Abstract
Background
Previous studies have reported the direct or indirect relationship between the renin-angiotensin-aldosterone system (RAAS) and atrial fibrillation (AF). However, in patients with “apparently” idiopathic AF without possible external influence, whether RAAS is dysregulated at an early stage of AF and its relationship with the recurrence of AF after ablation have not been studied.
Methods
This single-center, prospective, case-control study included apparently healthy individuals with AF (the case group) or paroxysmal supraventricular tachycardia (PSVT, the control group) referred for catheter ablation at the same period. The primary outcome was RAAS activation in these two groups. The secondary outcome was the 1-year recurrence of AF after ablation.
Results
This study included 51 “apparently” idiopathic AF and 91 patients with PSVT. A greater proportion of patients in the case group had plasma renin activity (PRA) levels < 1 ng/ml/h compared to the control group (25.5 % vs. 7.7 %, P = 0.003). PRA < 1 ng/ml/h was the only factor found to be associated with the diagnose of AF in both the univariate model (odds ratio [OR] 4.11, 95 % confidence interval [CI] 1.52–11.11, P = 0.005) and the model adjusted for age and sex (OR 3.98, 95 % CI 1.20–13.25, P = 0.024). A similar pattern was seen with paroxysmal AF. No significant difference in the components of RAAS was observed between 11 patients with the recurrence of AF and 40 without the recurrence at the 1-year follow-up.
Conclusions
This observational study revealed an association between low renin activity and the diagnosis of “apparently” idiopathic AF, particularly paroxysmal AF.
Keywords: Atrial fibrillation, The renin-angiotensin-aldosterone system, Aldosterone-renin ration, Plasma renin activity
1. Introduction
Atrial fibrillation (AF) is the most common arrythmia, which increases the risk of thromboembolism, heart failure, and death [1], [2], [3]. Enormous efforts have been made to uncover the mechanisms of AF. In the past, the term ‘idiopathic AF or lone AF’ was used to describe AF in young patients without underlying cardiovascular diseases (CVDs) or other comorbidities like pulmonary diseases or evidence of acute triggers for AF [4]. The robustness of this term has been questioned due to the tremendous variability in its definitions. In addition, it is conceivable that every patient with AF has a specific cause [5]. In the particular population with ‘apparently’ idiopathic AF, their early stage or yet subclinical vascular disease might be undetectable by routine diagnostics. Therefore, recent guidelines recommend that the use of the term ‘idiopathic AF or lone AF’ should be abandoned [6]. However, tracing identifiable predisposing factors or the presence of early stages of the underlying disease is still of high priority because it may pave the way for the development of new early treatment strategies or diagnostics for apparently healthy individuals with AF.
Recently, accumulating evidence pointed to an association between dysregulation of the renin-angiotensin-aldosterone system (RAAS) and AF. Hypertensive patients with primary aldosteronism are at a 12-fold higher risk of AF compared with patients with essential hypertension [7]. Findings from several clinical studies suggested that treatment with aldosterone receptor antagonists, also known as mineralocorticoid antagonists (MRAs), reduced the incidence of new-onset or recurrent AF in patients with left ventricular dysfunction [8], [9]. In addition, expression of renin was down-regulated in both cellular and animal models of rapid atrial depolarization [10]. It is unknown whether alternations of the RAAS exist in patients with “apparently” idiopathic AF. Moreover, since blockers of the RAAS, i.e., renin inhibitors, angiotensin-converting enzyme inhibitors (ACEIs), angiotensin receptor blockers (ARBs), angiotensin receptor/neprilysin inhibitors (ARNI), and MRAs used in patients with concomitant CVDs would affect the level of plasma renin and aldosterone to some extent, “apparently” idiopathic AF might be a fine pathophysiological model to identify the hidden pathophysiological mechanism of AF.
We therefore aimed to measure components of the RAAS in apparently healthy patients with AF with the intention of addressing the following questions: (1) Is the RAAS dysregulated in apparently healthy patients with AF? (2) Can any components of the RAAS be biomarkers for AF in apparently healthy patients? (3) Are baseline components of the RAAS associated with AF recurrence after ablation in apparently healthy patients with AF?
