Abstract
Purpose:
Cyclin E (CCNE1) has been proposed as a biomarker of sensitivity to adavosertib, a Wee1 kinase inhibitor, and a mechanism of resistance to HER2-targeted therapy.
Experimental Design:
Copy number and genomic sequencing data from The Cancer Genome Atlas and MD Anderson Cancer Center databases were analyzed to assess ERBB2 and CCNE1 expression. Molecular characteristics of tumors and patient-derived xenografts (PDX) were assessed by next-generation sequencing, whole-exome sequencing, fluorescent in situ hybridization, and IHC. In vitro, CCNE1 was overexpressed or knocked down in HER2+ cell lines to evaluate drug combination efficacy. In vivo, NSG mice bearing PDXs were subjected to combinatorial therapy with various treatment regimens, followed by tumor growth assessment. Pharmacodynamic markers in PDXs were characterized by IHC and reverse-phase protein array.
Results:
Among several ERBB2-amplified cancers, CCNE1 co-amplification was identified (gastric 37%, endometroid 43%, and ovarian serous adenocarcinoma 41%). We hypothesized that adavosertib may enhance activity of HER2 antibody–drug conjugate trastuzumab deruxtecan (T-DXd). In vitro, sensitivity to T-DXd was decreased by cyclin E overexpression and increased by knockdown, and adavosertib was synergistic with topoisomerase I inhibitor DXd. In vivo, the T-DXd + adavosertib combination significantly increased γH2AX and antitumor activity in HER2 low, cyclin E amplified gastroesophageal cancer PDX models and prolonged event-free survival (EFS) in a HER2-overexpressing gastroesophageal cancer model. T-DXd + adavosertib treatment also increased EFS in other HER2-expressing tumor types, including a T-DXd–treated colon cancer model.
Conclusions:
We provide rationale for combining T-DXd with adavosertib in HER2-expressing cancers, especially with co-occuring CCNE1 amplifications.
Translational Relevance.
This preclinical study of trastuzumab deruxtecan in combination with adavosertib demonstrates enhanced antitumor activity in HER2-expressing tumors. These findings provide supporting rationale for clinical pursuit of this combination in a select subset of patients with HER2-expressing solid tumors.
Introduction
Human epidermal growth factor receptor 2 (HER2) is a key oncogenic driver across several tumor types (1, 2). The development of HER2-targeted therapies has dramatically improved oncologic outcomes for patients with breast and gastric cancer, and trastuzumab-based regimens have become the standard-of-care for patients with HER2-positive disease (3–5). There is growing interest in HER2-directed therapeutics, as biomarker profiling efforts have demonstrated ERBB2 amplification/HER2 overexpression in several cancer types beyond breast and gastric cancer. Many of these therapies are now being evaluated in biomarker-selected clinical trials (6, 7). A major challenge in the treatment of patients with HER2-targeted therapies is the presence of intrinsic resistance or development of acquired resistance to therapy (8, 9). Several strategies have been used to combat resistance to trastuzumab, including treatment with HER2-directed antibody–drug conjugates (ADC), combinations with chemotherapy and/or immunotherapy, and by targeting co-alterations which may be implicated in therapeutic resistance (10–12). Trastuzumab deruxtecan (T-DXd) is a HER2-targeted ADC composed of the mAb trastuzumab linked to DXd, a potent topoisomerase I inhibitor payload, which induces DNA damage and apoptosis (13). Durable activity of T-DXd has been demonstrated in patients with HER2-positive (HER2+) breast and gastric cancer, and more recently HER2-low expressing breast cancers, and is being evaluated in additional tumor types (14–16).
Amplification or overexpression of cyclin E (CCNE1), a key regulator of the G1–S transition of the cell cycle, has been identified as a potential mechanism of intrinsic resistance to trastuzumab (17–19). Patients with HER2-positive breast cancer with high cyclin E expression have worse disease-specific survival in comparison with tumors with low cyclin E (20). Overexpression of cyclin E has also been implicated in resistance to trastuzumab in HER2-positive gastric cancer (8, 21). Preclinical studies have shown that HER2 signaling is closely linked to cell-cycle regulation, and that the antitumor activity of trastuzumab can be enhanced by targeting cell-cycle regulation in the setting of cyclin E amplification in vitro (20). Cyclin E overexpression and the resultant increase in cyclin-dependent kinase 2 (CDK2) activity have also been implicated in resistance to trastuzumab, and combinations with CDK2 inhibitors have been suggested as potential strategy to overcome intrinsic resistance in vivo (17).
An essential regulator of the G2–M cell-cycle checkpoint is Wee1 kinase, which negatively regulates cell-cycle progression by halting entry into mitosis to allow for activation of the DNA damage repair (DDR) pathway (22). Adavosertib (AZD1775, MK-1775), a Wee1 kinase inhibitor, has demonstrated enhanced activity preclinically in tumors with increased replicative stress and DDR pathway activation, including cyclin E overexpression (23). Wee1 kinase inhibition bypasses the ability of cells to arrest in G2 to repair their replicative stress mediated damage, resulting in the accumulation of DNA damage and subsequent mitotic catastrophe (24). Recent studies have found that Wee1 inhibition overcomes resistance to trastuzumab treatment (25, 26). In a recent multitumor basket trial, we demonstrated that patients with cyclin E amplification had a 27% objective response rate with adavosertib (27). Other clinical trials have evaluated adavosertib in combination with chemotherapy (28, 29).
Collectively, these studies have led to the hypothesis that T-DXd can have enhanced antitumor activity in combination with adavosertib in HER2 expressing tumors. We have directly tested this hypothesis by investigating the prevalence of ERBB2 and CCNE1 amplification across tumor types and examined the antitumor activity of T-DXd and adavosertib as single agents and combination in vitro and in vivo.
Materials and Methods
TCGA analysis
Copy-number data from the TCGA Pan-Cancer Atlas Hub was downloaded from UCSC Xena Functional Genomics Explorer (https://xenabrowser.net/) for “primary solid tumor” and “primary blood derived cancer—peripheral blood” samples. Discrete Independence Statistic Controlling for Observations with Varying Event Rates (DISCOVER), a statistical test for detecting co-occurrence and mutual exclusivity in cancer genomic data, was used to determine the co-occurrence of ERBB2 and CCNE1 amplification across tumor types (30). Tumor types with zero ERBB2 amplified cases were not reported.
MD Anderson Cancer Center genomic sequencing analysis
A prospectively maintained genomic database of patients at the University of Texas MD Anderson Cancer Center was queried for patients with ERBB2 amplification with and without CCNE1 amplification on patients who underwent comprehensive genomic profiling for routine clinical care. Pathologic diagnoses were abstracted from the electronic medical record. The analysis was performed under an Institutional Review Board approved protocol with waiver of informed consent.
Cell lines, drugs, antibodies, and other reagents
Breast cancer cell lines HCC-1954 (RRID: CVCL_1259), HCC-1569 (RRID: CVCL_1255), SK-BR-3 (RRID: CVCL_0033), BT-474 (RRID: CVCL_0179), MD-AMB-175VII (RRID: CVCL_1400), MDA-MB-436 (RRID: CVCL_0623), and MDA-MB-468 (RRID: CVCL_0419) were obtained from the ATTC. Gastric cancer cell line MKN7 (RRID: CVCL_1417) was obtained from the Japanese Collection of Research Bioresources Cell Bank (JCRB; NIBIO). Cell lines were mycoplasma-free tested using Lonza Mycoplasma Detection Kit. Cell line STR analyses were performed at cell line collection. Cell lines, except MKN7 which used RPMI1640 medium, were maintained in culture at 37°C and 5% CO2 in either DMEM or RPMI with 10% supplemental FBS. Cell line passages numbers were 2 and 4 between thawing and use. Adavosertib was obtained from the NCI Developmental Therapeutics Program, DXd was purchased from MedChemExpress, and both trastuzumab and T-DXd were obtained from The University of Texas MD Anderson Cancer Center Pharmacy. The following antibodies for Western blotting were purchased from Cell Signaling Technology: anti-cyclin B1 (#12231; RRID: AB_2783553), anti-cyclin E1 (#20808; RRID: AB_2783554), anti-CDK1 (#9116; RRID: AB_2074795), anti-phosphorylated CDK1 (#9114; RRID: AB_2074652), anti-CDK2 (#2546; RRID: AB_2276129), anti-phosphorylated CDK2 (#2561; RRID: AB_2078685), anti-phosphorylated CHK1 (#2348; RRID: AB_331212), anti-H2AX (#2595; RRID: AB_10694556), anti-γH2AX (#9718; RRID: AB_2118009), anti-HER2 (#2242; RRID: AB_331015), anti-p21 (#2947; RRID: AB_823586), anti-p27 (#2552; RRID: AB_10693314), anti-STING (#13647; RRID: AB_2732796), anti-phosphorylated TBK1 (#5483; RRID: AB_10693472), anti-TBK1 (#3504; RRID: AB_2255663), anti-phosphorylated IRF3 (#37829; RRID: AB_2799121), anti-IRF3 (#11904; RRID: AB_2722521), anti-phosphorylated IKKαβ (#2697; RRID: AB_2079382), anti-phosphorylated AKT (#4060; RRID: AB_2315049), anti-AKT (#9272; RRID: AB_329827), anti-phosphorylated ERK1/2 (#4370; RRID: AB_2315112), and anti-ERK1/2 (#9102; RRID: AB_330744). Anti-B-actin antibody (#A5441; RRID: AB_476744) was purchased from Sigma. Secondary antibodies for Goat-anti-Rabbit Alexa Fluor-680 (#A21076; RRID: AB_2535736) and Goat-anti-Mouse-Dylight-800 (#610145–121; RRID: AB_2556774) were purchased from Life Technologies and Rockland Immunochemicals, respectively.
Cell viability assay
Cells were seeded at a density of 1.0 to 2.0 × 105 cells per 100 μL per well into 96-well plates, based on previously established seeding conditions. Following cell adhesion for overnight, treatments were performed in triplicate with both monotherapy and combination therapy with serially diluted concentrations ranging from 0.1 to 10,000 nmol/L (DXd) or 0.2 to 20,000 nmol/L (adavosertib) or 0.012 to 1200 μg/mL (T-DXd) and incubated at 37°C for 72 hours. Fixation was performed with 50% trichloroacetic acid at 4°C for 1 hour, and cells were then stained with 0.4% sulforhodamine B (SRB) for 1 hour. Plates were washed, air dried for 30 to 60 minutes, and 10 mmol/L Tris base was added to solubilize the protein-bound SRB dye for 10 minutes. Absorbance measurements were performed with Synergy 4 (BioTek, RRID: SCR_019750) and optical density (OD) values were read at 490 nm. The IC50 and CI values were determined using CalcuSyn (RRID: SCR_020251). The CI were calculated on the basis of the Chou–Talalay model, with CI < 1.0, synergistic; CI = 1, additive; and CI > 1.0, antagonistic.
