Abstract
Purpose:
Limited effective treatments are currently available for central nervous system (CNS) metastasis (CM). This is largely driven by the inability of current therapeutics to penetrate the blood brain barrier (BBB) and the lack of preclinical models for testing new therapies. Here we study the efficacy of AZD1390, a BBB penetrating ataxia-telangiectasia mutated inhibitor, as a radiosensitizer for breast cancer CM treatment.
Experimental Design:
Three patient-derived xenograft (PDX) tumors including 2 HER2+ and 1 triple-negative breast cancer harboring DNA damage response (DDR) gene mutations, were implanted subcutaneously in the flank of mice to assess tumor growth inhibition by AZD1390 combined with radiation. Animal survival was further assessed by implanting the best responding PDX model orthotopically in the brain.
Results:
Pretreatment with AZD1390 followed by radiation therapy inhibited growth of PDX tumors implanted in the flank, and improved survival in orthotopic models with average survival of 222 days compared with 123 days in controls. Administration of AZD1390 posttreatment for 21 days had no further benefits. While the combination therapy resulted in sustained tumor inhibition, sporadic regrowth was observed in some mice 50 to 100 days posttreatment in all models. Gene expression comparing these tumors with complete responders demonstrated changes in upregulation of oncogenic proteins, which are potential drivers of tumor growth after treatment.
Conclusions:
Our results demonstrate that AZD1390 effectively sensitizes breast cancer CM to radiation therapy in DDR mutant tumors. This study demonstrates the potential of using AZD1390 as a novel therapeutic agent for patients with breast cancer CM.
Translational Relevance.
Central nervous system (CNS) metastasis (CM) has remained a major source of mortality for patients with breast cancer due to limited treatment options that can penetrate the blood brain barrier (BBB). AZD1390, a BBB-penetrant selective ataxia-telangiectasia mutated inhibitor has been shown to radiosensitize glioblastomas. Here we show that AZD1390, when used in combination with radiation therapy, significantly inhibited the growth of patient-derived xenograft models of breast cancer CM, all with various DNA damage response gene alterations. The combination significantly improved survival of the animals, and the response was sustained until the experimental endpoint up to three months post treatment in the majority of the tumors. The results here support the inclusion of patients with breast cancer CM in clinical trials with AZD1390 combined with radiotherapy.
Introduction
Central nervous system (CNS) metastasis (CM) is the most common type of intracranial malignancy, occurring in 15% to 40% of all patients with cancer with metastatic disease (1). Primary tumors originating from the lung, breast, and melanoma are most likely to metastasize to the brain (1–4). In recent years, there has been an increase in the incidence of CM, which is likely a result of prolonged survival of patients with cancer receiving aggressive treatments for their primary disease with new systemic therapies (1–3, 5–7).
There are few effective treatments for CM, which is a major source of morbidity and mortality. There is a critically unmet need for novel therapies for CM (8). Currently, local treatments of CM with whole-brain radiotherapy can increase survival by a few months, which can be further extended with stereotactic radiosurgery (1). To evoke a clinical response for patients with CM, drugs that are known to penetrate the blood brain barrier (BBB) are still needed (4). Several drugs have been shown to be effective in clinical trials for CM. This includes ALK inhibitors for non–small cell lung cancer (9), HER2 inhibitors for HER2+ breast cancer (10), and checkpoint inhibitors for melanoma (11). Other therapies being explored for breast cancer CM are targeted for specific genetic alterations, such as PARP inhibitors against cancers with deficiencies in DNA damage repair, and receptor tyrosine kinase inhibitors for HER2+ breast cancers (12). Thus far, none of these are curative and new treatments are still required for patients that do not harbor the targeted genetic alterations.
Historically, limiting progress in the treatment of CM is a lack of clinically relevant models, access to human tissue samples, and comprehensive molecular profiles that would provide insight into the underlying heterogenous biology (8). Patient-derived xenograft (PDX) models serve as valuable experimental therapeutic models to identify and test novel therapies. Our group has successfully established and characterized a large series of PDX models from different CM histologic subtypes (8). We and others have shown that PDX models preserve genetic features of their original patient tumors (PT) and are more accurate predictors of clinical outcomes as opposed to cell line xenograft models (8, 13–15).
Through comprehensive molecular analyses performed on PDX models and their donor patient tissues, our group identified molecular characteristics of CM tumors (8). Among the pathways most frequently altered was DNA damage response (DDR; refs. 1, 8). Higher incidence of deficiencies in DDR has been reported in the CM tumor compared with the primary tumor and has been suggested to promote metastasis to the CNS (16, 17). This may be a result of the frequent mutations in TP53 in breast cancer CM (18). Ataxia-telangiectasia mutated (ATM) is a serine/threonine protein kinase that plays a vital role in DDR, in particular after ionizing radiation (IR), by detecting DNA double-stranded breaks (DSB) and orchestrating their repair (19). Genetic ablation or pharmacologic inhibition of ATM results in tumor cell hypersensitivity to IR (20), and has been shown to improve overall survival in glioblastoma (GBM) mouse models (21), particularly in a TP53 mutant context (20, 21).
AZD1390 is a potent, orally bioavailable and selective inhibitor of ATM developed by AstraZeneca and has been specifically optimized for BBB penetration. The BBB-penetrant ability of AZD1390 has been demonstrated in mice, primates, and humans (20, 22, 23). AZD1390 can also penetrate the BBB of brain tumor-bearing mice and has been shown to preferentially localize to the tumors located within the brain (20, 23). The preferential localization of drugs to brain metastasis tumors has been shown previously (24) and is due in part to the leakier BBB of these tumors (25). AZD1390 is now in early clinical development as a radiosensitizer of CNS malignancies and has been shown to be an effective radiosensitizer in tumor cells of GBM and lung cancer cell lines grown in the brain (20). However, there is a lack of preclinical data validating the efficacy of AZD1390 as a novel therapeutic agent for CM. Here, we used three breast cancer CM PDX models (2 HER2+ and 1 triple-negative breast cancer (TNBC), all of which harbor TP53 mutations and exhibit other aberrant DDR profiles, to validate the efficacy of AZD1390 as a radiosensitizer of CM tumors. We show that AZD1390, when used in combination with radiation therapy, inhibits the growth of breast cancer CM PDX tumors in both ectopic flank implantation and orthotopic models. Our studies have shed light on the potential efficacy of AZD1390 in the treatment of breast cancer CM. Given its ability to evoke a strong clinical response for both primary and metastatic tumors of the CNS, this novel class of therapeutics demonstrates an encouraging potential to improve patient outcomes for CM.
Materials and Methods
Animal studies
All animal experiments were conducted in accordance with Institutional Animal Care and Use Committee guidelines and an approved protocol. Establishment of CM PDX models has been described previously (8). We used 3 different breast cancer metastasis PDX models, CM07 (ER+/PR−/HER2+), CM14 (ER−/PR−/HER2+), and CM16 (TNBC). The dissected PDX tumor was dissociated into single cells with the human tumor dissociation kit (Miltenyi) using the gentleMACS Octo Dissociator (Miltenyi) according to the manufacturer's instructions. For flank studies, tumor cells were resuspended in a 1:1 tumor/PBS slurry with reduced growth factor Matrigel (Corning). Then, 5×106 cells were injected subcutaneously into the right flank of 4- to 6-week-old female NOG mice (Taconic).