2. Methods
Given the low prevalence of AF in apparently healthy individuals, we conducted an unmatched case-control study among patients referred for radio-frequency catheter ablation. This study conformed to the principles outlined in the Declaration of Helsinki and was approved by the ethics committee of the First Affiliated Hospital of Nanjing Medical University (2021-SR-079). Each patient provided a written consent form.
2.1. Case definition and recruitment
In this prospective study, apparently healthy individuals were defined as adults aged 18–60 years, without history or risk factors of cardiovascular and pulmonary diseases, including coronary heart disease, hypertension, hyperthyroidism, structural heart disease, congestive heart failure, chronic obstructive pulmonary disease, diabetes mellitus, or an acute trigger of AF [11]. Patients with obstructive sleep apnea and current alcohol consumption were identified from self-reported history or self-reported information provided by the participants. During the 14-month recruitment period, apparently healthy patients with AF were consecutively included in the case group. These patients were further categorized into having paroxysmal and non-paroxysmal AF depending on whether their AF could spontaneously convert to sinus rhythm or revert to sinus rhythm with electrical or pharmacological cardioversion within 7 days of onset. It was noted that all the patients with paroxysmal AF were in sinus rhythm when admitted to the hospital and during blood sampling. To rule out the possible effect of drugs on RAAS, patients with usage of MRAs, ACEI/ARB/ARNI, β-blockers, calcium channel blockers and diuretics within 4 weeks of admission were excluded. All the controls and cases underwent computed tomography to rule out apparent adrenal adenomas or nodules before catheter ablation.
2.2. Recruitment of controls
For better compatibility, during the same recruitment period, all apparently healthy patients with paroxysmal supraventricular tachycardia (PSVT) were consecutively included in the control group. In addition, all the controls were in sinus rhythm when admitted to the hospital and during blood sampling. Patients with incessant PSVT were excluded.
2.3. Blood sampling
Fasting peripheral blood was drawn at 8 a.m. after patients remained upright for at least 2 h. Blood samples were centrifuged and plasma was stored at −20 °C. Plasma renin activity (PRA) was measured by radioimmunoassay using a commercial angiotensin-I kit (The North Institute of Biotechnology, Beijing, China). Plasma aldosterone concentration (PAC) was measured in a fully automated chemiluminescent analyzer (LIAISON) using reagents provided by DiaSorin (LIAISON Direct Renin Assay; DiaSorin). The normal range of PRA and PAC was 0.93–6.56 ng/ml/h and 6.5–30.0 ng/dL, respectively. The aldosterone-renin ratio (ARR) was calculated by dividing PAC by PRA. The coefficient of variation within and between batches were less than 10 % and 15 % respectively.
2.4. Ablation procedure
The atrial ablation procedure adopted in the case group has been described previously [12]. After constructing a three-dimensional electroanatomical geometry using the CARTO system (Biosense Webster), wide antral circumferential radiofrequency ablation was performed on both sides of the pulmonary veins. This procedure utilized an open irrigated-tip catheter (ThermoCool SF, ThermoCool SmartTouch, Biosense Webster) operating in a power-controlled mode set at 30–45 W, with a temperature limited to 43 °C. The ablation index (AI)-guided ablation process around pulmonary veins was performed point-by-point with a targeted AI value of over 500 for the anterior wall, over 450 for the roof and inferior wall, and over 400 for the posterior wall. Low voltage zones < 0.5 mV mapped during sinus rhythm were subjected to homogenization using an ablation catheter. Verification of electric isolation was conducted using a mapping catheter (Pentaray, Biosense Webster). In the control group, conventional accessory pathway ablation or slow pathway modification was performed based on the findings of the electrophysiology study, utilizing the ThermoCool radiofrequency ablation catheter (Biosense Webster). Transthoracic echocardiography was performed in all the patients before ablation.