Apoptosis assay
Cells were seeded at a density of 5.0 × 105 cells per plates into 6 cm plates. Treatments were performed the next day in triplicate with the following doses: adavosertib 1,000 nmol/L, DXd 100 nmol/L, and T-DXd 20 μg/mL. After 72 hours, cells supernatant and trypsinized attached cells were combined, and then washed with phosphate-buffered saline (PBS). Cell pellets were resuspended with Annexin-V-FLUOS buffer (Roche), transferred to 5mL round-bottom cell-strainer tubes (Falcon), and centrifuged. Annexin-V-Fluorescein was added to each tube and incubated for 10 minutes at 25°C followed by propidium iodide, which was incubated for 1 minute at 25°C. Annexin-V-FLUOS buffer was then added and cells were assessed by flow cytometry.
Isogenic knockdown of cyclin E
HEK-293 cells were seeded at a density of 1.0 × 105 cells per well overnight and transfected using Lipofectamine 3000 (Thermo Fisher Scientific), the following day with viral plasmids for CCNE1 shRNA [nonsilencing control and knockdown #32 (V2LHS_642332)] purchased from shRNA and ORFeome Core Facility at MDACC. Single cell colonies were then established, and cyclin E knockdown was confirmed by Western blot analysis. Viral media was harvested 48 to 72 hours following transfection. MKN7 cells were seeded in 6-well plates at a density of 1.0 × 105 cells per well overnight and infected with CCNE1 shRNA lentivirus for 2 to 3 days followed by puromycin selection for three passages. Cyclin E western blotting was then performed to confirm adequate knockdown of cyclin E expression.
Overexpression of cyclin E
Inducible Cyclin E expression plasmid pCW57.1-CCNE1 (RRID: Addgene_164144) was transfected into HEK293 cells along with virus packaging vectors. Lentivirus were collected. The HER2-positive breast cancer cell line HCC1954 were transduced with cyclin E virus. After blasticidin selection, cells were treated doxycycline at 50, 500, and 5,000 ng/mL for 2 days. Cell lysates were prepared to detect cyclin E expression by immunoblotting. For cell viability assays, cells were treated with drugs in the presence or absence of doxycycline.
Western blot analysis
Immunoblotting was performed to evaluate the effects of DXd and T-DXd in combination with adavosertib in MKN7 cells. Cells were treated for 6, 12, 24, or 72 hours with monotherapy or combination therapy at clinically relevant doses. Cells were dissociated with trypsin, washed with PBS, and lysis was performed in 1× Laemmli buffer. Protein concentration of cell lysates was quantified using the Pierce BCA Protein Assay Kit (Thermo Fisher Scientific) and equal protein was loaded into the gel. Protein was then transferred to a 2.0 μm nitrocellulose membrane (Bio-Rad Laboratories) and blocked with 0.1% casein blocking buffer (Thermo Fisher Scientific) at room temperature for 1 hour. Primary antibodies were prepared at 1:1,000 dilution in 5% bovine serum albumin (BSA) and membranes were incubated at room temperature overnight. Membranes were then washed in TBST for 3 × 5 minutes, incubated with secondary antibodies (anti-rabbit and/or anti-mouse) formulated at 1:10,000 dilution in 5% BSA for one hour, and washed TBST for 3 × 5 minutes. Immunoblot visualization was performed using the Odyssey IR imaging system (Li-Cor Biosciences).
In vivo studies
Animal experiments were approved by the Institutional Animal Care and Use Committee at the University of Texas MD Anderson Cancer Center. Tumors samples were obtained by image-guided biopsy from patients and implanted into the flanks of female NSG mice (The Jackson Laboratory). Informed written consent’ was obtained from each subject that participated in the PDX development protocol, which was approved by the MD Anderson Institutional Review Board; studies were conducted in accordance with the Declaration of Helsinki and the U.S. Common Rule. Once tumors were adequate size, they were passaged into athymic nu/nu mice. Treatments were initiated when tumors were approximately 200 to 400 mm3 in size. Mice were euthanized when experimental endpoint was reached or when tumor diameter reached 2,000 mm. Tumor volume (Vt) was calculated as Vt (mm3) = (width)2 × length)/2 and % change in Vt from baseline was calculated as (Vt,day0 – Vt,dayX)/Vt,Day0. Relative treatment-to-control (T/C) ratio was calculated as (Vt,day21/Vt,day0)/(Vc,day21/Vc,day0), where t is the treatment and c is the control. Event-free survival (EFS-2) was defined as the which tumor volume doubled in size from baseline.
Treatment and schedule
To evaluate the antitumor activity of T-DXd in combination with adavosertib, we randomized mice (N = 4–5) to four treatment arms: untreated control, adavosertib (60 mg/kg), T-DXd (1, 3, or 10 mg/kg), and adavosertib (60 mg/kg) + T-DXd (1, 3, or 10 mg/kg). Adavosertib was delivered by oral gavage 5 days on with 2 days off and formulated in 5% methylcellulose vehicle. T-DXd was diluted in sterile water and delivered intravenously by tail vein injection on day 1 of each 21-day cycle. For the sequencing study in PDX.003.204, adavosertib was delivered in four schedules (A, B, C, and D) per 21-day cycle: days 1–5, 8–12, and 15–19 (schedule A), days 1–2, 8–9, and 15–16 (schedule B), days 1–3 (schedule C), and days 2–3 (schedule D).
Whole-exome sequencing
Fragments of flash frozen PDX tissues were lysed in buffer containing protease K and homogenized. DNA was then extracted using the Qiagen DNA Mini Kit per manufacturer's protocol. Normal DNA was also purified from whole blood samples using Qiagen Blood Mini Kit (Qiagen) per manufacturer's protocol. Extracted genomic DNA was then quantified with Qubit 2.0 DNA HS Assay (Thermo Fisher Scientific) and quality assessed by 1% standard agarose gel. Library preparation and exome capture was performed using SureSelectXT Low Input Kit and Agilent All Exome V4 (Agilent). Library quantity assessed with QuantStudio 5 System (Applied Biosystems, RRID: SCR_020240) Illumina 8-nt dual-indices were used. Equimolar pooling of libraries was performed on the basis of QC values and sequenced on an Illumina NovaSeq S4 (Illumina) with a read length configuration of 150 PE for 100M PE reads (50M in each direction). Whole-exome sequencing of samples was performed using the Institute of Personalized Cancer Therapy Cancer Genomic Laboratory or by Amadera Health.
Immunohistochemistry
Tumors were collected at the conclusion of each experiment and rapidly fixed in 10% neutral-buffered formalin for 24 hours and washed with ethanol. Tissues were formalin fixed and paraffin embedded by the MDACC Research Histology Core Facility. HER2 IHC was performed using by the MDACC Clinical Laboratory Improvement Amendments (CLIA) certified lab and reviewed by a pathologist.
Reverse-phase protein array (RPPA)
Tumor tissues of PDXs used for PD analysis [PDX.003.204 (n = 5) and PDX.003.213 (n = 5)] were collected following treatment with T-DXd and/or adavosertib. At the conclusion of each experiment, tumor fragments were flash frozen in liquid nitrogen and stored at −80°C. Tumor pieces of approximately 3 × 3 mm in size were placed in bead lysis tubes for protein extraction. RPPA conducted by the MD Anderson Functional Proteomics Core Facility. Proteins were quantified and normalized for loading and differential expression of proteins was assessed.
Statistical analysis
For in vitro and in vivo experiments, statistical comparisons, including Student t test and Pearson correlation test, and figures were generated using Prism version 8 (GraphPad). Log-rank test was used to compared EFS-2 curves for in vivo studies.
Data availability
The data that support the findings of this study are available from the corresponding author upon reasonable request.
Results
ERBB2 and CCNE1 amplification across solid tumors
To investigate the prevalence of ERBB2 and co-occurring CCNE1 amplification, we assessed sequencing data across tumor types included in The Cancer Genome Atlas (TCGA) Pan-Cancer cohort. A total of 706 patients were identified with ERBB2 amplification and 161 (22.8%) of these had co-amplification of CCNE1. We observed co-occurring amplification of ERBB2 CCNE1 in stomach adenocarcinoma (37% of ERBB2 amplified patients; 8.4% total patients), ovarian serous cystadenocarcinoma (41.1% of ERBB2 amplified patients; 3.9% total patients), and uterine corpus endometrioid carcinoma (42.9% of ERBB2 amplified patients, 4.9% total patients; Table 1).
Table 1.
Co-amplification of ERBB2 and CCNE1 across tumor types in the cancer genome atlas.
| ERBB2 AMP | CCNE1 AMP | Co-AMP | Total | ||
|---|---|---|---|---|---|
| Tumor type | N | N | N | % | N |
| Breast invasive carcinoma | 169 | 76 | 18 | 10.70% | 1,099 |
| Stomach adenocarcinoma | 100 | 67 | 37 | 37.00% | 440 |
| Uterine corpus endometrioid carcinoma | 63 | 73 | 27 | 42.90% | 547 |
| Ovarian serous cystadenocarcinoma | 56 | 203 | 23 | 41.10% | 595 |
| Bladder urothelial carcinoma | 47 | 52 | 10 | 21.30% | 414 |
| Esophageal carcinoma | 42 | 21 | 9 | 21.40% | 184 |
| Lung squamous cell carcinoma | 38 | 90 | 10 | 26.30% | 524 |
| Lung adenocarcinoma | 36 | 68 | 6 | 16.70% | 553 |
| Colon adenocarcinoma | 36 | 11 | 3 | 8.30% | 504 |
| Cervical and endocervical cancer | 23 | 13 | 3 | 13.00% | 295 |
| Head and neck squamous cell carcinoma | 19 | 13 | 2 | 10.50% | 524 |
| Pancreatic adenocarcinoma | 17 | 12 | 2 | 11.80% | 184 |
| Uterine carcinosarcoma | 15 | 25 | 5 | 33.30% | 56 |
| Rectum adenocarcinoma | 14 | 1 | 0 | 0.00% | 169 |
| Sarcoma | 11 | 50 | 4 | 36.40% | 263 |
| Liver hepatocellular carcinoma | 6 | 18 | 1 | 16.70% | 376 |
| Prostate adenocarcinoma | 4 | 3 | 0 | 0.00% | 499 |
| Kidney papillary cell carcinoma | 3 | 0 | 0 | 0.00% | 303 |
| Glioblastoma multiforme | 1 | 11 | 0 | 0.00% | 607 |
| Brain lower grade glioma | 1 | 4 | 0 | 0.00% | 515 |
| Testicular germ cell tumor | 1 | 2 | 0 | 0.00% | 150 |
| Skin cutaneous melanoma | 1 | 3 | 0 | 0.00% | 104 |
| Adrenocortical cancer | 1 | 7 | 1 | 100.00% | 90 |
| Diffuse large B-cell lymphoma | 1 | 0 | 0 | 0.00% | 48 |
| Cholangiocarcinoma | 1 | 0 | 0 | 0.00% | 36 |
| Total | 706 | 823 | 161 | 22.80% | 9,079 |
We then examined the frequency of ERBB2 and CCNE1 amplifications across tumor types in a clinical genomic database of patients at the University of Texas MD Anderson Cancer Center. We identified a total of 455 patients with ERBB2 amplification and of these, 60 (13.2%) had co-occurring amplification of CCNE1. Similarly, ERBB2 amplified tumors frequently had CCNE1 co-amplification in this cohort, including 38.1% of gastroesophageal, 25% of endometrial, and 15.4% of ovarian cancers. Taken together, these findings suggest that solid tumors with ERBB2 often have co-occurring amplification of CCNE1, thus identifying a subset of ERBB2 amplified patients may benefit from therapeutic strategies which target both alterations.