Tumor volume was calculated using length × width × width × 0.5. Treatment was initiated when the average size of all tumors was 70 mm3. Mice were randomized into treatment groups where average tumor volume was similar between all groups (n = 5–6). Treatment groups were, A: Vehicle-treated control, B: 20 mg/kg/day AZD1390 by oral gavage for 4 days, C: 2.5 Gy radiation per day for 4 days, D: combination of AZD1390 followed by radiation 1 hour after AZD1390 administration for 4 days, and E: combination of AZD1390 and radiation as in group D, followed by adjuvant treatment of 20 mg/kg/day AZD1390 for 21 days. Treatment regimen was designed on the basis of previous pharmacokinetics/pharmacodynamics profile, where it has been shown that the radiosensitizing effect of AZD1390 peaks at 1 hour after dosing (20, 23). Mice were euthanized when tumors reached a diameter of 15 mm, when the tumor has ulcerated, when the mouse exhibited signs of pain or distress, or at study endpoint (CM07: 120 days, CM14: 140 days, CM16: 90 days). Residual tumors were resected when animals were euthanized; half was preserved in 10% formalin, and half was flash frozen.
For intracranial studies, dissociated single tumor cells from CM14 were resuspended in PBS. 1.5 × 105 cells were injected intracranially into the brain parenchyma of the right cerebrum as previously described (8). Treatment was initiated at 20 days, when tumors were expected to have grown in the brain (8). Mice were randomized into treatment groups where average mouse weight was similar between all groups (n > 6 per group). Groups A–D were used as described for flank tumors. Mice were euthanized when there was a loss of 10% body weight within a week, low body condition score of 1, or other signs of pain or distress identified by a veterinarian. Brains were harvested and preserved in 10% formalin. 5 μm tissue sections were stained with hematoxylin and eosin (H&E). H&E staining was assessed by a pathologist. AZD1390 was provided by AstraZeneca for all animal experiments.
Tumor irradiation
Radiation therapy was performed using the XRAD 320iX X-ray irradiation chamber (Precision X-ray). Mice were anesthetized with a ketamine-xylazine cocktail and kept on a heating pad to maintain body temperature. Just prior to irradiation, mice were placed in a holding fixture and the appropriate lead shield (Precision X-ray) was placed over the fixture to target the X-ray over the flank or the head. Irradiation was performed at 250 kV, 16 mA, with a 50 cm focus skin distance using a 2-mm Al filter. Mice were irradiated at a dose rate of approximately 2 Gy/min for a total of 2.5 Gy. This was repeated for 4 consecutive days for a total dose of 10 Gy. The dose of 10 Gy has been routinely used for in vivo irradiation studies, and was shown to be effective when combined with AZD1390 in GBM (20).
MRI
MRI was performed at one timepoint on a selected subset of animals that were still alive 134 days posttreatment. MRI was performed using a 7T 24 cm bore horizontal MRI system (MR Solutions). A scout image was taken to center the position of the rat brain. The final image acquired with the following parameters: TR/TE eff = 5000/60 ms, voxel size of 0.07 × 0.07 × 0.5 mm³, FOV of 16 mm (width) × 18 mm (height) × 16 mm (depth), matrix size of 228 × 256 × 32, and slice thickness of 0.5 mm. Animals were maintained in anesthetized with isoflurane using a nose cone delivery system. Image analysis was performed on ImageJ (RRID:SCR_003070). A region of interest (ROI) was manually drawn over regions with detectable tumor signal for all slices. Tumor volume was calculated by multiplying the total area of the ROIs by slice thickness.
IHC
The antibody for γH2AX was purchased from Abcam (ab2893, RRID:AB_303388). Antigen retrieval was performed with a citrate-based antigen unmasking solution (Vector Labs). Sections were blocked with 10% normal goat serum, stained with the primary antibody diluted in TBST with 2% goat serum overnight at 4°C. Sections were incubated with biotinylated secondary antibody (Vector Labs), Vectastain Elite ABC reagent (Vector Labs), developed using DAB substrate (Vector Labs) and counterstained with Mayer's Hematoxylin.
Nucleic acid extraction
Tumors were homogenized with the Bullet Blender (NextAdvance) for 3 minutes at full speed in RNase-free RINO tubes (NextAdvance). Genomic DNA and total RNA were extracted simultaneously using the AllPrep DNA/RNA Mini kit (Qiagen). Nucleic acids were quantified using the TapeStation genomic DNA or RNA ScreenTape on the 4200 TapeStation System (Agilent)
Next-generation sequencing
Whole-exome sequencing raw data files of PDX models was obtained from a previously published dataset (8). Exome reads were aligned against Human GRCh37 reference genome using BWA-MEM (RRID:SCR_017619). Aligned exome reads were then used to identify copy-number aberrations and somatic variants. Somatic variant calling was performed with Seurat (26) and Strelka (27). Genetic alterations in 126 DDR genes were identified, including genes responsible for base excision repair, mismatch repair, nucleotide excision repair, homologous recombination (HR), non-homologous end joining, and general DNA damage sensing and response.
RNA sequencing (RNA-seq) libraries were prepared using the KAPA mRNA HyperPrep Kit for Illumina Platforms (Roche). mRNA capture and subsequent library preparation were performed according to the manufacturer's recommended protocol. Equimolar pools of libraries were created at a concentration of 5 nmol/L. Single-end sequencing was performed on the Illumina HiSeq 2500 platform for an average read length of 75 bp.
Sequencing data was aligned using STAR (RRID:SCR_004463) and read counting was performed by htseq-count. Differential gene expression was performed using the Limma package in R (28). Filtered differential expression gene lists were uploaded to Ingenuity Pathway Analysis (IPA; RRID:SCR_008653; Qiagen) and analyzed using the Core Analysis workflow, or separately with gene set enrichment analysis (GSEA; UC San Diego, Broad Institute).
Data availability
RNA-seq data presented in this article has been deposited in NCBI's Gene Expression Omnibus (RRID:SCR_005012) under accession number GSE224899. All data generated have been presented in article and raw data is available from the corresponding author upon reasonable request.
Results
DNA damage repair deficiency in breast cancer CM PDX models and matching donor human tissue
Our lab has previously established and characterized PDX models of CM derived from primary breast cancers (8). Three breast cancer CM PDX models were selected for this study based on alterations in TP53 or other DDR genes: CM07 (ER+/PR−/Her2+), CM14 (ER−/PR−/HER2+), and CM16 (TNBC). Whole-exome sequencing data of these PDX models at multiple passages and on the original PT (8) was analyzed for 126 DDR genes, revealing genetic alterations in each PT and corresponding PDX (Fig. 1A). Deficiencies in DDR have been shown to enhance radiation therapy and in some cases is a prerequisite for treatment with radiation sensitizers (19, 20). For AZD1390, in vitro studies in GBM cell lines demonstrated that TP53 mutation was required for its efficacy, while in vivo studies in GBM PDXs demonstrated responses in 3/4 of the TP53 wild-type PDXs, indicating that TP53 may not be the only determinant of response to AZD1390 (20).