2.5. Follow-up
All the patients in the case group received a 7-day Holter monitoring one year post-ablation. Recurrence was defined as any episode of atrial arrhythmia lasting longer than 30 s. Patients with PSVT in the control group did not have scheduled follow-up visits unless palpitations recurred.
2.6. Statistical analysis
Continuous variables are described as mean ± standard deviation (SD) and median (interquartile range) based on their distribution. Categorical variables are described as frequencies (percentage). For continuous variables, the two groups were compared using the Student’s t-test or the Mann-Whitney U test, as appropriate. Between-group difference for categorical variables were compared using the Chi-square test or the Fisher’s exact test. Other than the common recognized threshold of ≥ 30 ng/dL:ng/ml/h, our study set a lower ARR threshold of ≥ 10 ng/dL:ng/ml/h to detect subclinical differences.
The univariate logistic regression model was used to test possible factors associated with AF or paroxysmal AF, which were further adjusted for age and sex. Since the patients included in this study had no conventional cardiovascular risk factors or comorbidities, no additional variables were adjusted in the multivariable regression model. Statistical analysis was performed using SPSS (IBM SPSS Statistics version 25.0, IBM Corporation, Armonk, NY). A two-sided P-value of less than 0.05 was considered significant.
3. Results
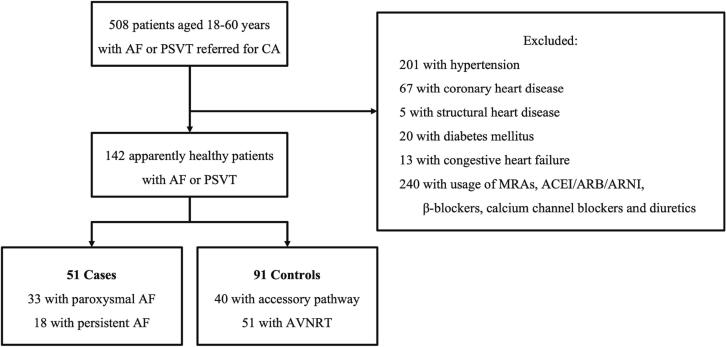
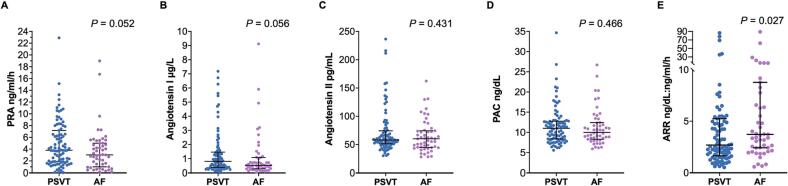
This study included 51 apparently healthy patients with AF (the case group) and 91 patients with PSVT (the control group). The screening process was illustrated in Fig. 1. Table 1 displays the clinical characteristics of the two groups. Patients in the case group were older (48.0 ± 8.5 years vs. 39.3 ± 11.6 years, P < 0.001), more likely to be male (74.5 % vs.46.2 %, P = 0.001) and have higher body mass index (BMI) (24.8 ± 2.3 kg/m2 vs. 23.6 ± 3.6 kg/m2, P = 0.018) compared to those in the control group. In addition, patients in the case group had a larger left atrial diameter (37.6 ± 5.2 mm vs. 31.3 ± 4.0 mm, P < 0.001). As shown in Figure 2, patients in the case group had borderline lower levels of PRA (3.0 ng/ml/h vs. 3.8 ng/ml/h, P = 0.052) and angiotensin I (0.5 μg/L vs. 0.8 μg/L, P = 0.056), as well as a higher level of ARR (3.7 ng/dL:ng/ml/h vs. 2.7 ng/dL:ng/ml/h, P = 0.027). Moreover, the proportion of patients with PRA < 1 ng/ml/h was significantly higher in the case group compared to the control group (25.5 % vs. 7.7 %, P = 0.003). There were no significant differences in the levels of angiotensin II and PAC between the two groups.
Fig. 1.