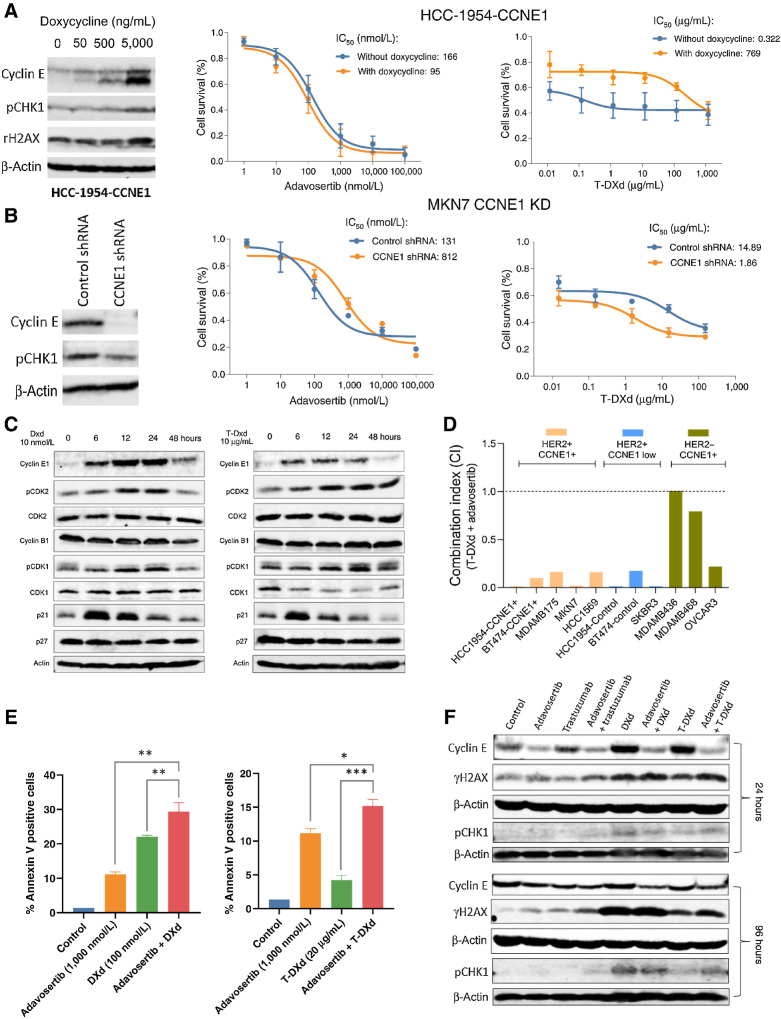
Cyclin E overexpression modulates sensitivity to T-DXd in HER2-positive cell lines
We used the Wee1 kinase inhibitor adavosertib to target cyclin E overexpression based on preclinical (23) and clinical findings (31). We also assessed the impact of cyclin E expression on the sensitivity to T-DXd in different cell line models. To this end, we generated a cyclin E inducible (via doxycycline) HER2+ HCC-1954 cell line and subjected it to single agent treatments with adavosertib and T-DXd (Fig. 1A; Supplementary Fig. S1A). Results revealed that while overexpression of cyclin E induced by doxycline at 5,000 ng/mL led to a slight decrease in a IC50 of adavosertib, it desensitized cells to T-DXd with a IC50 that increased more than 2,000-fold (Fig. 1A). Conversely, knockdown of cyclin E in the gastric cancer cell line MKN7 with amplification of ERBB2 and CCNE1 led to increased sensitivity to T-DXd (Fig. 1B; Supplementary Fig. S1B). To confirm this finding, we screened more cell lines with varying status of HER2 and cyclin E expression by immunoblotting (Supplementary Fig. S1C). Taking this cell line panel with varying HER2 and cyclin E status together, cell viability assay showed that cell lines in which HER2 and cyclin E are co-expressed were less sensitive to T-DXd as demonstrated by significantly higher IC50s than those with HER2+/cyclin E low or and higher than HER2−/cyclin E+ status. But HER2+/cyclin E+ cell lines were more sensitive to adavosertib than HER2+/cyclin E low cell lines (Supplementary Figs. S1D and S1E). Taken together, these findings suggest that cyclin E overexpression may deem cancer cells less sensitive to T-DXd therapy. Wee1 was expressed in all cell lines tested and its expression did not correlate with adovasertib sensitivity (Supplementary Fig. S1F).
Figure 1.
DXd is synergistic in combination with adavosertib and induces expression of cyclin E in vitro. A, Effects of cyclin E overexpression on cell sensitivity to adavosertib and T-DXd. HER2+ HCC-1954 cells were transfected with a viral expression cassette for cyclin E. In the absence or presence of doxycycline, cells were treated with adavosertib or T-DXd at individual dose ranges, or their combination, for 72 hours. Immunoblotting was performed to detect cyclin E, pCHK1, and γH2AX. Cell viability was measured using SRB assay. Drug IC50s and combination index (CI) were calculated using CalcuSyn. B, Effects of cyclin E knockdown on cell sensitivity to adavosertib and T-DXd. HER2+ MKN7 cells were transfected with CCNE1 shRNA virus. Immunoblotting was performed to detect cyclin E and pCHK1. Cell viability assays with adavosertib and T-DXd were performed as described above. C, Immunoblot of cell-cycle regulatory proteins in MKN7 cells following treatment with DXd and T-DXd for 6, 12, 24, and 48 hours. D, Combination index of T-DXd and adavosertib measured by cell viability assay in a panel of 11 cell lines. E, Apoptosis assay of DXd and T-DXd in combination with adavosertib in MKN7 cells using Annexin V labeling. F, Immunoblot of cyclin E, γH2AX, and pCHK1 in MKN7 cells following treatment with DXd and T-DXd in combination with adavosertib for 24 or 96 hours. CCNE1, cyclin E1; DXd, deruxtecan; ERBB2, Erb-B2 receptor tyrosine kinase 2; T-DXd, trastuzumab deruxtecan.
DXd and T-DXd upregulate cyclin E expression in vitro
Next, we assessed the impact of T-DXd and its topoisomerase I inhibitor payload, DXd, on cell cycle and DNA damage response pathways related to CCNE1/Wee1, in the MKN7 cell line (Fig. 1C; Supplementary Figs. S1G–S1L). Cells were treated up to 48 hours with 10 nmol/L of DXd or 10 μg/mL of T-DXd. Cyclin E expression was induced from 6 to 24 hours following treatment with both DXd and T-DXd. Phosphorylated cyclin-dependent kinase 2 (pCDK-2) was also upregulated at 12 to 24 hours with DXd and from 12 to 48 hours with T-DXd. The CDK inhibitor p21 (p21) was also upregulated at 6 to 12 hours following treatment with DXd and T-DXd. These findings suggest that in a background of ERBB2 and CCNE1 amplification, DXd and T-DXd can further induce the expression cyclin E in vitro.
Adavosertib is synergistic with DXd in HER2-positive cell lines
We next tested the hypothesis that DXd and T-DXd may sensitize cancer cells to agents that can target increased replicative stress, such as adavosertib. We examined the activity of combination therapies of DXd and adavosertib by cell viability assays in a panel of cell lines with varying HER2/cyclin E status. Notably, we observed synergy in HER2+ cell lines with or w/o cyclin E overexpression, with combination index (CI) ranging from 0.004 to 0.28, but not in HER2−/cyclin E-expressing cell lines (Fig. 1D). We did not systematically test the combination of adovasertib with trastuzumab, however in MKN7 cells, we did not observe synergy with this combination (data not shown).
Apoptosis was assessed by annexin V staining to determine the effects of adavosertib in combination with DXd or T-DXd in MKN7 cells. Although both DXd and T-DXd were capable of inducing apoptosis (5–20%), combination of these agents with adavosertib significantly enhanced their apoptotic effects (15–30%) as evidenced by further increased population percentage of annexin V positive cells (Fig. 1E). We then treated MKN7 cells with the adavosertib in combination with DXd (10 nmol/L), T-DXd (10 μg/mL), and trastuzumab (20 μg/mL) to assess the impact of these combinations on expression of cyclin E and phosphorylated (Ser-139 residue) histone variant H2AX (γH2AX), a marker of DNA damage. Immunoblotting showed that treatment with DXd and T-DXd upregulated cyclin E expression at both early (24 hours) and late (96 hours) time points, which was abrogated by adavosertib (Fig. 1F; Supplementary Fig. S2A). Both γH2AX and phosphorylated CHK1 (pCHK1) levels were upregulated by both DXd and T-DXd. Further, γH2AX was enhanced by combination treatment with adavosertib and DXd or T-DXd and pCHK1 was enhanced by adavosertib and T-DXd combination at both time points (Fig. 1F; Supplementary Figs. S2B and S2C). Similarly, although doxycycline dose-dependently induced overexpression of cyclin E in 72 hours, it also increased pCHK1 and γH2AX levels at its highest concentration (Fig. 1A; Supplementary Figs. S2D and S2E). Consistently, in MKN7 cells with cyclin E knockdown pCHK1 expression was decreased, compared with control MKN7 cells (Fig. 1B; Supplementary Fig. S2F).
Taken together, these results suggest that transient induction of cyclin E expression by DXd or T-DXd may sensitize cells to Wee1 kinase inhibition, as evident by enhanced DNA damage pathway activation and apoptosis when combined with adavosertib.