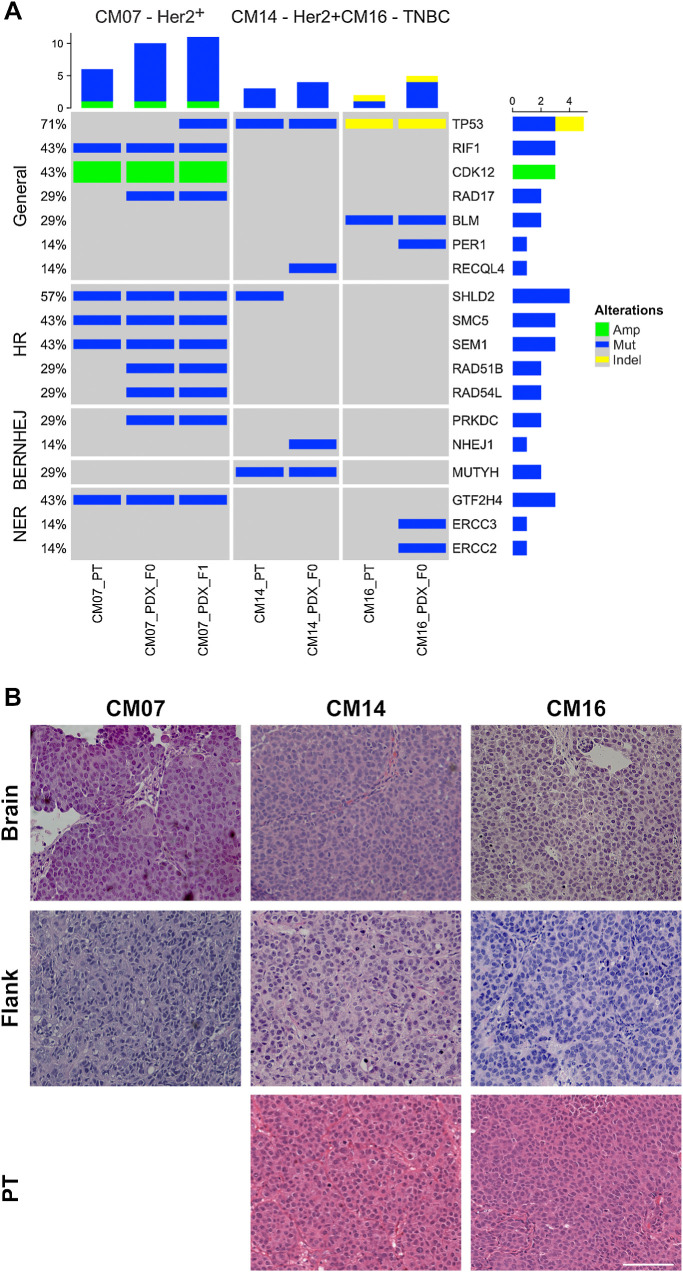
Figure 1.
Characterization of brain metastasis PDX models. A, Oncoprint of DNA damage repair genomic alterations found in PDXs and their original PT. The three PDX models and their original PT were previously characterized by whole-exome sequencing. Genetic alterations across 126 DDR genes in various repair pathways were identified in the PT and PDX. Only genes with alterations are shown in the figure. B, H&E staining of PDX tumors implanted subcutaneously into the flank and by intracardiac injection into the brain, along with the PT. Tumor histology was consistent in PDX models implanted in the flank, brain and PT. Scale bar represents 100 μm.
TP53 alterations were present in CM14 and CM16 in the PT and PDX (Fig. 1A), with a missense mutation in CM14 and indel in CM16. TP53 mutations were not called in CM07 PT or the F0 generation in the PDX due to low variant allele frequency. However, analysis of subsequent passages of CM07 revealed the presence of a TP53 mutation, which was enriched in the PDX. Therefore, all 3 PDX models were TP53 mutant. In addition, each PDX model and corresponding PT had additional mutations in DDR genes. CM07 harbors 3 mutations in HR genes including SHLD2, SMC5, and SEM1, suggesting deficiency in one of the pathways involved in double-stranded DNA damage repair induced by radiation therapy. CM16 has mutations in BLM, a RecQ helicase involved in DNA DSB repair. CM14 has mutations in MUTYH, which is involved in break excision repair. Even though all 3 models are mutant for TP53, they also had other deficiencies in DDR.
AZD1390 inhibits tumor growth in flank PDX models of breast CM
The in vivo efficacy of AZD1390 was first screened in 3 CM PDX models: CM07, CM14, and CM16, subcutaneously in the flank. Preclinical testing of PDX models has most commonly been performed in the flank and reliably mimics patient response to therapies in co-clinical trials (29). We have shown that the histology and molecular properties of CM PDXs remain consistent whether implanted subcutaneously in the flank or orthotopically in the brain, and both sites of implantations could be used for preclinical studies (8). Here, the histology of the PDX models was assessed by a pathologist (Fig. 1B). Patient tumor was not available for CM07. Overall, there were no substantial differences between the PT, flank- and brain-implanted PDXs. All tumors showed histologic features characteristic of metastatic breast carcinoma. Tumors demonstrated sheeted and nested growth patterns, and had a somewhat monotonous appearance with mild to moderate pleomorphism. Cells were of moderate size and contained round, centrally located nuclei with partially open chromatin and prominent nucleoli. Some cells demonstrated multiple nucleoli, and mitotic figures and karyorrhectic nuclear debris were present. This demonstrates that PDXs remained consistent regardless of the site of implantation, and that flank models can accurately reflect the characteristics of the original PT.
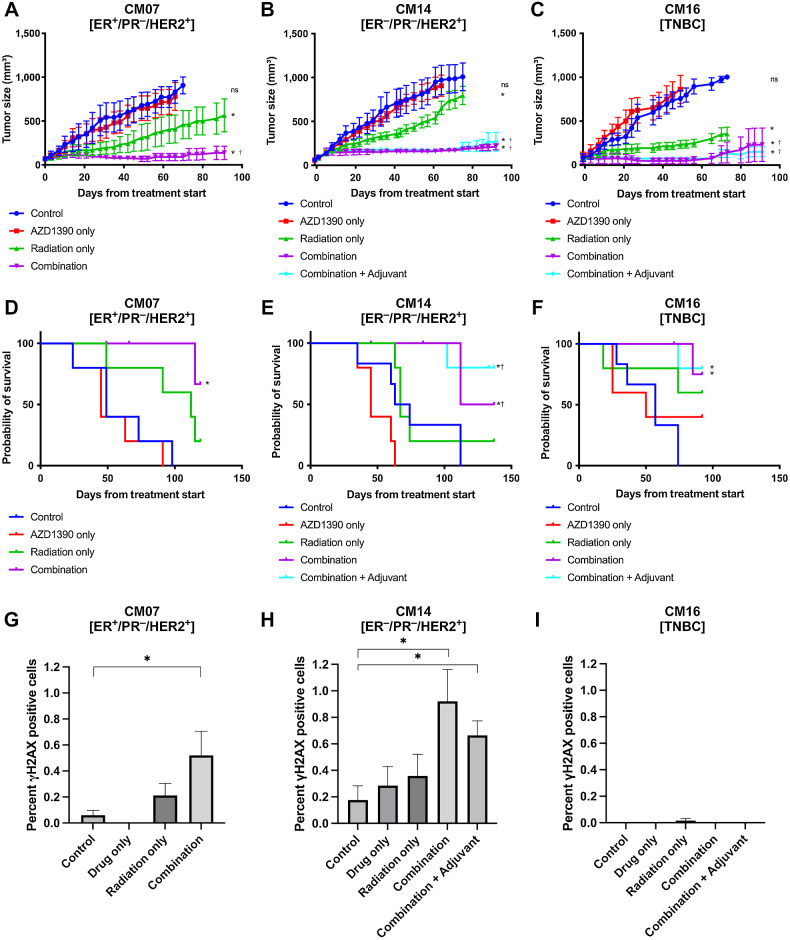
Animals were given 20 mg/kg of AZD1390 an hour prior to administering 2.5 Gy of IR per day, for a total of 4 days, only AZD1390 or radiation alone, or none as control. An additional group of animals treated with the combination of AZD1390 and radiation were given AZD1390 in an adjuvant setting for 21 days. No toxicity was observed in the animals in all treatment groups, including the combination and adjuvant groups. In comparison to the radiation only treatment group, pre-treatment with AZD1390 followed by a fractionated regimen of radiation therapy was able to inhibit tumor growth significantly in all 3 PDX models (Fig. 2A–C). A significant reduction of tumor growth was first observed in the combination group in comparison to the radiation group between day 20 to 40 after initiation of treatment for CM14 and CM16, and around day 70 for CM07. The combination treatment inhibited tumor growth by an average of 81%. CM07 (ER+/HER2+) exhibited growth inhibition of 85%, compared with 8% for tumors treated with AZD1390 alone or 39% for tumors treated with radiation alone. CM14 (ER−/HER2+) had the highest inhibition relative to radiation treatment, with combination therapy inhibiting tumor growth by 80%, compared with 22% growth inhibition with radiation alone and 13% with AZD1390 alone. This indicated that the radiosensitizing effect of AZD1390 was strongest in CM14. AZD1390 inhibited tumor growth by 77% in CM16 (TNBC) in the combination group, compared with 0% with AZD1390 alone and 58% with radiation alone. Growth inhibition was sustained until the experiment endpoint in 75% of the tumors, which corresponded with an average of 65% survival of the animals in the combination group to the experimental endpoint compared with 0% in the control group (Fig. 2D–F).