Consort diagram depicting the screening process. Abbreviations: PSVT, paroxysmal supraventricular tachycardia; AF, atrial fibrillation; CA, catheter ablation; MRAs, mineralocorticoid antagonists; ACEIs, angiotensin-converting enzyme inhibitor; ARB, angiotensin receptor blocker; ARNI, angiotensin receptor/neprilysin inhibitor; AVNRT, atrioventricular nodal reentry tachycardia.
Table 1.
Clinical characteristics of apparent healthy individuals with PSVT, AF and paroxysmal AF.
| PSVT n = 91 |
AF n = 51 |
Paroxysmal AF n = 33 |
P value ‡ | P value § | |
|---|---|---|---|---|---|
| Age, years* | 39.3 ± 11.6 | 48.0 ± 8.5 | 48.6 ± 8.3 | <0.001 | <0.001 |
| Male, n (%)† | 42 (46.2) | 38 (74.5) | 21 (63.6) | 0.001 | 0.085 |
| Current drinker, n (%)† | 0 | 0 | 0 | NA | NA |
| Obstructive sleep apnea, n (%)† | 0 | 0 | 0 | NA | NA |
| Body mass index, kg/m2* | 23.6 ± 3.6 | 24.8 ± 2.3 | 24.4 ± 2.3 | 0.018 | 0.255 |
| Serum potassium, mmol/L* | 4.1 ± 0.3 | 4.0 ± 0.3 | 4.0 ± 0.3 | 0.779 | 0.301 |
| LAD, mm* | 31.3 ± 4.0 | 37.6 ± 5.2 | 35.6 ± 4.6 | <0.001 | <0.001 |
| LVDd, mm* | 45.8 ± 3.8 | 47.9 ± 3.6 | 47.7 ± 3.5 | 0.002 | 0.014 |
| LVEF, %* | 64.4 ± 2.2 | 62.9 ± 3.5 | 63.4 ± 2.4 | 0.002 | 0.023 |
| RAAS components | |||||
| PRA, ng/ml/h * | 3.8 (1.9, 7.2) | 3.0 (1.0, 5.0) | 2.7 (0.7, 5.25) | 0.052 | 0.025 |
| PRA < 1 ng/ml/h, n (%) | 7 (7.7) | 13 (25.5) | 11 (33.3) | 0.003 | 0.001 |
| Angiotensin I, μg/L* | 0.8 (0.4, 1.5) | 0.5 (0.3, 1.1) | 0.5 (0.3, 0.9) | 0.056 | 0.032 |
| Angiotensin II, pg/ml* | 58.1 (51.3, 74.2) | 60.2 (44.7, 74.4) | 53.4 (44.6, 77.1) | 0.431 | 0.244 |
| PAC, ng/dL* | 11.0 (8.3, 12.8) | 10.0 (8.3, 12.4) | 9.8 (7.7, 12.1) | 0.466 | 0.287 |
| ARR, ng/dL:ng/ml/h * | 2.7 (1.6, 5.2) | 3.7 (2.4, 8.8) | 3.7 (2.6, 11.0) | 0.027 | 0.015 |
| ARR > 30 ng/dL:ng/ml/h, n (%)† | 5 (5.5) | 2 (3.9) | 2 (6.1) | 1.000 | 1.000 |
| ARR > 10 ng/dL:ng/ml/h, n (%)† | 10 (11.0) | 11 (21.6) | 10 (30.3) | 0.088 | 0.010 |
*Values are means ± standard deviations or medians (interquartiles). †Values are numbers (percentages). ‡: PSVT vs. AF. §: PSVT vs. Paroxysmal AF.
Abbreviations: PSVT, paroxysmal supraventricular tachycardia; AF, atrial fibrillation; LAD, left atrial diameter; LVDd, left ventricular diastolic diameter; LVEF, left ventricular ejection fraction; RAAS, renin-angiotensin-aldosterone system; PRA, plasma renin activity; PAC, plasma aldosterone concentration; ARR, aldosterone–renin ratio.
Fig. 2.
Scatter plot of components of RAAS in apparent healthy individuals with PSVT and AF. Abbreviations: PSVT, paroxysmal supraventricular tachycardia; AF, atrial fibrillation; RAAS, renin-angiotensin-aldosterone system; PRA, plasma renin activity; PAC, plasma aldosterone concentration; ARR, aldosterone–renin ratio.