Adovasertib has been proposed to enhance antitumor immune response in part by activating stimulator of IFN genes (STING) signaling (32). Therefore, we assessed the effect of the therapy on STING signaling in HER2+ cell lines. We found in BT-474 cells that T-DXd treatment led to an increase in STING expression as well as an increase of a STING downstream markers pIRF3 at 24 and 72 hours, but this was not substantially enhanced by the combination (Supplementary Fig. S2G). We observed enhancement in expression of other STING markers by the combination treatment at 24 hours, including pTBK1 in BT-474 cells and pIKKαβ in HCC-1954 cells (Supplementary Figs. S2G and S2H).
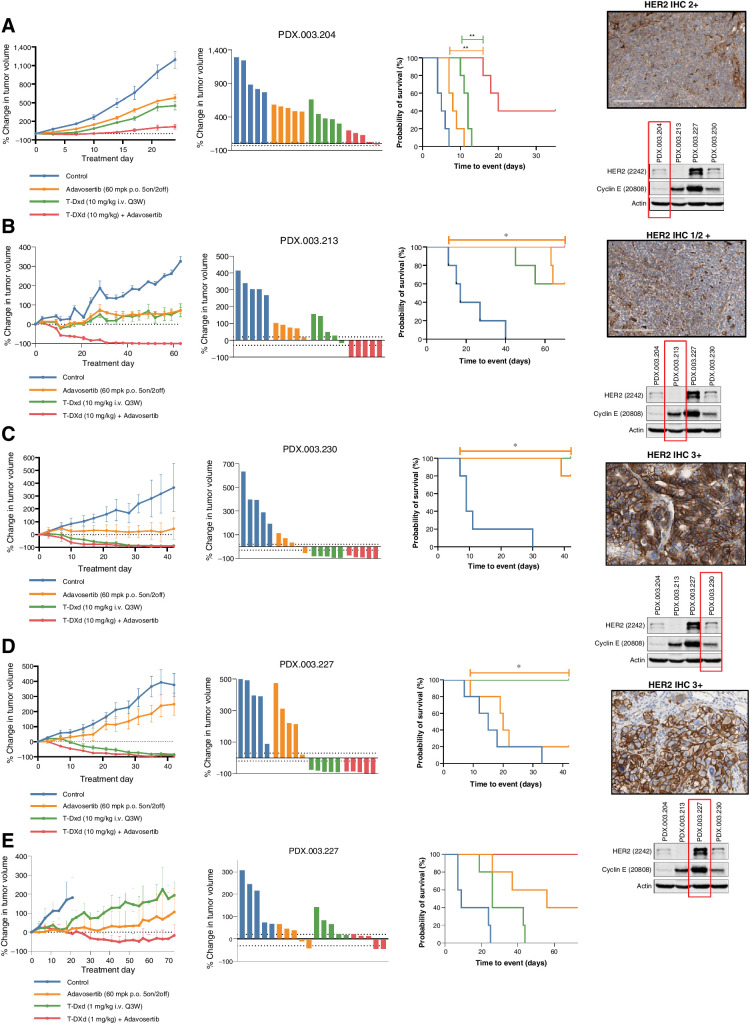
In vivo activity of T-DXd and adavosertib in HER2-low (IHC 1/2+) expressing gastroesophageal cancers
On the basis of the synergistic effects observed between DXd and adavosertib in vitro, we selected a panel of PDX models with HER2 expression and/or ERBB2 amplification, both with and without CCNE1 co-amplification, to assess the antitumor activity of T-DXd in combination with adavosertib in vivo (Supplementary Table S1). Models selected were developed from patients with gastric/GEJ cancers who had previously been treated with 5-FU and oxaliplatin, as well as trastuzumab for their HER2-positive disease. Two of the gastric/GEJ PDX models (PDX.003.204 and PDX.003.227) were developed from patients who had received topoisomerase inhibitor irinotecan as monotherapy or as a component of the FOLFIRI regimen [folinic acid, fluorouracil (5-FU), and irinotecan]. The PDX models were molecularly characterized by whole-exome sequencing (WES), HER2 IHC, as well as Western blotting (Fig. 2A–D).
Figure 2.
In vivo activity of T-DXd in combination with adavosertib in HER2 low (1/2+) and high (3+) expressing gastroesophageal cancers. A, Adavosertib (60 mg/kg p.o. 5on/2off) enhanced tumor growth inhibition when combined with T-DXd (10 mg/kg i.v. every 3 weeks) in a HER2 low, cyclin E amplified gastroesophageal cancer PDX model PDX.003.204. Right panel demonstrates HER2 IHC and immunoblotting for HER2, Cyclin E1, and actin. B, Adavosertib (60 mg/kg p.o. 5on/2off) in combination with T-DXd (10 mg/kg i.v. every 3 weeks) led to enhanced tumor regression in a HER2 low, cyclin E amplified gastroesophageal cancer PDX model PDX.003.213, with an ERBB2 V777 L and G778A mutation. C, T-DXd (10 mg/kg IV every 3 weeks) induced durable tumor regression alone and in combination with adavosertib in an ERBB2 amplified and HER2 overexpressing gastroesophageal PDX cancer model PDX.003.230. Adavosertib alone also demonstrated significant antitumor activity. D and E, T-DXd (10 mg/kg i.v. every 3 weeks) induced durable tumor regression in an ERBB2 amplified and HER2 overexpressing gastroesophageal PDX cancer model PDX.003.227 with concomitant cyclin E amplification/expression alone and when T-DXd was combined with adavosertib (60 mg/kg p.o. 5on/2off) (D). Antitumor activity of lower dose T-DXd (1 mg/kg i.v. every 3 weeks) was enhanced with the combination with adavosertib (60 mg/kg p.o. 5on/2off) (E). PDX, patient-derived xenograft; T-DXd, trastuzumab deruxtecan.
We first assessed the combination of T-DXd with adavosertib in PDXs with low HER2 expression. PDX.003.204 (HER2 2+ IHC, CCNE1 amplified) demonstrated modest tumor growth inhibition with T-DXd monotherapy as shown by a relative treatment-to-control (T/C) ratio 0.48, and event-free survival (time to tumor doubling, EFS-2) of 12 days versus 5 days (P = 0.002). The antitumor activity was significantly enhanced compared with T-DXd when adavosertib was combined with T-DXd (T/C ratio 0.18; EFS-2 20 days vs. 12 days, P = 0.002; Fig. 2A). PDX.003.213 (HER2 1/2+, FISH negative, CCNE1 amplified) similarly showed only growth inhibition but not regression with either adavosertib (T/C ratio 0.68; EFS-2 not estimable) or T-DXd (T/C ratio 0.66; EFS-2 not estimable) monotherapies. However, the activity of T-DXd was dramatically enhanced when combined with adavosertib, leading to near complete tumor regression in all mice treated (T/C ratio 0.17; EFS-2 not estimable; Fig. 2B). Although this HER2-low expressing model did not demonstrate ERBB2 amplification on WES, ERBB2 mutations (V777L, G778A) were identified in the tyrosine kinase domain. The volumetric analysis data (T/C ratio and EFS-2) can be found in Supplementary Table S2.
We recently reported that, preclinically, in the setting of transient cyclin E induction, sequential treatment of adavosertib has enhanced activity to concurrent treatment (23). We assessed if such induction of cyclin E by DXd and T-DXd (Fig. 1C and F) would provide treatment benefit by sequential treatment of T-DXd + adavosertib. We first evaluated this sequencing in vitro by treating MKN7 cells for 48 hours with DXd (10 nmol/L) or T-DXd (10 μg/mL) both as monotherapy or in combination with adavosertib, concurrently and sequentially (Supplementary Fig. S3C). For each treatment schedule, expression levels of cyclin E, HER2, and γH2AX were assessed in the residual tumors, following 24 and 48 hours of therapy. When treated with T-DXd, HER2 expression was downregulated in monotherapy or in combination with adavosertib. Both DXd and T-DXd induced expression of cyclin E, and this effect was abrogated when combined with adavosertib. Although γH2AX expression was upregulated following treatment with DXd or T-DXd in combination with adavosertib, there was no appreciable difference in concurrent or sequential treatment.
Considering the potential for overlapping toxicity with T-DXd + adavosertib in anticipation of future clinical studies, treatment sequencing was further assessed in vivo to determine if this combination was equally as efficacious with less frequent dosing of adavosertib. We selected PDX.003.204, a GEJ adenocarcinoma model with HER2 2+ by IHC and CCNE1 amplification on WES, for treatment based on the enhanced combinatorial activity demonstrated in Fig. 2A. Mice were treated with T-DXd and adavosertib monotherapy, and adavosertib was administered in four different schedules (A, B, C, and D) when combined with T-DXd per 21-day cycle: days 1 to 5, 8 to 12, and 15 to 19 (schedule A), days 1 to 2, 8 to 9, and 15 to 16 (schedule B), days 1 to 3 (schedule C), and days 2 to 3 (schedule D; Supplementary Fig. S3A). The greatest tumor growth inhibition and longest time to tumor doubling was observed in schedule A (5 day on/2 day off dosing of adavosertib; Supplementary Fig. S3B).
In vivo activity of T-DXd and adavosertib in HER2-high (IHC 3+) gastroesophageal cancers
We next selected two gastroesophageal PDX models with HER2 overexpression (IHC 3+) and ERBB2 amplification. We tested PDX.003.230, a model that is HER2 3+ and ERBB2 amplified. This model was also noted to have an ERBB2 T733I mutation of the kinase domain and has been previously reported in gastric cancer, and found to be weakly transforming, but resistant to lapatinib (33, 34). PDX.003.230 demonstrated near complete tumor regression in all mice with both T-DXd 10 mg/kg (T/C ratio 0.36; EFS-2 not estimable) and T-DXd 10 mg/kg + adavosertib (T/C ratio 0.11; EFS-2 not estimable). Additionally, PDX.003.230 demonstrated robust sensitivity to adavosertib monotherapy (T/C ratio 0.46; EFS-2 not estimable vs. 9 days, P = 0.002), which was notable considering that no other models were sensitive to this agent alone (Fig. 2C).
PDX.003.227 (HER2 3+ IHC, ERBB2, and CCNE1 amplified) had high levels of cyclin E expression on Western blotting but was not sensitive to adavosertib monotherapy. However, this model demonstrated near complete tumor regression in all mice with both T-DXd 10 mg/kg (T/C ratio 0.20; EFS-2 not estimable) and T-DXd 10 mg/kg + adavosertib (T/C ratio 0.09; EFS-2 not estimable; Fig. 2D). Reducing the dose of T-DXd to 1 mg/kg also induced significant tumor growth inhibition (T/C ratio 0.59; EFS-2 26 days vs. 9 days, P = 0.013), although the combination of adavosertib and T-DXd had greater antitumor activity than T-DXd, achieving tumor regression (T/C ratio 0.33; EFS-2 not estimable vs. 26 days, P = 0.002; Fig. 2E).