Figure 2.
Effect of AZD1390 and IR on flank implanted PDX models. A–C, Tumor growth curves and (D–F) Kaplan Meier survival curves of the 3 CM PDX models, beginning at the first day of treatment. A and D, CM07 and (B and E) CM14 are HER2+ tumors, while (C and F) CM16 is a model of TNBC. Animals were treated with 2 mg/kg/day OD AZD1390 and/or 2 Gy/day of IR for 4 days. Adjuvant therapy included additional 21 days of 2 mg/kg/day AZD1390 post-radiation. Tumor growth curves were terminated when 50% of the animals have been euthanized. G–I, Quantification of γH2AX positive cells in the 3 CM PDX models at study endpoint. Error bars represent standard error. Asterisks (*) indicate whether the tumor size at 90 days was statistically significant (P < 0.05) compared with the control group, dagger (†) indicates significance when compared with radiation group.
The response to IR alone varied between the PDX models (Fig. 2A–C). However, the combination treatment was able to substantially inhibit tumor growth in all breast cancer CM PDXs regardless of their sensitivity to radiation. The adjuvant treatment did not further decrease tumor growth in CM14 and CM16 (Fig. 2B and C), and subsequently excluded for CM07. IHC staining of γH2AX was employed to assess the degree of DNA damage induced by inhibiting ATM at the study endpoint (Fig. 2G–I; Supplementary Fig. S1). More DSBs were observed in the combination group compared with the control in CM07 and CM14, suggesting AZD1390-mediated inhibition of DNA damage repair results in long-term changes in genomic stability of the tumor, resulting in accumulation of residual DNA damage in the tumors, which could not be repaired. No DSB was detected in CM16, regardless of treatment group. Because peak activation of DDR generally occurs 24 hours after radiation treatment and diminishes after several days (20), it was not surprising that for some models, no DSBs were observed at the experimental endpoint. The presence of long-term genomic instability in some tumors suggests that they may have long-term sensitivity to DNA-damaging treatments, including additional doses of radiation. The inhibition of tumor growth shown in our in vivo flank implantation study validates AZD1390’s ability to radiosensitize PDX models of ER+, HER2+, and TNBC CM, regardless of their baseline sensitivity to radiation therapy.
AZD1390 inhibits tumor growth in orthotopic CM PDX model
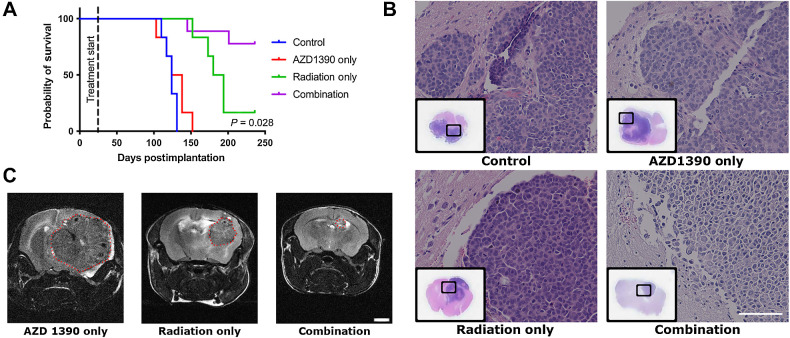
Because CM14 (ER−/HER2+) was the PDX model with the best overall response to the combination treatment from the flank study, it was selected to move forward in a subsequent orthotopic in vivo efficacy study. CM14 was implanted directly into the brains of immunodeficient mice and treated with AZD1390 and/or IR. Mice treated with AZD1390 combined with radiation had the highest rate of survival in the orthotopic model, having an average survival rate of over 222 days, with 77% surviving until the experimental endpoint of 236 days, compared with 123 days in the control and 188 days in the radiation group (Fig. 3A). This was consistent with the flank CM14 model, where the 67% of the combination tumors surviving until the experiment endpoint (Fig. 2D–F). The combination treatment also resulted in an 87% improvement in survival compared with the control group in the flank study, and 78% improvement in the orthotopic study. The presence of brain tumors was confirmed by histologic analysis of the brain at study endpoint (Fig. 3B). The size of the tumor in the brain was also measured using MRI in a selected subset of animals 134 days posttreatment, following euthanasia (Fig. 3C). Tumor volume calculated from the MRI scans showed that the tumors treated with radiation only had significantly larger tumors compared with those that received the combination treatment, with average volume of 53 mm³ and 0.5 mm³ respectively. These data further confirm growth inhibition after the radiosensitizing effects of AZD1390 in breast cancer CM PDX tumors.
Figure 3.
Effect of AZD1390 and IR on orthotopically implanted CM14 PDX in the brain. A, The Kaplan–Meier curve of animals implanted with orthotopic brain tumor. Treatment was initiated 20 days after implantation. P value of the survival of radiation only compared with combination groups is indicated. B, H&E staining of representative brain sections collected at study endpoint, from all 3 PDX tumors. Scale bars represent 100 μm. C, MRI scan of the entire mouse brain taken 134 days posttreatment, and image from one coronal section is shown here. MRI was performed for one animal in each group. No animals from the control group were alive at 134 days, and no images were acquired. Scale bars represent 2 mm.
Gene expression differences between stable and resistant tumors
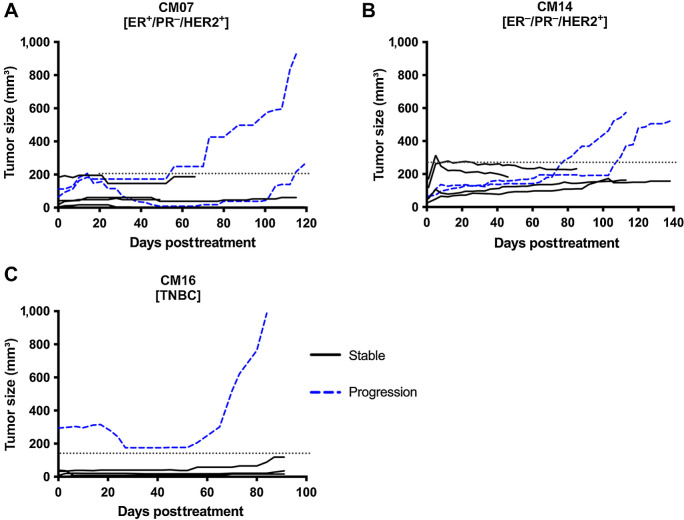
Although both the combination and adjuvant treatments were able to significantly inhibit tumor growth, some mice presented signs of tumor progression over the course of the flank study (Fig. 4A–C). Most mice treated with the combination therapy sustained tumor inhibition throughout the duration of the study and tumors from these mice are referred to as stable tumors. However, around 50 to 100 days posttreatment, 33%, 33%, and 25% of the tumors in CM07, CM14, and CM16, respectively, started regrowing – an indication of tumor progression and/or resistance. To identify the possible underlying molecular causes of tumor progression, we performed RNA-seq analysis to compare stable vs. progressing tumors from the combination group in each model. Differential gene expression was performed on the stable versus progression groups for CM07 and CM14. However, because there was only 1 tumor that progressed in CM16 (Fig. 4C), no differential analysis was performed.
