):131–137. doi: 10.1016/0003-2697(81)90168-8. [DOI] [PubMed] [Google Scholar]
- Felsted R. L., Leavitt R. D., Bachur N. R. Purification of the phytohemagglutinin family of proteins from red kidney beans (Phaseolus vulgaris) by affinity chromatography. Biochim Biophys Acta. 1975 Sep 9;405(1):72–81. doi: 10.1016/0005-2795(75)90316-5. [DOI] [PubMed] [Google Scholar]
- Fournet B., Leroy Y., Wieruszeski J. M., Montreuil J., Poretz R. D., Goldberg R. Primary structure of an N-glycosidic carbohydrate unit derived from Sophora japonica lectin. Eur J Biochem. 1987 Jul 15;166(2):321–324. doi: 10.1111/j.1432-1033.1987.tb13517.x. [DOI] [PubMed] [Google Scholar]
- Gleeson P. A., Clarke A. E. Antigenic determinants of a plant proteoglycan, the Gladiolus style arabinogalactan-protein. Biochem J. 1980 Nov 1;191(2):437–447. doi: 10.1042/bj1910437. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gleeson P. A., Schachter H. Control of glycoprotein synthesis. J Biol Chem. 1983 May 25;258(10):6162–6173. [PubMed] [Google Scholar]
- Johnson K. D., Chrispeels M. J. Substrate Specificities of N-Acetylglucosaminyl-, Fucosyl-, and Xylosyltransferases that Modify Glycoproteins in the Golgi Apparatus of Bean Cotyledons. Plant Physiol. 1987 Aug;84(4):1301–1308. doi: 10.1104/pp.84.4.1301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaladas P. M., Goldberg R., Poretz R. D. Rabbit anti-carbohydrate antibody elicited by the lymphocyte mitogenic glycoprotein from Wistaria floribunda seeds. Mol Immunol. 1983 Jul;20(7):727–735. doi: 10.1016/0161-5890(83)90050-0. [DOI] [PubMed] [Google Scholar]
- King J., Laemmli U. K. Polypeptides of the tail fibres of bacteriophage T4. J Mol Biol. 1971 Dec 28;62(3):465–477. doi: 10.1016/0022-2836(71)90148-3. [DOI] [PubMed] [Google Scholar]
- Knecht D. A., Dimond R. L., Wheeler S., Loomis W. F. Antigenic determinants shared by lysosomal proteins of Dictyostelium discoideum. Characterization using monoclonal antibodies and isolation of mutations affecting the determinant. J Biol Chem. 1984 Aug 25;259(16):10633–10640. [PubMed] [Google Scholar]
- Kornfeld R., Kornfeld S. Assembly of asparagine-linked oligosaccharides. Annu Rev Biochem. 1985;54:631–664. doi: 10.1146/annurev.bi.54.070185.003215. [DOI] [PubMed] [Google Scholar]
- Laurière C., Laurière M., Sturm A., Faye L., Chrispeels M. J. Characterization of beta-fructosidase, an extracellular glycoprotein of carrot cells. Biochimie. 1988 Nov;70(11):1483–1491. doi: 10.1016/0300-9084(88)90285-4. [DOI] [PubMed] [Google Scholar]
- Rubin R. W., Warren R. W. Quantitation of microgram amounts of protein in SDS-mercaptoethanol-tris electrophoresis sample buffer. Anal Biochem. 1977 Dec;83(2):773–777. doi: 10.1016/0003-2697(77)90084-7. [DOI] [PubMed] [Google Scholar]
- Sojar H. T., Bahl O. P. A chemical method for the deglycosylation of proteins. Arch Biochem Biophys. 1987 Nov 15;259(1):52–57. doi: 10.1016/0003-9861(87)90469-3. [DOI] [PubMed] [Google Scholar]
- Sturm A., Van Kuik J. A., Vliegenthart J. F., Chrispeels M. J. Structure, position, and biosynthesis of the high mannose and the complex oligosaccharide side chains of the bean storage protein phaseolin. J Biol Chem. 1987 Oct 5;262(28):13392–13403. [PubMed] [Google Scholar]
- Trimble R. B., Maley F. Optimizing hydrolysis of N-linked high-mannose oligosaccharides by endo-beta-N-acetylglucosaminidase H. Anal Biochem. 1984 Sep;141(2):515–522. doi: 10.1016/0003-2697(84)90080-0. [DOI] [PubMed] [Google Scholar]
- Van Kuik J. A., Sijbesma R. P., Kamerling J. P., Vliegenthart J. F., Wood E. J. Primary structure of a low-molecular-mass N-linked oligosaccharide from hemocyanin of Lymnaea stagnalis. 3-O-methyl-D-mannose as a constituent of the xylose-containing core structure in an animal glycoprotein. Eur J Biochem. 1986 Nov 3;160(3):621–625. doi: 10.1111/j.1432-1033.1986.tb10083.x. [DOI] [PubMed] [Google Scholar]
- van Kuik J. A., van Halbeek H., Kamerling J. P., Vliegenthart J. F. Primary structure of the low-molecular-weight carbohydrate chains of Helix pomatia alpha-hemocyanin. Xylose as a constituent of N-linked oligosaccharides in an animal glycoprotein. J Biol Chem. 1985 Nov 15;260(26):13984–13988. [PubMed] [Google Scholar]