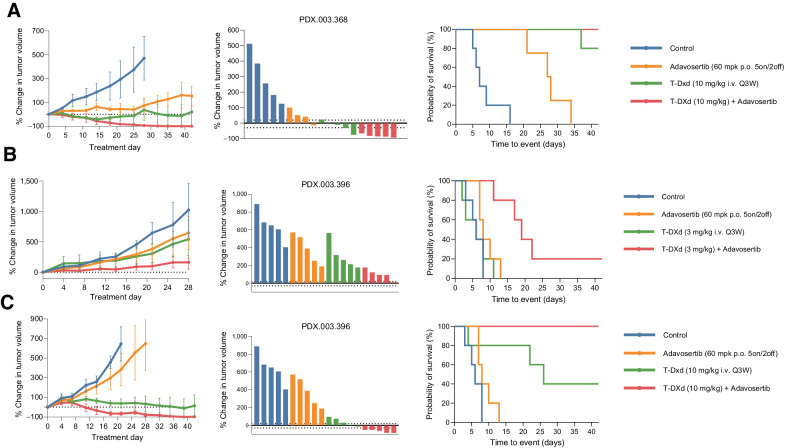
Adavosertib enhances efficacy of T-DXd in other tumor types and in the setting of prior T-DXd exposure
In addition to the four HER2-expressing gastric/GEJ models, we tested an endometrial neuroendocrine carcinoma model and a colorectal adenocarcinoma model. Endometrial neuroendocrine carcinoma model PDX.003.368 was HER2 3+ and ERBB2 amplified as well as CCNE1 amplified (Figs. 3A). This PDX was generated from a patient who had previously been treated with trastuzumab + pertuzumab and cisplatin + etoposide. This model exhibited sensitivity to adavosertib monotherapy (T/C ratio 0.37; EFS-2 27.5 days vs. 7 days, P = 0.002), as well as T-DXd at 3 mg/kg (T/C ratio 0.20; EFS-2 not estimable vs. 7 days, P = 0.002). Regression was observed with the combination of adavosertib with T-DXd at 3 mg/kg (T/C ratio 0.06; EFS-2 not estimable).
Figure 3.
In vivo activity of T-DXd in combination with adavosertib in other HER2 high (3+) expressing tumors. A, T-DXd (3 mg/kg i.v. every 3 weeks) induced durable tumor regression in an ERBB2 amplified/HER2 overexpressing and CCNE1 amplified endometrial neuroendocrine carcinoma PDX, PDX.003.368. B and C, Adavosertib (60 mg/kg p.o. 5on/2off) in combination with T-DXd (3 and 10 mg/kg i.v. every 3 weeks) led to enhanced tumor regression in a ERBB2 amplified/HER2 overexpressing colorectal cancer model PDX.003.396, which was developed from a patient who had progressed after treatment with T-DXd. PDX, patient-derived xenograft; T-DXd, trastuzumab deruxtecan.
Although T-DXd has exhibited substantial activity in clinical trials, most patients ultimately progress. We thus investigated the activity of T-DXd when combined with adavosertib in PDX.003.396, a HER2 overexpressing/amplified colorectal cancer PDX model developed from a patient who was previously treated with T-DXd and had prolonged stable disease, but ultimately progressed after cycle 14 (Fig. 3B and C). This PDX model was HER2 3+ by IHC with ERBB2 amplification but without CCNE1 amplification on WES. We tested T-DXd at both 3 mg/kg (Fig. 3B) and 10 mg/kg (Fig. 3C) in combination with adavosertib. This model had limited sensitivity to T-DXd at 3 mg/kg (T/C ratio 0.55; EFS-2 6 days vs. 6 days, P = 0.916), but T-DXd at 3 mg/kg + adavosertib led to prolonged time to tumor doubling (T/C ratio 0.27; EFS-2 19 days vs. 6 days, P = 0.006). T-DXd monotherapy at 10 mg/kg induced tumor growth inhibition in comparison with control (T/C ratio 0.19; EFS-2 26 days vs. 6 days, P = 0.034) but when combined with adavosertib, durable tumor regression was observed (T/C ratio 0.04; EFS-2 not estimable with combination vs. 26 days with T-DXd, P = 0.049). Notably, in this model previously exposed to T-DXd, T-DXd monotherapy had significant antitumor activity, but objective responses were not achieved even with higher dose of T-DXd. However, adovasertib significantly enhanced T-DXd activity, by enhancing antitumor activity and EFS of low-dose T-DXd, leading to objective responses and prolongation of EFS with high-dose T-DXd.
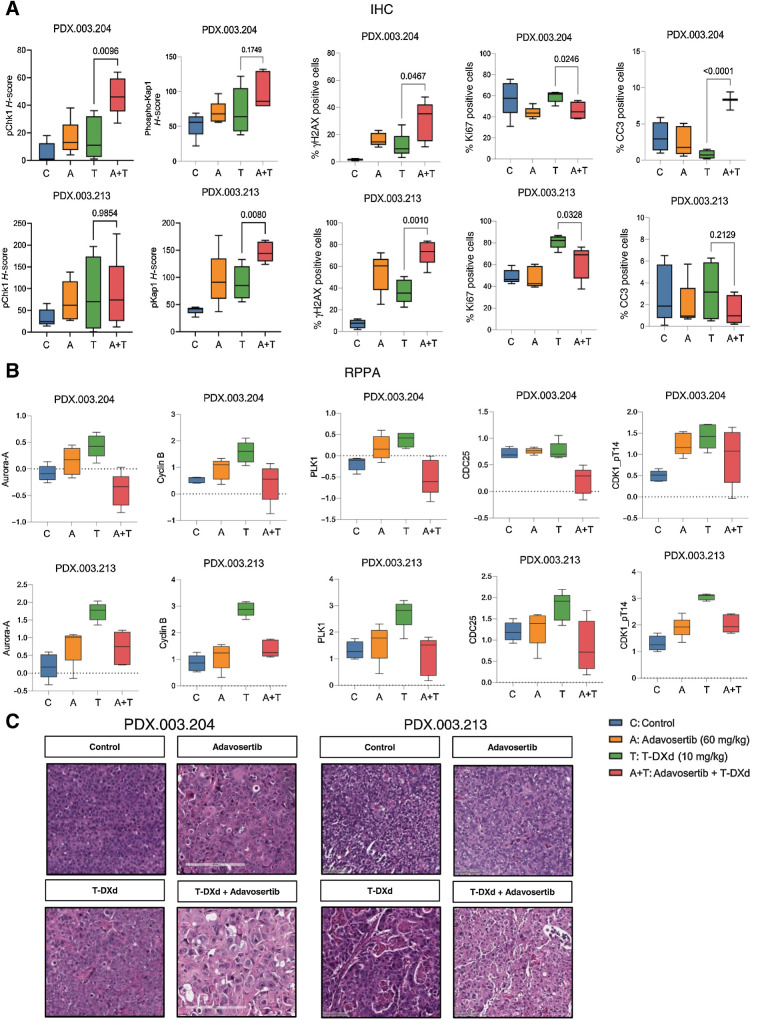
Pharmacodynamic effects of T-DXd in combination with adavosertib
To evaluate the pharmacodynamic effects of T-DXd in combination with adavosertib, we selected two HER2-low expressing gastric/GEJ models (PDX.003.204 and PDX.003.213; see Fig. 2). For biomarker analysis, mice were treated with adavosertib, T-DXd, or T-DXd + adavosertib and euthanized 10 days following treatment. IHC was performed to evaluate DNA damage response (γH2AX, phosphorylated Kap1, p-CHK1), cellular proliferation (Ki67), and apoptosis (cleaved caspase 3) markers. Single-agent treatments with adavosertib or T-DXd resulted in an increase in γH2AX, which was not statistically significant. However, combination therapy with T-DXd + adavosertib demonstrated statistically significant increase in γH2AX as compared with T-DXd or adavosertib alone in both models (Fig. 4A). Phosphorylated Chk1 had higher expression following treatment with T-DXd + adavosertib in comparison with T-DXd monotherapy in PDX.003.204 (H-score: mean 16.0 T-DXd vs. 47.2 T-DXd + adavosertib, P = <0.01), although this was not significantly different in PDX.003.213. There was higher expression of phosphorylated Kap1 in PDX.003.213 (H-score: mean 89.8 T-DXd vs. 146.8 T-DXd + adavosertib, P = 0.008), although this effect was not observed in PDX.003.204 (Fig. 4A; refs. 35–37).
Figure 4.
Pharmacodynamic effects of adavosertib in combination with T-DXd following 10-day treatment. Mice were treated for 10 days with adavosertib, T-DXd, or T-DXd + adavosertib, or were untreated (N = 5). A, PD marker assessment by IHC for adavosertib, T-DXd, and adavosertib + T-DXd tumors assessing phosphorylated Chk1, phosphorylated Kap1, γH2AX, Ki67, and cleaved caspase 3 (CC3). B, Differential expression of proteins assessed by RPPA revealed alterations in expression of several proteins involved in G2–M transition, including Aurora-A, cyclin B, PLK1, CDC25, and phosphorylated CDK1. C, Morphologic features on H&E staining observed following 10-day treatments with adavosertib, T-DXd, or T-DXd + adavosertib. CCNE1, Cyclin E; ERBB2, Erb-B2 receptor tyrosine kinase 2; H&E, hematoxylin and eosin; PD, pharmacodynamic.
To further investigate the combination of T-DXd and adavosertib, we then compared differential expressed proteins (DEP) with monotherapy and combination therapy by reverse-phase protein array analysis (RPPA; Fig. 4B). RPPA was conducted at 10 days to not only capture downstream effects but also in vivo adaptive responses that evolve. Unsupervised hierarchical clustering was performed on pairwise group comparisons for both PDX models assessed (Supplementary Figs. 4 and 5). Using an FDR of 0.05, we identified 19 DEPs between the T-DXd versus T-DXd + adavosertib arms in PDX.003.213 and 63 DEPs between these arms in PDX.003.204. We focused our assessment on overlapping DEPs between T-DXd and T-DXd + adavosertib treatment arms in both PDXs. Several proteins implicated in cell-cycle progression and the G2–M transition, including aurora kinase A, cyclin B, PLK1, CDC25, and CDK1 (phospho-T14) were significantly different when comparing T-DXd to T-DXd + adavosertib (Fig. 4B).
Additionally, we evaluated morphologic features characterized on hematoxylin and eosin (H&E) staining (Fig. 4C; Supplementary Table S3). In both PDXs tested, mitotic index was high in all treatment arms, and nuclear grade was high in T-DXd + adavosertib arms in comparison to untreated and monotherapy groups. In PDX.003.213, necrosis was enhanced when T-DXd was combined with adavosertib in comparison to PDX.003.204 where there was no difference in control versus treatment arms. Moreover, anisonucleosis (variation in nuclear size as a manifestation of nuclear injury) was observed in both PDX models following treatment with T-DXd and adavosertib.