Figure 4.
Growth curves of PDX tumors that were stable or progressed. A–C, Growth curve of individual tumors in the AZD1390 and radiation combination group. Solid lines represent tumors that were stable and did not grow after treatment, while blue dashed lines represent tumors that grew back and progressed. Dotted line indicates threshold used to determine stable and progression tumors for each PDX model.
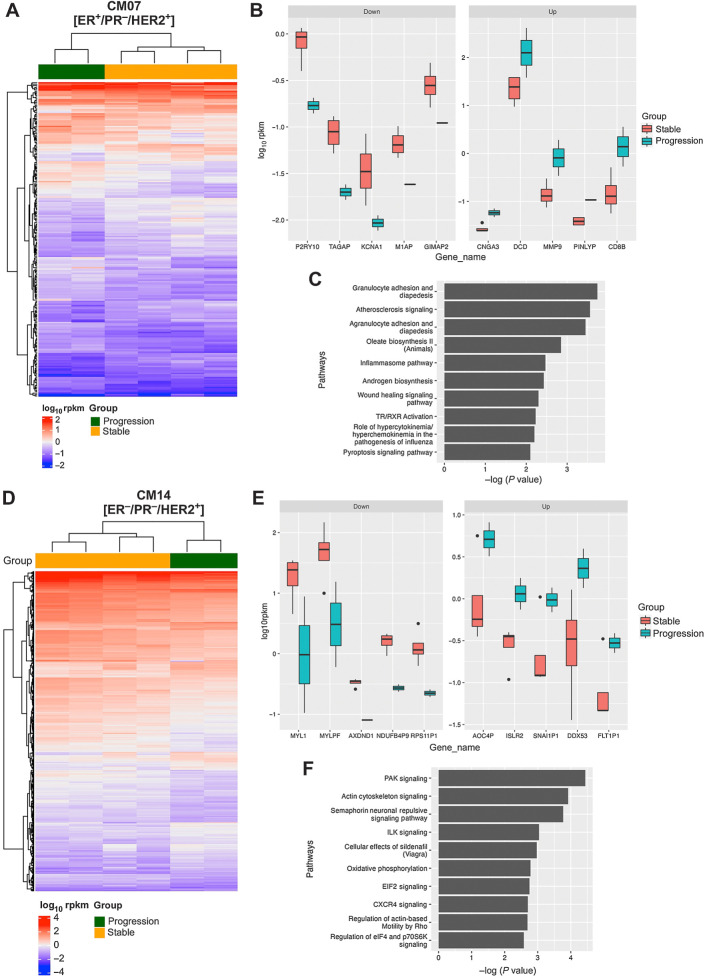
There were 226 significant differentially expressed genes identified in CM07 (ER+/HER2−) between stable and progressing tumors treated in the combination group (Fig. 5A and B). Among the top upregulated genes, dermcidin (DCD) had the highest average expression, indicating that stable CM07 tumors had relatively high baseline expression of DCD, and that progressing tumors significantly increased DCD expression in response to treatment. To identify changes in the signaling pathways associated with tumor progression, IPA was performed on the differentially expressed genes identified in CM07 (Fig. 5C). MMP-9 upregulation was involved in 4 of the top 10 canonical pathways revealed by IPA. These 4 pathways include granulocyte adhesion and diapedesis, atherosclerosis signaling, agranulocyte adhesion and diapedesis, and the wound healing signaling pathway.
Figure 5.
Gene expression analysis of CM07 and CM14 tumors that progressed after combination treatment. A, Heat map of the 226 differentially expressed genes identified between stable and progression samples for CM07. B, The top 5 upregulated and top 5 downregulated differentially expressed genes in CM07 (C) The top canonical pathways for CM07 progressing tumors identified by IPA. D, Heat map of the 447 differentially expressed genes identified between stable and progression samples for CM14. E, The top 5 upregulated and top 5 downregulated differentially expressed genes in CM14. F, The top canonical pathways for CM14 progressing tumors identified by IPA.
Differential gene expression was similarly performed on stable versus progression groups for CM14 (ER−/HER2+), which revealed 447 significant differentially expressed genes (Fig. 5D and E). IPA was similarly performed on the differentially expressed genes identified in CM14 (Fig. 5F). In CM14 progressing tumors, the top downregulated genes MYL1 and MYLPF were found in 6 of the top 10 pathways (PAK signaling, actin cytoskeleton signaling, semaphorin neuronal repulsive signaling pathway, cellular effects of Sildenafil, CXCR4 signaling, and regulation of actin-based motility by Rho). Changes in the PAK signaling pathway were driven by upregulation of ARHGAP10 and ITGA1, along with downregulation of ITGB6, MYL1, MYLPF, MYL5, and PIK3C2G.
Common differentially expressed genes and pathways shared between CM07 and CM14
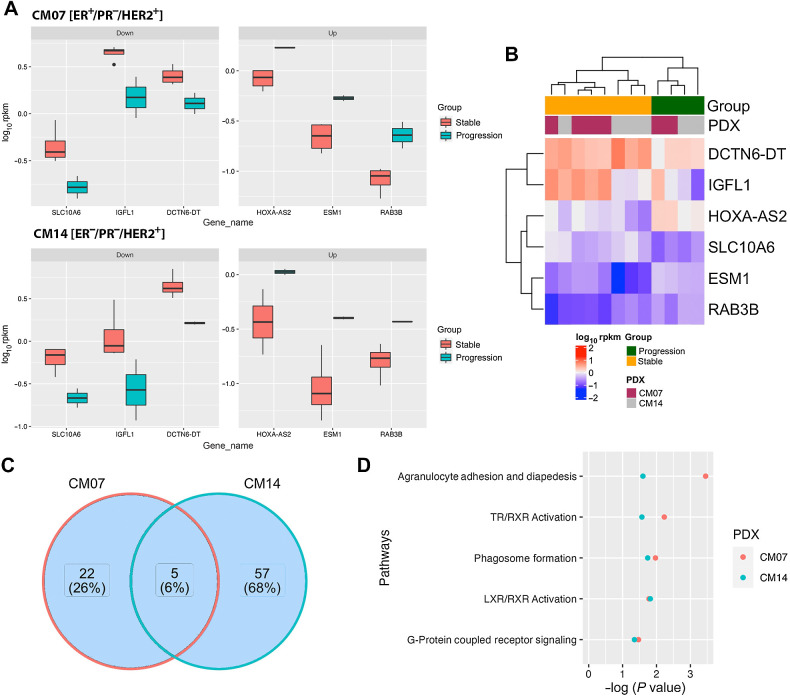
There were six common differentially expressed genes in both CM07 and CM14 progressing tumors (Fig. 6A and B). Of these genes, HOXA-AS2 and RAB3B, a member of the RAS oncogene family, are both upregulated. Both genes have been shown to be oncogenic and promote resistance to DNA-damaging chemotherapy (30, 31). There were five common pathways shared between CM07 and CM14 (Fig. 6C and D). Nuclear receptor signaling pathways were significantly altered in both models, including thyroid receptor (TR), liver X receptor (LXR), and retinoid X receptor (RXR), in which RAB3B is involved. These nuclear receptors play an important role in controlling proliferation by promoting apoptosis of breast cancer (32, 33). While the expression levels of TR, LXR, and RXR were unchanged, changes in downstream signaling components implicates nuclear receptor activation through changes in metabolites responsible for their activation, thereby promoting continued tumor proliferation. Changes in the agranulocyte adhesion and diapedesis pathway were also identified in both CM07 and CM14 (Fig. 6D). It is possible that tumor progression in both models may have also been driven by changes in this pathway because progressing tumors would have potentially enhanced their motility and resistance to apoptosis as a result.