As shown in Table 2, PRA < 1 ng/ml/h was found to be the only factor associated with the diagnose of AF in both the univariate model (odds ratio [OR] 4.11, 95 % confidence interval [CI] 1.52–11.11, P = 0.005) and the model adjusted for age, sex and BMI (OR 3.86, 95 % CI 1.17–12.76, P = 0.027).
Table 2.
Logistic regression analysis showing the association between the components of the RAAS and AF versus PSVT.
| Unadjusted |
Adjusted for age, sex and BMI |
|||||
|---|---|---|---|---|---|---|
| Odds ratio | 95 % confidence interval | P Value | Odds ratio | 95 % confidence interval | P Value | |
| PRA, ng/ml/h | 0.92 | 0.83–1.02 | 0.094 | |||
| PRA < 1 ng/ml/h | 4.11 | 1.52–11.11 | 0.005 | 3.86 | 1.17–12.76 | 0.027 |
| Angiotensin I, μg/L | 0.90 | 0.71–1.14 | 0.363 | |||
| Angiotensin II, pg/ml | 0.99 | 0.98–1.01 | 0.295 | |||
| PAC, μg/dL | 0.98 | 0.91–1.06 | 0.665 | |||
| ARR, ng/dL:ng/ml/h | 1.00 | 0.99–1.02 | 0.980 | |||
| ARR > 30 ng/dL:ng/ml/h | 1.42 | 0.27–7.62 | 0.679 | |||
| ARR > 10 ng/dL:ng/ml/h | 2.23 | 0.87–5.68 | 0.094 | |||
Abbreviations: PSVT, paroxysmal supraventricular tachycardia; AF, atrial fibrillation; RAAS, renin-angiotensin-aldosterone system; PRA, plasma renin activity; PAC, plasma aldosterone concentration; ARR, aldosterone–renin ratio; BMI, body mass index.
Among the 51 AF patients in the case group, 33 were classified as having paroxysmal AF. Given that both PSVT and paroxysmal AF share characteristics of occasional and paroxysmal episodes of tachyarrhythmia, we conducted a further comparison of clinical characteristics between patients with PSVT and paroxysmal AF (Table 1). In the case group, patients with paroxysmal AF were older and had a larger left atrial diameter, a larger left ventricular diastolic diameter, and a lower left ventricular ejection fraction, compared to the control group (48.6 ± 8.3 years vs. 39.3 ± 11.6 years, P < 0.001; 35.6 ± 4.6 mm vs. 31.3 ± 4.0 mm, P < 0.001; 47.7 ± 3.5 mm vs. 45.8 ± 3.8 mm, P = 0.014; and 63.4 ± 2.4 % vs. 64.4 ± 2.2 %, P = 0.023, respectively). Patients with paroxysmal AF had lower levels of PRA (2.7 ng/ml/h vs. 3.8 ng/ml/h, P = 0.025), and angiotensin I (0.5 μg/L vs. 0.8 μg/L, P = 0.032), as well as a higher level of ARR (3.7 ng/dL:ng/ml/h vs. 2.7 ng/dL:ng/ml/h, P = 0.015) compared with those with PSVT. Moreover, the proportion of patients with paroxysmal AF with PRA < 1 ng/ml/h and ARR > 10 ng/dL:ng/ml/h was higher compared with the control group (33.3 % vs. 7.7 %, P = 0.001 and 30.3 % vs. 11.0 %, P = 0.010, respectively). There were no significant differences in the levels of angiotensin II and PAC between the two groups.
Moreover, PRA < 1 ng/ml/h remained as the only factor found to be associated with the diagnose of paroxysmal AF both in the univariate model (OR 6.00, 95 % CI 2.08–17.28, P = 0.001) and in the model adjusted for age, sex and BMI (OR 4.66, 95 % CI 1.35–16.1, P = 0.015), as shown in Table 3.
Table 3.