Discussion
Presence of cyclin E amplification in HER2-driven malignancies has been proposed as a mechanism of resistance to HER2-targeted therapeutics (4, 10). In this study, we found that co-amplification of CCNE1 and ERRB2 is commonly found in gastric/GEJ, uterine, and ovarian cancers. We demonstrated that in HER2-expressing, cyclin E amplified tumors the antitumor activity of T-DXd could be enhanced in combination with adavosertib both in vitro and in vivo. Furthermore, we found that DXd, the topoisomerase I inhibitor payload of T-DXd, induced cyclin E upregulation and thus may sensitize cancers to Wee1 kinase inhibition. These findings provide supporting rationale for the combination of T-DXd with adavosertib in HER2-expressing, cyclin E amplified solid tumors.
Although HER2-targeted therapies have been transformative in the care of patients with HER2-expressing tumors, intrinsic and acquired resistance remains a challenge, providing an opportunity to develop more potent HER2-targeted agents such as ADCs, bispecific antibodies and “add-on” strategies (combinations with other molecularly targeted agents or chemotherapy; ref. 10). Amplification/overexpression of cyclin E has been proposed as a mechanism of resistance to HER2-targeted therapies, which has been demonstrated by a worse clinical benefit from trastuzumab in HER2/cyclin E-positive breast cancers (9), and poorer prognosis in gastric cancer (21) However, a recent report has suggested cyclin E amplification is associated with better progression-free survival in HER2-positive gastric cancer (38). Therefore, further study is needed to determine the effect of cyclin E amplification on the antitumor activity HER2-targeted therapies in the clinic, especially in the context of T-DXd treatment.
Preclinical studies have revealed that transient knockdown of HER2 or treatment with anti-HER2 antibodies can downregulate cyclin E expression and furthermore, this interaction may be mediated by (i) effects of HER2 on the G1 phase of the cell cycle and (ii) HER2-mediated localization of the CDK inhibitors p21 and p27 (20, 39, 40). Mittendorf and colleagues demonstrated that targeting both HER2 and cyclin E with trastuzumab in combination with roscovitine, a CDK2 inhibit. or, led to a synergistic antitumor effect (20). In this study, we combined T-DXd with adavosertib, an inhibitor of Wee1 which regulates CDK2 activity. We found that within the TCGA and MD Anderson databases, many ERBB2-amplified tumors also had concomitant CCNE1 amplification, providing rationale for targeting both HER2 and cyclin E. We focused on targeting Wee1 with adovasertib specifically, as we had already demonstrated preclinically that adovasertib has activity in cyclin E amplified cells in vitro and in vivo, and we had demonstrated the antitumor activity of adovasertib in a clinical trial selecting for cyclin E amplification (23, 41). We realize that there are other inhibitors of cell cycle in development including inhibitors of CDK2 alone or in combination with other cyclin-dependent kinases, as well as inhibitors of PKMYT1 kinases. Further work is needed to determine if other combinations may also enhance the activity of T-DXd.
Considering the promising antitumor activity observed clinically with T-DXd, we focused our study on combinatorial strategies to enhance the activity of T-DXd in HER2 expressing tumors (14, 15). We found that DXd, the topoisomerase I inhibitor payload of T-DXd, and adavosertib were synergistic in vitro and that apoptosis was enhanced through this combination. Topoisomerase I inhibitors function to stabilize the topoisomerase I-DNA cleavable complexes thus inducing double-stranded breaks and activation of the DDR (13). Overexpression of cyclin E has been shown to induce deregulation of the G1–S transition, leading to premature entry into mitosis and induce replicative stress (24). Wee1 kinase is a key regulator of the G2–M transition, which functions through inhibition of CDK1 and halting entry into mitosis when the DDR is activated (23, 24). Adavosertib has demonstrated enhanced activity in the setting of cyclin E overexpression and furthermore has been studied in combination with other therapies which induce DNA damage (chemotherapy and/or radiation; refs. 28, 29, 31). In HER2-positive cell lines, we identified that overexpression of cyclin E was associated with reduced sensitivity to T-DXd. One possible explanation for the enhanced antitumor activity observed in our study is that tumors with baseline increased activity of the DDR pathway (cyclin E amplification/overexpression) are then subjected to further DNA damage via DXd/T-DXd, and following Wee1 kinase inhibition with adavosertib, negative regulation of the cell cycle is relieved, thus leading to mitotic catastrophe. We also demonstrated that DXd/T-DXd both induced upregulation of cyclin E protein expression, which may further sensitize cancer cells to adavosertib, beyond cells with cyclin E amplification/overexpression.
Of the PDX models tested in our study, one model (PDX.003.213) with HER2 1/2+ expression by IHC, ERBB2 V777 L and G778A mutations, and cyclin E amplification demonstrated stable disease following treatment with T-DXd, but durable tumor regression when this was combined with adavosertib. Notably, ERBB2 V777 L is a known activating ERBB2 mutation, and while the functional significance of G778A has not been experimentally characterized, multiple activating missense mutations in the αC-β4 loop have been reported including those in proximity and at the same codon (35–37). Another model (PDX.003.204) with HER2 2+ expression and cyclin E amplification also demonstrated prolonged growth inhibition with T-DXd and adavosertib in comparison with T-DXd monotherapy. Conversely, when using the same dose of T-DXd (10 mg/kg) in HER2 overexpressing PDX models (PDX.003.227, PDX.003.230), near complete tumor regression was achieved with T-DXd monotherapy, suggesting that tumors with HER2 1+ or 2+ expression may especially derive benefit from combination strategies with T-DXd. A study by Modi and colleagues of T-DXd in HER2-low breast cancer demonstrated promising clinical activity with T-DXd monotherapy, and this activity was confirmed in the randomized DESTINY-Breast04 trial showing prolonged progression-free and overall survival in this population over physician's choice chemotherapy (16, 42) However, the activity of T-DXd in HER2-low tumors may vary by tumor site of origin. In a phase II trial of T-DXd in previously treated gastric cancers, a subgroup analysis identified that patients with HER2 3+ expression derived the greatest benefit though activity was still observed in HER2 2+ expressing patients (15). In contrast, the activity of T-DXd in HER2-low colorectal cancer is limited, as there were no confirmed objective responses in patients with HER2 2+/FISH negative or HER2 IHC 1+ on the phase II trial (43).
Activity of T-DXd has also been assessed in phase II study of ERBB2-mutant non–small cell lung cancer, which demonstrated a confirmed objective response rate (ORR) of 55%, including patients with both HER2 extracellular and kinase domain mutations (44). This has led to the recent FDA approval of T-DXd for patients with non–small cell lung cancer with activating ERBB2 mutations. Further investigation is warranted to determine the mechanism by which ERBB2 mutations confer sensitivity to T-DXd, although preclinical studies suggest that this may be in part due to enhanced ADC internalization (45). The finding that adavosertib dramatically enhanced antitumor activity in PDX.003.213, which bears ERBB2 mutations raises the possibility that adavosertib may also enhance activity of T-DXd in ERBB2-mutant tumors. It would be of special interest to study the combination in ERBB2-mutant non–small cell lung cancer, were T-DXd is already FDA-approved.
Although we provide supporting rationale for the combination of T-DXd, there are several limitations to this study, and additional considerations which are relevant for clinical translation. The primary setting which we investigated this combination was in gastric/GEJ tumors, where T-DXd has been approved by the FDA in the second-line setting. Several clinical trials are underway evaluating T-DXd in non-breast/gastroesophageal tumors, which have demonstrated signal in HER2-expressing solid tumors (46). Further study is warranted to determine the generalizability of T-DXd and adavosertib to other HER2-expressing tumor types. This combination may be especially interesting in gynecologic tumors, where CCNE1 amplification is a known poor prognostic factor (47, 48), and adovasertib and T-DXd have been associated with significant clinical activity in CCNE amplified and HER2-expressing tumors, respectively (27, 49–51).
There is great interest in determining whether genomic co-occurring alterations such as alterations in MAPK or PI3K axis, TP53 or DNA damage response genes affect the antitumor activity of anti-HER2 agents, or activity of deruxtecan or adovasertib. Although our models were genomically characterized (Supplementary Table S1), the panel was not large enough to perform genotype–phenotype correlation in the context of these agents in monotherapy or in combination. We have primarily focused our functional studies on cyclin E and have not performed rescue experiments by forced overexpression of other cell-cycle components to overcome the combination. We have not studied the role of replication stress as a predictive biomarker and have not tested the effect of T-DXd and adovasertib alone or in combination on replication stress. Further, as our in vivo models were immunocompromised, although we found that T-DXd affected STING signaling, we were unable to assed effect of these treatments on the immune system in vivo.
In this study we examined the potential role of adavosertib as a therapeutic strategy to overcome primary refractory versus acquired resistance to T-DXd. Clinically there are three important scenarios to consider: (i) patients with HER2-low expressing tumors (ERBB2 non-amplified, HER2 1–2+ IHC), where efficacy of T-DXd is more variable, (ii) patients with ERBB2-amplified and/or HER2-overexpressing tumors, and (iii) patients with acquired resistance to T-DXd. In our study we demonstrated that adovasertib enhanced antitumor activity of T-DXd in HER2- low tumors. In ERBB2-amplified and/or HER2-overexpressing tumors, it would be important to determine whether the combination can overcome intrinsic resistance, and for patients who have a response, whether it can deepen the responses, and/or prolong the durability of the responses. PDX models tested with HER2 3+ expression exhibited sensitivity to T-DXd (particularly at higher doses), which limited assessment of combinatorial activity, although some combinatorial effects were seen in HER2-high PDXs in experiments with lower T-DXd doses. It is hard to clearly benchmark dose and schedule that will parallel clinical practice, however, as clinically some of T-DXd toxicities (such as interstitial lung disease) may be dose dependent, potentiating the antitumor activity of lower doses of T-DXd may also be worth pursuing. Although we examined the antitumor activity of T-DXd + adavosertib in the setting of prior T-DXd exposure, we found that T-DXd had some antitumor activity in these the colorectal adenocarcinoma models, and thus they did not have true acquired resistance, but enhanced activity was still observed when combined with adavosertib.
In summary, our findings suggest that adavosertib can enhance the activity of T-DXd in HER2-expressing cancers with amplification of CCNE1, particularly in the setting of HER2-low expression (HER2 1+ or 2+). We found that that T-DXd + adavosertib led to upregulation of several biomarkers associated with DNA damage and apoptosis in comparison with T-DXd monotherapy. Furthermore, we identified that DXd induced upregulation of cyclin E, which may sensitize cells to adavosertib in tumors with low cyclin E-expression at baseline. These findings provide supporting rationale for clinical translation of this combination in patients with HER2-expressing cyclin E amplified tumors. Further study is warranted to determine if the combination of T-DXd and adavosertib can overcome acquired resistance as well as intrinsic resistance to T-DXd.