Figure 6.
Common genes and pathways shared by both PDX models in progression vs. stable tumors. A, The 3 downregulated and 3 upregulated differentially expressed genes shared between both models. B, Heat map of the 6 differentially expressed genes shared between CM07 and CM14. C, The number of canonical pathways identified from each model and the number of pathways shared between both models. D, The 5 pathways that are shared between CM07 and CM14 progressing tumors.
GSEA was also performed on CM07 and CM14 separately. Pathways with similar directional changes were identified (Supplementary Fig. S2). Lower enrichment score in UV and DDR pathways in the progression samples suggests that rescue of DDR activity was not responsible for tumor progression. Enrichment in oncogenic pathways such as Hedgehog and KRAS signaling was observed, along with enrichment in coagulation and angiogenesis, all of which could promote tumor proliferation. While there were differing pathways identified in the GSEA compared with the IPA analysis, the enrichment in oncogenic pathways was found both sets of analyses, suggesting that while there may be some tumor dependent changes that drive tumor progression, there are also commonalities that could be targeted in a clinical setting.
Discussion
In this study, we are the first to show that the combination of AZD1390 with IR significantly decreased tumor growth and increased survival in PDX models of breast cancer CM, indicative of radiosensitization by AZD1390. Specifically, we tested both HER2+, with varying ER status, and TNBC PDX models, which are the two subtypes of breast cancer with the highest incidence of CM (34). Pretreatment with AZD1390 resulted in significant inhibition in all three PDX tumors, regardless of their hormone and HER2 receptor status, genetic background, initial radiosensitivity, and was not associated with toxicity in mice. The ability of AZD1390 to radiosensitize tumors with low initial radiosensitivity was previously observed in GBM tumors (20). While the total IR dose (10 Gy) used in this study was lower than the typical 30 Gy for whole brain radiation therapy in humans, the strong radiosensitizing effects of AZD1390 observed in this study suggests that lower IR doses could be used with AZD1390, and is expected to reduce the incidence of adverse side effects of radiation therapy. Previous studies have demonstrated that harboring a TP53 mutation may be a predictor for AZD1390 response (20). This is potentially a result of p53-deficient cells lacking the ability to arrest after DNA damage induced by IR. ATM inhibition intensifies this effect by inhibiting the repair of DSBs leading to cell death through catastrophic mitosis (20). We have shown that the incidence of TP53 mutations and deletions in CM is 100% in both patients and matching PDX models (8), which suggests that the majority of patients with CM could benefit from the AZD1390/IR combination strategy.
We have previously demonstrated that the molecular and histologic profile of the original PT was preserved in the PDX model even when implanted subcutaneously in the flank (4). In addition, flank tumor growth latency periods correlated with patient survival. We demonstrated that there was a 73% concordance in copy-number alterations, 84% concordance in mutations and 86% correlation in gene expression profile compared with the original PT (8). While orthotopic models are most ideal, flank models are still most commonly used in preclinical studies for their ease of handling, implanting, and monitoring. In this study, we demonstrated that mice with flank-implanted tumors responded similarly to tumors implanted intracranially. This corroborates other studies showing similar response in ectopic models with co-clinical studies (29).
This is the first use of the ATM inhibitor AZD1390 in CM PDX models. Other ATM inhibitors have been tested as radiosensitizers for cancers with mixed results. The efficacy of ATM inhibitor M3541 was unclear in the phase I clinical trial due to its poor pharmacokinetic profile (35). KU-60019 was shown to be an effective radiosensitizers in glioma cell lines (36), but has yet to be tested in clinical trials. AZD1390 was able to radiosensitize PDX glioma tumors grown ectopically and orthotopically in the brain (20). In addition to radiosensitizers, ATM inhibitors have also been shown to have other tumor inhibitory effects and are able to synergize with chemotherapy and immunotherapy. ATM inhibition has been shown to sensitize lung cancer to cisplatin treatment (37), while ATM inhibitor AZD0156 has been shown to overcome chemotherapy resistance in neuroblastoma (38). As immunocompromised NOG mice were used in this study, the effect of AZD1390 and radiation on the immunogenicity of the tumor was unknown. However, studies have demonstrated that ATM inhibition can enhance immunogenicity, and sensitizes the tumor to checkpoint inhibitors (39, 40). This suggests that the efficacy of AZD1390 is not limited to radiosensitization of CNS tumors, and that it should also be tested in combination with chemotherapy and immunotherapy.
While AZD1390 with IR was highly efficacious in inhibiting tumor growth initially, in 31% of animals, the tumor progressed and continued growing 80+ days after treatment. This was observed previously in GBM tumors (20). Gene expression analysis of the progressing tumors revealed an upregulation of multiple oncogenes, both shared and unique to the various models. This includes DCD and MMP-9 in CM07, DDX53 in CM14, and RAB3B and HOXA-AS2 in both models. Many of these genes, including DCD and MMP-9, are upregulated in breast cancers, and are associated with enhanced cell proliferation and resistance to apoptosis (41, 42). RAB3B knockdown in hepatocellular carcinoma has been shown to promote sensitivity to chemotherapies that inhibit DNA replication (30), which promotes DNA damage and ATM activation through replication stress (43). In contrast, genes directly related to DNA damage repair were unchanged. This suggests that tumors could be overcoming IR and AZD1390 therapy through reactivation of cell cycle progression and inhibition of apoptosis via upregulation of oncogenic pathways, rather than the rescue of DNA damage repair pathways by activation alternative repair pathways.
In addition to upregulation of oncogenic proteins, other possible mechanisms of progression after AZD1390 and IR treatment were identified. Downregulation of AKR1C3 in CM07 progressing tumors drove changes in both the androgen biosynthesis and TR/RXR activation pathways. AKR1C3 expression increases androgen levels and androgen receptor activation (44), which could promote cell growth in HER2+ breast cancer (45). Furthermore, changes in the PAK signaling pathway were potentially driven by upregulation of ARHGAP10 and ITGA1. ARHGAP10 has been suggested to have a vital role in multiple cellular processes, such as cell migration, adhesion, and actin cytoskeleton reorganization (46). ARHGAP10 is also associated with EMT and metastasis in cancer progression (47, 48). When considering the future direction of ATM inhibitors, it is of interest to examine these potential markers of tumor progression and their relevance to developing additional adjuvant therapies. Functional validation of these markers in a larger cohort will be required to determine the ideal targets to prevent progression after treatment.
AstraZeneca is now supporting early clinical development of AZD1390 including an oncology phase I trial in combination with standard of care radiation treatment in patients with GBM and CM metastasis and also other extracranial metastases in a separate phase I trial. The potential clinical impact is significant and incorporating ATM inhibitors as a combination therapy can mitigate the significant unmet need in the treatment of varying subtypes of breast CMs. In addition, the high prevalence of TP53 and other DDR mutations in breast CMs suggests that the majority of tumors presented in the clinic are likely to respond to the combination of AZD1390 and radiotherapy.