Logistic regression analysis showing the association between the components of the RAAS and paroxysmal AF versus PSVT.
| Unadjusted |
Adjusted for age, sex and BMI |
|||||
|---|---|---|---|---|---|---|
| Odds ratio | 95 % confidence interval | P Value | Odds ratio | 95 % confidence interval | P Value | |
| PRA, ng/ml/h | 1.14 | 1.00–1.30 | 0.057 | |||
| PRA < 1 ng/ml/h | 6.00 | 2.08–17.28 | 0.001 | 4.66 | 1.35–16.1 | 0.015 |
| Angiotensin I, μg/L | 1.24 | 0.88–1.73 | 0.217 | |||
| Angiotensin II, pg/ml | 1.00 | 0.99–1.02 | 0.349 | |||
| PAC, μg/dL | 0.98 | 0.90–1.07 | 0.617 | |||
| ARR, ng/dL:ng/ml/h | 1.00 | 0.98–1.01 | 0.614 | |||
| ARR > 30 ng/dL:ng/ml/h | 1.11 | 0.21–6.02 | 0.904 | |||
| ARR > 10 ng/dL:ng/ml/h | 3.52 | 1.31–9.49 | 0.013 | 2.43 | 0.80–7.38 | 0.117 |
Abbreviations: RAAS, renin-angiotensin-aldosterone system; PSVT, paroxysmal supraventricular tachycardia; AF, atrial fibrillation; BMI, body mass index; PRA, plasma renin activity; PAC, plasma aldosterone concentration; ARR, aldosterone–renin ratio.
Among the 91 patients in the control group, 40 was finally diagnosed with atrioventricular reentrant tachycardia and 51 with atrioventricular nodal reentry tachycardia. First pass isolation, which refers to the successful and complete electrical isolation of pulmonary veins in a single encirclement, was achieved in 44 patients with AF (86.2 %). At the 1-year follow-up, among the 51 patients in the case group, 11 (21.7 %) had recurrence of atrial arrhythmia and 3 received redo procedures. None of the patients in the control group had arrhythmia recurrence during the follow-up. However, no significant difference was observed in any of the variables when comparing patients with and without AF recurrence (Table S1).
4. Discussion
In the present study, we found that (1) ARR was higher in apparently healthy patients with AF compared with their controls; (2) The presence of PRA less than 1 ng/ml/h was identified as the only factor associated with the diagnose of AF, especially paroxysmal AF, within this group of apparently healthy patients.
Renin, secreted by juxtaglomerular kidney cells, is primarily regulated through negative long feedback mechanisms. Factors such as increased renal artery pressure, high salt intake, and elevated levels of aldosterone can suppress renin secretion [13]. In this study, we found that low renin activity was associated with “apparently” idiopathic AF. Several possible explanations can be considered.
The secretion of atrial natriuretic peptide (ANP) by stretched atrial walls in patients with AF promotes the excretion of sodium and water while inhibiting the secretion of renin, angiotensin, and aldosterone [14], [15]. The supporting evidence for this statement is derived from previous studies. In patients with left ventricular dysfunction who underwent coronary artery bypass grafting, continuous low-dose infusion of human ANP was found to decrease postoperative renin activity [16]. Animal studies have also provided evidence in this regard. In both normal dogs and dogs with acute low output heart failure, the administration of synthetic ANP resulted in a reduction of renin secretion [17]. Additionally, a comparison between dogs with non-filtering kidneys and normal dogs showed that ANP inhibited renin release by increasing sodium delivery to the macula densa [18].
Another plausible explanation for the observed low renin activity in cases of “apparently” idiopathic AF is the presence of concealed high aldosteronism. In our study, blood samples were collected at 8 a.m. following a minimum of 2 h in an upright posture, which is likely to represent the peak aldosterone level throughout the day. However, it is important to acknowledge that the secretion of aldosterone follows a circadian rhythm and plasma aldosterone levels fluctuate during the day [19]. We speculate that hyperaldosteronism might be concealed in cases of “apparently” idiopathic AF, even though there was no significant difference in PAC between the two groups. This could also partially explain why AF occurrence could be reduced when treated with MRA [8], [20], [21].