Supplementary Material
Supplemental Tables 1 - 3
Supplemental Figures 1 - 5
Acknowledgments
This work was supported by the following: NIH Training of Academic Surgical Oncologists (Award No. 5T32CA009599–32), University of Texas PDX Development and Trial Center (Award No. 5U54CA224065–04), Center for Clinical and Translational Science (Award No. 5UL1TR003167–03), and the MD Anderson Cancer Center support grant (Award No. P30 CA016672). We thank Ms. Susanna Brisendine for preparing submission documents and submission.
The publication costs of this article were defrayed in part by the payment of publication fees. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Footnotes
Note: Supplementary data for this article are available at Clinical Cancer Research Online (http://clincancerres.aacrjournals.org/).
Authors' Disclosures
T.P. DiPeri reports grants from NIH/NCI during the conduct of the study. T.A. Yap reports grants and personal fees from Acrivon, Artios, AstraZeneca, Bayer, BeiGene, Blueprint, Clovis, EMD Serono, F-Star, Genentech, ImmuneSensor, Merck, Pfizer, and Sanofi; grants from BioNTech, BMS, Boundless Bio, Constellation, Cyteir, Eli Lilly, Forbius, GlaxoSmithKline, Haihe, Ideaya, Ionis, Ipsen, Jounce, Karyopharm, KSQ, Kyowa, Mirati, Novartis, Ribon Therapeutics, Regeneron, Repare, Rubius, Scholar Rock, Seattle Genetics, Tesaro, Vivace, and Zenith; personal fees from AbbVie, Adagene, Almac, Aduro, Amphista, Athena, Atrin, Avoro, Axiom, Baptist Health Systems, Blueprint Medicines, Boxer, Bristol Myers Squibb, C4 Therapeutics, Calithera, Cancer Research UK, Circle Pharma, CUHK Committee, Cybrexa, Dark Blue Therapeutics, Diffusion, Ellipses.life, Genentech, Genmab, Gerson and Lehrman Group, Glenmark, GLG, Globe Life Sciences, GSK, Guidepoint, Idience, Ignyta, I-Mab, Institut Gustave Roussy, Intellisphere, Janssen, Kyn, LRG1, MEI Pharma, Mereo, Natera, Nexys, Novocure, OHSU, OncoSec, Ono Pharma, Panangium, Pegascy, PER, Piper-Sandler, Pliant Therapeutics, Prolynx, Radiopharm Theranostics, resTORbio, Roche, Schrodinger, Synthis Therapeutics, Terremoto Biosciences, Tessellate Bio, TD2, Theragnostics, Tome Biosciences, Varian, Versant, Vibliome, Xinthera, Zai Labs, Zentalis, and ZielBio; and personal fees and other support from Seagen during the conduct of the study. E.E. Dumbrava reports grants from Bayer HealthCare Pharmaceuticals, Immunocore, Amgen, Aileron Therapeutics, Compugen, TRACON Pharmaceuticals, Unum Therapeutics, Gilead Immunomedics, BOLT Therapeutics, Aprea Therapeutics, Bellicum Pharmaceuticals, PMV Pharma, Triumvira, Seagen, Mereo BioPharma 5, Sanofi, Rain Oncology, Astex Therapeutics, Sotio, Poseida, Mersana Therapeutics, Genentech, and Boehringer Ingelheim; and other support from BOLT Therapeutics, Mersana Therapeutics, Orum Therapeutics, Summit Therapeutics, Catamaran Bio, PMV Pharma, ASCO, LFSA Association, Rain Oncology, and Banner MD Anderson Cancer Center during the conduct of the study. K. Keyomarsi reports grants and nonfinancial support from Repare, Schrodinger, Apeiron, and Blueprint and grants from NCI and CPRIT during the conduct of the study as well as grants and nonfinancial support from Repare, Schrodinger, Apeiron, and Blueprint and grants from NCI and CPRIT outside the submitted work. F. Meric-Bernstam reports personal fees from AbbVie, Aduro BioTech, Alkermes, AstraZeneca, Daiichi Sankyo, Calibr (a division of Scripps Research), Debiopharm, Ecor1 Capital, eFFECTOR Therapeutics, F. Hoffman-La Roche, GT Apeiron, Genentech, Harbinger Health, IBM Watson, Infinity Pharmaceuticals, Jackson Laboratory, Kolon Life Science, Lengo Therapeutics, Menarini Group, OrigiMed, PACT Pharma, Parexel International, Pfizer, Protai Bio, Samsung Bioepis, Seattle Genetics, Tallac Therapeutics, Tyra Biosciences, Xencor, Zymeworks, Black Diamond, Biovica, Eisai, Fog Pharma, Immunomedics, Inflection Biosciences, Karyopharm Therapeutics, Loxo Oncology, Mersana Therapeutics, OnCusp Therapeutics, Puma Biotechnology, Sanofi, Silverback Therapeutics, Spectrum Pharmaceuticals, Theratechnologies, and Zentalis; grants from Aileron Therapeutics, AstraZeneca, Bayer Healthcare Pharmaceutical, Calithera Biosciences, Curis, CytomX Therapeutics, Daiichi Sankyo, Debiopharm International, eFFECTOR Therapeutics, Genentech, Guardant Health, Klus Pharma, Takeda Pharmaceutical, Novartis, Puma Biotechnology, and Taiho Pharmaceutical Co.; and other support from European Organisation for Research and Treatment of Cancer (EORTC), European Society for Medical Oncology (ESMO), and Cholangiocarcinoma Foundation outside the submitted work. No disclosures were reported by the other authors.
Authors' Contributions
T.P. DiPeri: Conceptualization, data curation, formal analysis, investigation, visualization, methodology, writing–original draft. K.W. Evans: Formal analysis, supervision, investigation, visualization, writing–review and editing. M.G. Raso: Formal analysis, investigation, writing–review and editing. M. Zhao: Formal analysis, investigation, visualization, writing–review and editing. Y.Q. Rizvi: Writing–review and editing. X. Zheng: Data curation, software, formal analysis, investigation, visualization, writing–review and editing. B. Wang: Investigation, writing–review and editing. B.P. Kirby: Investigation, writing–review and editing. K. Kong: Data curation, writing–review and editing. M. Kahle: Data curation, writing–review and editing. T.A. Yap: Investigation, writing–review and editing. E.E. Dumbrava: Investigation, writing–review and editing. J.A. Ajani: Investigation, writing–review and editing. S. Fu: Investigation, writing–review and editing. K. Keyomarsi: Investigation, writing–review and editing. F. Meric-Bernstam: Conceptualization, resources, data curation, software, formal analysis, supervision, funding acquisition, validation, investigation, visualization, methodology, writing–original draft, project administration, writing–review and editing.
References
- 1. Yarden Y. Biology of HER2 and its importance in breast cancer. Oncology 2001;61Suppl 2:1–13. [DOI] [PubMed] [Google Scholar]
- 2. Menard S, Pupa SM, Campiglio M, Tagliabue E. Biologic and therapeutic role of HER2 in cancer. Oncogene 2003;22:6570–8. [DOI] [PubMed] [Google Scholar]
- 3. Slamon DJ, Leyland-Jones B, Shak S, Fuchs H, Paton V, Bajamonde A, et al. Use of chemotherapy plus a monoclonal antibody against HER2 for metastatic breast cancer that overexpresses HER2. N Engl J Med 2001;344:783–92. [DOI] [PubMed] [Google Scholar]
- 4. Meric-Bernstam F, Johnson AM, Dumbrava EEI, Raghav K, Balaji K, Bhatt M, et al. Advances in HER2-targeted therapy: novel agents and opportunities beyond breast and gastric cancer. Clin Cancer Res 2019;25:2033–41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Bang Y-J, Van Cutsem E, Feyereislova A, Chung HC, Shen L, Sawaki A, et al. Trastuzumab in combination with chemotherapy versus chemotherapy alone for treatment of HER2-positive advanced gastric or gastro-oesophageal junction cancer (ToGA): a phase 3, open-label, randomised controlled trial. Lancet 2010;376:687–97. [DOI] [PubMed] [Google Scholar]
- 6. Hainsworth JD, Meric-Bernstam F, Swanton C, Hurwitz H, Spigel DR, Sweeney C, et al. Targeted therapy for advanced solid tumors on the basis of molecular profiles: results from MyPathway, an open-label, Phase IIa multiple basket study. J Clin Oncol 2018;36:536–42. [DOI] [PubMed] [Google Scholar]
- 7. Meric-Bernstam F, Hurwitz H, Raghav KPS, McWilliams RR, Fakih M, VanderWalde A, et al. Pertuzumab plus trastuzumab for HER2-amplified metastatic colorectal cancer (MyPathway): an updated report from a multicentre, open-label, phase 2a, multiple basket study. Lancet Oncol 2019;20:518–30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Lee JY, Hong M, Kim ST, Park SH, Kang WK, Kim K-M, et al. The impact of concomitant genomic alterations on treatment outcome for trastuzumab therapy in HER2-positive gastric cancer. Sci Rep 2015;5:9289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Vernieri C, Milano M, Brambilla M, Mennitto A, Maggi C, Cona MS, et al. Resistance mechanisms to anti-HER2 therapies in HER2-positive breast cancer: current knowledge, new research directions and therapeutic perspectives. Crit Rev Oncol Hematol 2019;139:53–66. [DOI] [PubMed] [Google Scholar]
- 10. Goel S, Wang Q, Watt AC, Tolaney SM, Dillon DA, Li W, et al. Overcoming therapeutic resistance in HER2-positive breast cancers with CDK4/6 inhibitors. Cancer Cell 2016;29:255–69. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Coats S, Williams M, Kebble B, Dixit R, Tseng L, Yao N-S, et al. Antibody-drug conjugates: future directions in clinical and translational strategies to improve the therapeutic index. Clin Cancer Res 2019;25:5441–8. [DOI] [PubMed] [Google Scholar]
- 12. Mitani S, Kawakami H. Emerging targeted therapies for HER2 positive gastric cancer that can overcome trastuzumab resistance. Cancers (Basel) 2020;12:400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Ogitani Y, Aida T, Hagihara K, Yamaguchi J, Ishii C, Harada N, et al. DS-8201a, a novel HER2-targeting ADC with a novel DNA topoisomerase I inhibitor, demonstrates a promising antitumor efficacy with differentiation from T-DM1. Clin Cancer Res 2016;22:5097–108. [DOI] [PubMed] [Google Scholar]
- 14. Modi S, Saura C, Yamashita T, Park YH, Kim S-B, Tamura K, et al. Trastuzumab deruxtecan in previously treated HER2-positive breast cancer. N Engl J Med 2020;382:610–21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Shitara K, Bang Y-J, Iwasa S, Sugimoto N, Ryu M-H, Sakai D, et al. Trastuzumab deruxtecan in previously treated HER2-positive gastric cancer. N Engl J Med 2020;382:2419–30. [DOI] [PubMed] [Google Scholar]