Supplementary Material
Supplemental Figure 1. Immunochemistry (IHC) staining of γH2AX at experimental endpoint
Supplemental Figure 2. Gene set enrichment analysis (GSEA) of progression versus stable tumors
Acknowledgments
We would like to acknowledge the University of Southern California Norris Comprehensive Cancer Center Translational Pathology Core (NCI grant # P30CA014089) for their histology services and the Molecular Genomics Core (NCI grant # P30CA014089) and Keck Genomics Platform for their sequencing services.
This work was supported partially by AstraZeneca and partially by the National Cancer Institute, Center for Reducing Cancer Health Disparities of the National Institutes of Health under award numbers U54CA233396, U54CA233444, & U54CA233465, which support the Florida-California Cancer Research, Education and Engagement (CaRE2) Health Equity Center.
The publication costs of this article were defrayed in part by the payment of publication fees. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Footnotes
Note: Supplementary data for this article are available at Clinical Cancer Research Online (http://clincancerres.aacrjournals.org/).
Authors' Disclosures
P. Hamerlik reports other support from AstraZeneca during the conduct of the study; other support from AstraZeneca outside the submitted work. B. Salhia reports grants from AstraZeneca during the conduct of the study; personal fees from AstraZeneca outside the submitted work; in addition, B. Salhia has a patent for “Salhia, B. SYSTEMS AND METHODS FOR PRECLINICAL MODELS OF METASTASIS, United States. Serial# 14/668,260. File Date: 3/25/15. Issued as patent number 10,525,148 on 01/07/2020. Issued to Translational Genomics Research Institute.” No disclosures were reported by the other authors.
Authors' Contributions
B.Y. Tew: Formal analysis, investigation, methodology, writing–original draft, writing–review and editing. A.J. Kalfa: Investigation, writing–original draft, writing–review and editing. Z. Yang: Formal analysis, methodology, writing–original draft, writing–review and editing. K.M. Hurth: Formal analysis, writing–review and editing. T. Simon: Visualization, writing–original draft, writing–review and editing. E. Abnoosian: Investigation. S.T. Durant: Conceptualization, funding acquisition, visualization, methodology, writing–original draft, project administration, writing–review and editing. P. Hamerlik: Conceptualization, visualization, methodology, writing–original draft, project administration, writing–review and editing. B. Salhia: Conceptualization, resources, data curation, formal analysis, supervision, funding acquisition, validation, investigation, visualization, methodology, writing–original draft, project administration, writing–review and editing.
References
- 1. Salhia B, Kiefer J, Ross JT, Metapally R, Martinez RA, Johnson KN, et al. Integrated genomic and epigenomic analysis of breast cancer brain metastasis. PLoS One 2014;9:e85448. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Cruz-Munoz W, Kerbel RS. Preclinical approaches to study the biology and treatment of brain metastases. Semin Cancer Biol 2011;21:123–30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Owonikoko TK, Arbiser J, Zelnak A, Shu HK, Shim H, Robin AM, et al. Current approaches to the treatment of metastatic brain tumours. Nat Rev Clin Oncol 2014;11:203–22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Amin S, Baine MJ, Meza JL, Lin C. Association of immunotherapy with survival among patients with brain metastases whose cancer was managed with definitive surgery of the primary tumor. JAMA Netw Open 2020;3:e2015444. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Svokos KA, Salhia B, Toms SA. Molecular biology of brain metastasis. Int J Mol Sci 2014;15:9519–30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Wong ET, Berkenblit A. The role of topotecan in the treatment of brain metastases. Oncologist 2004;9:68–79. [DOI] [PubMed] [Google Scholar]
- 7. Amsbaugh MJ, Kim CS. Brain metastasis. StatPearls; 2022. [PubMed] [Google Scholar]
- 8. Tew BY, Legendre C, Schroeder MA, Triche T, Gooden GC, Huang Y, et al. Patient-derived xenografts of central nervous system metastasis reveal expansion of aggressive minor clones. Neuro Oncol 2020;22:70–83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Chow LQM, Barlesi F, Bertino EM, van den Bent MJ, Wakelee HA, Wen PY, et al. ASCEND-7: efficacy and safety of ceritinib treatment in patients with ALK-positive non–small cell lung cancer metastatic to the brain and/or leptomeninges. Clin Cancer Res 2022;28:2506–16. [DOI] [PubMed] [Google Scholar]
- 10. Murthy RK, Loi S, Okines A, Paplomata E, Hamilton E, Hurvitz SA, et al. Tucatinib, trastuzumab, and capecitabine for HER2-positive metastatic breast cancer. N Engl J Med 2020;382:597–609. [DOI] [PubMed] [Google Scholar]
- 11. Tawbi HA, Forsyth PA, Hodi FS, Algazi AP, Hamid O, Lao CD, et al. Long-term outcomes of patients with active melanoma brain metastases treated with combination nivolumab plus ipilimumab (CheckMate 204): final results of an open-label, multicenter, phase II study. Lancet Oncol 2021;22:1692–704. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Bailleux C, Eberst L, Bachelot T. Treatment strategies for breast cancer brain metastases. Br J Cancer 2021;124:142–55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. DeRose YS, Wang G, Lin YC, Bernard PS, Buys SS, Ebbert MT, et al. Tumor grafts derived from women with breast cancer authentically reflect tumor pathology, growth, metastasis and disease outcomes. Nat Med 2011;17:1514–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Klinghammer K, Raguse JD, Plath T, Albers AE, Joehrens K, Zakarneh A, et al. A comprehensively characterized large panel of head and neck cancer patient-derived xenografts identifies the mTOR inhibitor everolimus as potential new treatment option. Int J Cancer 2015;136:2940–8. [DOI] [PubMed] [Google Scholar]
- 15. Lee HW, Lee JI, Lee SJ, Cho HJ, Song HJ, Jeong DE, et al. Patient-derived xenografts from non–small cell lung cancer brain metastases are valuable translational platforms for the development of personalized targeted therapy. Clin Cancer Res 2015;21:1172–82. [DOI] [PubMed] [Google Scholar]
- 16. Diossy M, Reiniger L, Sztupinszki Z, Krzystanek M, Timms KM, Neff C, et al. Breast cancer brain metastases show increased levels of genomic aberration-based homologous recombination deficiency scores relative to their corresponding primary tumors. Ann Oncol 2018;29:1948–54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Sun J, Wang C, Zhang Y, Xu L, Fang W, Zhu Y, et al. Genomic signatures reveal DNA damage response deficiency in colorectal cancer brain metastases. Nat Commun 2019;10:3190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Lo Nigro C, Vivenza D, Monteverde M, Lattanzio L, Gojis O, Garrone O, et al. High frequency of complex TP53 mutations in CNS metastases from breast cancer. Br J Cancer 2012;106:397–404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Bensimon A, Aebersold R, Shiloh Y. Beyond ATM: the protein kinase landscape of the DNA damage response. FEBS Lett 2011;585:1625–39. [DOI] [PubMed] [Google Scholar]
- 20. Durant ST, Zheng L, Wang Y, Chen K, Zhang L, Zhang T, et al. The brain-penetrant clinical ATM inhibitor AZD1390 radiosensitizes and improves survival of preclinical brain tumor models. Sci Adv 2018;4:eaat1719. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Biddlestone-Thorpe L, Sajjad M, Rosenberg E, Beckta JM, Valerie NC, Tokarz M, et al. ATM kinase inhibition preferentially sensitizes p53-mutant glioma to ionizing radiation. Clin Cancer Res 2013;19:3189–200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Jucaite A, Stenkrona P, Cselenyi Z, De Vita S, Buil-Bruna N, Varnas K, et al. Brain exposure of the ATM inhibitor AZD1390 in humans-a positron emission tomography study. Neuro Oncol 2021;23:687–96. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Talele S, Zhang W, Chen J, Gupta SK, Burgenske DM, Sarkaria JN, et al. Central nervous system distribution of the ataxia-telangiectasia mutated kinase inhibitor AZD1390: implications for the treatment of brain tumors. J Pharmacol Exp Ther 2022;383:91–102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Morikawa A, Peereboom DM, Thorsheim HR, Samala R, Balyan R, Murphy CG, et al. Capecitabine and lapatinib uptake in surgically resected brain metastases from metastatic breast cancer patients: a prospective study. Neuro Oncol 2015;17:289–95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Yonemori K, Tsuta K, Ono M, Shimizu C, Hirakawa A, Hasegawa T, et al. Disruption of the blood brain barrier by brain metastases of triple-negative and Basal-type breast cancer but not HER2/neu-positive breast cancer. Cancer 2010;116:302–8. [DOI] [PubMed] [Google Scholar]
- 26. Christoforides A, Carpten JD, Weiss GJ, Demeure MJ, Von Hoff DD, Craig DW. Identification of somatic mutations in cancer through Bayesian-based analysis of sequenced genome pairs. Bmc Genomics 2013;14:302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Kim S, Scheffler K, Halpern AL, Bekritsky MA, Noh E, Kallberg M, et al. Strelka2: fast and accurate calling of germline and somatic variants. Nat Methods 2018;15:591–4. [DOI] [PubMed] [Google Scholar]
- 28. Ritchie ME, Phipson B, Wu D, Hu Y, Law CW, Shi W, et al. limma powers differential expression analyses for RNA-sequencing and microarray studies. Nucleic Acids Res 2015;43:e47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Koga Y, Ochiai A. Systematic review of patient-derived xenograft models for preclinical studies of anticancer drugs in solid tumors. Cells 2019;8:418. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Tsunedomi R, Yoshimura K, Kimura Y, Nishiyama M, Fujiwara N, Matsukuma S, et al. Elevated expression of RAB3B plays important roles in chemoresistance and metastatic potential of hepatoma cells. BMC Cancer 2022;22:260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Wang Q, Zhang W, Deng C, Lin S, Zhou Y. HOXA-AS2 may predict the prognosis of solid tumors among Chinese patients: a meta-analysis and bioinformatic analysis. Front Oncol 2022;12:1030825. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Crowe DL, Chandraratna RA. A retinoid X receptor (RXR)-selective retinoid reveals that RXR-alpha is potentially a therapeutic target in breast cancer cell lines, and that it potentiates antiproliferative and apoptotic responses to peroxisome proliferator-activated receptor ligands. Breast Cancer Res 2004;6:R546–55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Ju X, Huang P, Chen M, Wang Q. Liver X receptors as potential targets for cancer therapeutics. Oncol Lett 2017;14:7676–80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Kim YJ, Kim JS, Kim IA. Molecular subtype predicts incidence and prognosis of brain metastasis from breast cancer in SEER database. J Cancer Res Clin Oncol 2018;144:1803–16. [DOI] [PubMed] [Google Scholar]
- 35. Waqar SN, Robinson C, Olszanski AJ, Spira A, Hackmaster M, Lucas L, et al. Phase I trial of ATM inhibitor M3541 in combination with palliative radiotherapy in patients with solid tumors. Invest New Drugs 2022;40:596–605. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Golding SE, Rosenberg E, Valerie N, Hussaini I, Frigerio M, Cockcroft XF, et al. Improved ATM kinase inhibitor KU-60019 radiosensitizes glioma cells, compromises insulin, AKT and ERK prosurvival signaling, and inhibits migration and invasion. Mol Cancer Ther 2009;8:2894–902. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Shen M, Xu Z, Xu W, Jiang K, Zhang F, Ding Q, et al. Inhibition of ATM reverses EMT and decreases metastatic potential of cisplatin-resistant lung cancer cells through JAK/STAT3/PD-L1 pathway. J Exp Clin Cancer Res 2019;38:149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Koneru B, Farooqi A, Nguyen TH, Chen WH, Hindle A, Eslinger C, et al. ALT neuroblastoma chemoresistance due to telomere dysfunction-induced ATM activation is reversible with ATM inhibitor AZD0156. Sci Transl Med 2021;13:eabd5750. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Hu M, Zhou M, Bao X, Pan D, Jiao M, Liu X, et al. ATM inhibition enhances cancer immunotherapy by promoting mtDNA leakage and cGAS/STING activation. J Clin Invest 2021;131:e139333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Zhang Q, Green MD, Lang X, Lazarus J, Parsels JD, Wei S, et al. Inhibition of ATM increases interferon signaling and sensitizes pancreatic cancer to immune checkpoint blockade therapy. Cancer Res 2019;79:3940–51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Bancovik J, Moreira DF, Carrasco D, Yao J, Porter D, Moura R, et al. Dermcidin exerts its oncogenic effects in breast cancer via modulation of ERBB signaling. BMC Cancer 2015;15:70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Joseph C, Alsaleem M, Orah N, Narasimha PL, Miligy IM, Kurozumi S, et al. Elevated MMP9 expression in breast cancer is a predictor of shorter patient survival. Breast Cancer Res Treat 2020;182:267–82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Kurz EU, Douglas P, Lees-Miller SP. Doxorubicin activates ATM-dependent phosphorylation of multiple downstream targets in part through the generation of reactive oxygen species. J Biol Chem 2004;279:53272–81. [DOI] [PubMed] [Google Scholar]
- 44. Dozmorov MG, Azzarello JT, Wren JD, Fung KM, Yang Q, Davis JS, et al. Elevated AKR1C3 expression promotes prostate cancer cell survival and prostate cell-mediated endothelial cell tube formation: implications for prostate cancer progression. BMC Cancer 2010;10:672. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. He L, Du Z, Xiong X, Ma H, Zhu Z, Gao H, et al. Targeting androgen receptor in treating HER2 positive breast cancer. Sci Rep 2017;7:14584. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Lin LL, Yang F, Zhang DH, Hu C, Yang S, Chen XQ. ARHGAP10 inhibits the epithelial-mesenchymal transition of non–small cell lung cancer by inactivating PI3K/Akt/GSK3beta signaling pathway. Cancer Cell Int 2021;21:320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Barcellos KS, Bigarella CL, Wagner MV, Vieira KP, Lazarini M, Langford PR, et al. ARHGAP21 protein, a new partner of alpha-tubulin involved in cell-cell adhesion formation and essential for epithelial-mesenchymal transition. J Biol Chem 2013;288:2179–89. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Sousa S, Cabanes D, Archambaud C, Colland F, Lemichez E, Popoff M, et al. ARHGAP10 is necessary for alpha-catenin recruitment at adherens junctions and for Listeria invasion. Nat Cell Biol 2005;7:954–60. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplemental Figure 1. Immunochemistry (IHC) staining of γH2AX at experimental endpoint
Supplemental Figure 2. Gene set enrichment analysis (GSEA) of progression versus stable tumors
Data Availability Statement
RNA-seq data presented in this article has been deposited in NCBI's Gene Expression Omnibus (RRID:SCR_005012) under accession number GSE224899. All data generated have been presented in article and raw data is available from the corresponding author upon reasonable request.