- 16. Modi S, Jacot W, Yamashita T, Sohn J, Vidal M, Tokunaga E, et al. Trastuzumab deruxtecan in previously treated HER2-low advanced breast cancer. N Engl J Med 2022;387:9–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Scaltriti M, Eichhorn PJ, Cortés J, Prudkin L, Aura C, Jiménez J, et al. Cyclin E amplification/overexpression is a mechanism of trastuzumab resistance in HER2+ breast cancer patients. Proc Natl Acad Sci U S A 2011;108:3761–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Luhtala S, Staff S, Tanner M, Isola J. Cyclin E amplification, over-expression, and relapse-free survival in HER-2-positive primary breast cancer. Tumour Biol 2016;37:9813–23. [DOI] [PubMed] [Google Scholar]
- 19. Kim J, Fox C, Peng S, Pusung M, Pectasides E, Matthee E, et al. Preexisting oncogenic events impact trastuzumab sensitivity in ERBB2-amplified gastroesophageal adenocarcinoma. J Clin Invest 2014;124:5145–58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Mittendorf EA, Liu Y, Tucker SL, McKenzie T, Qiao N, Akli S, et al. A novel interaction between HER2/neu and cyclin E in breast cancer. Oncogene 2010;29:3896–907. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Alsina M, Landolfi S, Aura C, Caci K, Jimenez J, Prudkin L, et al. Cyclin E amplification/overexpression is associated with poor prognosis in gastric cancer. Ann Oncol 2015;26:438–9. [DOI] [PubMed] [Google Scholar]
- 22. Schmidt M, Rohe A, Platzer C, Najjar A, Erdmann F, Sippl W. Regulation of G2–M transition by inhibition of WEE1 and PKMYT1 kinases. Molecules 2017;22:2045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Chen X, Low K-H, Alexander A, Jiang Y, Karakas C, Hess KR, et al. Cyclin E overexpression sensitizes triple-negative breast cancer to wee1 kinase inhibition. Clin Cancer Res 2018;24:6594–610. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Fu S, Wang Y, Keyomarsi K, Meric-Bernstam F, Meric-Bernstein F. Strategic development of AZD1775, a wee1 kinase inhibitor, for cancer therapy. Expert Opin Investig Drugs 2018;27:741–51. [DOI] [PubMed] [Google Scholar]
- 25. Jin M-H, Nam A-R, Bang J-H, Oh K-S, Seo H-R, Kim J-M, et al. WEE1 inhibition reverses trastuzumab resistance in HER2-positive cancers. Gastric Cancer 2021;24:1003–20. [DOI] [PubMed] [Google Scholar]
- 26. Sand A, Piacsek M, Donohoe DL, Duffin AT, Riddell GT, Sun C, et al. WEE1 inhibitor, AZD1775, overcomes trastuzumab resistance by targeting cancer stem-like properties in HER2-positive breast cancer. Cancer Lett 2020;472:119–31. [DOI] [PubMed] [Google Scholar]
- 27. Fu S, Yao S, Yuan Y, Previs RA, Elias AD, Carvajal RD, et al. Multicenter Phase II Trial of the WEE1 inhibitor adavosertib in refractory solid tumors harboring CCNE1 amplification. J Clin Oncol 2023;41:1725–34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Oza AM, Estevez-Diz M, Grischke E-M, Hall M, Marmé F, Provencher D, et al. A biomarker-enriched, randomized Phase II trial of adavosertib (AZD1775) plus paclitaxel and carboplatin for women with platinum-sensitive TP53-mutant ovarian cancer. Clin Cancer Res 2020;26:4767–76. [DOI] [PubMed] [Google Scholar]
- 29. Cuneo KC, Morgan MA, Sahai V, Schipper MJ, Parsels LA, Parsels JD, et al. Dose escalation trial of the wee1 inhibitor adavosertib (AZD1775) in combination with gemcitabine and radiation for patients with locally advanced pancreatic cancer J Clin Oncol 2019;37:2643–50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Canisius S, Martens JW, Wessels LF. A novel independence test for somatic alterations in cancer shows that biology drives mutual exclusivity but chance explains most co-occurrence. Genome Biol 2016;17:261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Fu S, Yao S, Yuan Y, Previs RA, Elias AD, Carvajal R, et al. Abstract 974: Phase II trial of the wee1 inhibitor adavosertib in advanced refractory solid tumors with CCNE1 amplification. Cancer Res 2021;81(13 Supplement):974–. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Taniguchi H, Caeser R, Chavan SS, Zhan YA, Chow A, Manoj P, et al. WEE1 inhibition enhances the antitumor immune response to PD-L1 blockade by the concomitant activation of STING and STAT1 pathways in SCLC. Cell Rep 2022;39:110814. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Trowe T, Boukouvala S, Calkins K, Cutler RE, Fong R, Funke R, et al. EXEL-7647 inhibits mutant forms of ErbB2 associated with lapatinib resistance and neoplastic transformation. Clin Cancer Res 2008;14:2465–75. [DOI] [PubMed] [Google Scholar]
- 34. Lee JW, Soung YH, Seo SH, Kim SY, Park CH, Wang YP, et al. Somatic mutations of ERBB2 kinase domain in gastric, colorectal, and breast carcinomas. Clin Cancer Res 2006;12:57–61. [DOI] [PubMed] [Google Scholar]
- 35. Citri A, Gan J, Mosesson Y, Vereb G, Szollosi J, Yarden Y. Hsp90 restrains ErbB-2/HER2 signalling by limiting heterodimer formation. EMBO Rep 2004;5:1165–70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Xu W, Yuan X, Xiang Z, Mimnaugh E, Marcu M, Neckers L. Surface charge and hydrophobicity determine ErbB2 binding to the Hsp90 chaperone complex. Nat Struct Mol Biol 2005;12:120–6. [DOI] [PubMed] [Google Scholar]
- 37. Fan YX, Wong L, Ding J, Spiridonov NA, Johnson RC, Johnson GR. Mutational activation of ErbB2 reveals a new protein kinase autoinhibition mechanism. J Biol Chem 2008;283:1588–96. [DOI] [PubMed] [Google Scholar]
- 38. Kwon HJ, Park Y, Nam SK, Kang E, Kim K‐K, Jeong I, et al. Genetic and immune microenvironment characterization of HER2-positive gastric cancer: Their association with response to trastuzumab-based treatment. Cancer Med 2023;12:10371–84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Yang HY, Zhou BP, Hung MC, Lee MH. Oncogenic signals of HER-2/neu in regulating the stability of the cyclin-dependent kinase inhibitor p27. J Biol Chem 2000;275:24735–9. [DOI] [PubMed] [Google Scholar]
- 40. Le X-F, Betrosian I, Mao W, Murray M, Lu Z, Keyomarsi K, et al. Anti-HER2 antibody trastuzumab inhibits CDK2-mediated NPAT and histone H4 expression via the PI3K pathway. Cell Cycle 2006;5:1654–61. [DOI] [PubMed] [Google Scholar]
- 41. Fu S, Yao S, Yuan Y, et al. Multicenter phase II trial of the WEE1 inhibitor adavosertib in refractory solid tumors harboring CCNE1 amplification. J Clin Oncol 2022:JCO2200830. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Modi S, Park H, Murthy RK, Iwata H, Tamura K, Tsurutani J, et al. Antitumor activity and safety of trastuzumab deruxtecan in patients with HER2-low-expressing advanced breast cancer: results from a Phase Ib study. J Clin Oncol 2020;38:1887–96. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Siena S, Di Bartolomeo M, Raghav K, Masuishi T, Loupakis F, Kawakami H, et al. Trastuzumab deruxtecan (DS-8201) in patients with HER2-expressing metastatic colorectal cancer (DESTINY-CRC01): a multicentre, open-label, phase 2 trial. Lancet Oncol 2021;22:779–89. [DOI] [PubMed] [Google Scholar]
- 44. Li BT, Smit EF, Goto Y, et al. Trastuzumab deruxtecan in HER2-mutant non-small-cell lung cancer. N Engl J Med 2021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Li BT, Michelini F, Misale S, Cocco E, Baldino L, Cai Y, et al. HER2-mediated internalization of cytotoxic agents in ERBB2 amplified or mutant lung cancers. Cancer Discov 2020;10:674–87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Tsurutani J, Iwata H, Krop I, Jänne PA, Doi T, Takahashi S, et al. Targeting HER2 with trastuzumab deruxtecan: a dose-expansion, Phase I study in multiple advanced solid tumors. Cancer Discov 2020;10:688–701. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Kang E‐Y, Weir A, Meagher NS, Farrington K, Nelson GS, Ghatage P, et al. CCNE1 and survival of patients with tubo-ovarian high-grade serous carcinoma: an ovarian tumor tissue analysis consortium study. Cancer 2023;129:697–713. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Chan AM, Enwere E, McIntyre JB, Wilson H, Nwaroh C, Wiebe N, et al. Combined CCNE1 high-level amplification and overexpression is associated with unfavourable outcome in tubo-ovarian high-grade serous carcinoma. J Pathol Clin Res 2020;6:252–62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Mauricio D, Bellone S, Mutlu L, McNamara B, Manavella DD, Demirkiran C, et al. Trastuzumab deruxtecan (DS-8201a), a HER2-targeting antibody-drug conjugate with topoisomerase I inhibitor payload, shows antitumor activity in uterine and ovarian carcinosarcoma with HER2/neu expression. Gynecol Oncol 2023;170:38–45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Nishikawa T, Hasegawa K, Matsumoto K, Mori M, Hirashima Y, Takehara K, et al. Trastuzumab deruxtecan for human epidermal growth factor receptor 2-expressing advanced or recurrent uterine carcinosarcoma (NCCH1615): the STATICE trial. J Clin Oncol 2023;41:2789–99. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Yagishita S, Nishikawa T, Yoshida H, Shintani D, Sato S, Miwa M, et al. Co-clinical study of [fam-] trastuzumab deruxtecan (DS8201a) in patient-derived xenograft models of uterine carcinosarcoma and its association with clinical efficacy. Clin Cancer Res 2023. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplemental Tables 1 - 3
Supplemental Figures 1 - 5
Data Availability Statement
The data that support the findings of this study are available from the corresponding author upon reasonable request.