4.1. RAAS and AF recurrence
The absence of a difference in baseline renin levels between cases with AF recurrence and without recurrence could be attributed to the multifaceted nature of AF recurrence, which might be influenced by a myriad of factors beyond the baseline components of the RAAS alone. These factors could include patient-specific characteristics, genetic predispositions, lifestyle factors, and other unexplored variables [22], [23], [24]. Therefore, our study's focus on renin levels alone may not fully capture the entire picture. In addition, only 51 patients with AF and 13 patients with recurrent AF were included in our analysis. The small sample size may not be sufficient to show differences in renin levels.
4.2. Clinical implication
RAAS inhibitors that affect the atrial-remodeling process are recommended as upstream therapy for antiarrhythmic properties [6]. However, the evidence supporting of RAAS inhibitors as the upstream therapy of AF primarily comes from patients with structural heart disease or hypertension [25], [26], [27], [28], [29]. Thus, the reduction in AF events may be driven indirectly, through the improvement of heart failure or the control of blood pressure. In the context of our study, which exclusively including apparently healthy individuals, we demonstrated the dysregulation of RAAS in this such population for the first time. Gaining a comprehensive understanding the mechanisms of “apparently” idiopathic AF is of great value for improving the diagnosis, management, and prevention of this condition. Although our study did not fully elucidate the exact causal relationship between dysregulation of the RAAS and “apparently” idiopathic AF, it does provide insights into the potential benefit of targeted interventions to modulate the RAAS in affected individuals. Such interventions may prevent the development of AF or improve outcomes in those already diagnosed with “apparently” idiopathic AF. However, it is important to acknowledge that further research is needed to confirm the clinical benefits of such approaches.
Furthermore, both PSVT and paroxysmal AF could present as short-term palpitation. While PSVT is not immediately life-threatening, AF is associated with much higher risk of stroke and heart failure, necessitating early restoration of sinus rhythm. long-term monitoring to capture an ECG during palpitation episodes is inaccessible for most patients due to their poor compliance. The adjusted logistic analysis of our study revealed a significant association between low renin activity and paroxysmal “apparently” idiopathic AF, suggesting that low PRA level in apparently healthy patients with palpitations provided hints for the presence of paroxysmal AF. Therefore, our findings hold the potential to identify individuals at higher risk of having AF, facilitating the implementation of more effective screening measures.
4.3. Strength and limitation
One notable strength of our study is that we specifically focused on patients with “apparently” idiopathic AF who were still at the early stage of AF and ruled out patients using antihypertensive medications. A common methodological flaw observed in previous studies investigating the relationship between the RAAS and AF is the failure to account for the influence of antihypertensive drugs in their regression models [30].
There were several limitations to be acknowledged in our study. Firstly, due to its observational nature, we were unable to establish a causal relationship between low renin levels and “apparently” idiopathic AF. Secondly, the assessment of circulating PRA and PAC was limited to peripheral blood samples. Blood tests from samples closer to the heart, such as the coronary sinus, could provide a more robust demonstration of the association between renin activity and “apparently” idiopathic AF. Thirdly, we adopted the definition of idiopathic AF from the 2010 ESC guidelines for the management of atrial fibrillation with no context mentioning exercise. Therefore, physical activity was not included in the current study. Fourthly, only left atrial diameter was routinely measured in our center, leaving us with no choice but to use left atrial diameter rather than indexed left atrial volume as a measurement of left atrial size. Lastly, since the study population was relatively small and highly selected, our findings necessitate confirmation in larger and more diverse population.
5. Conclusion
This observational study revealed a correlation between low renin activity and the diagnosis of “apparently” idiopathic AF, particularly paroxysmal “apparently” idiopathic AF. However, no significant association was found between the baseline components of RAAS and the recurrence of AF.
Funding
This work was supported by grant from National Natural Science Foundation of China (No. 82270329) and Department of Science and Technology of Guangdong Province (2019B020230004).
Declaration of Competing Interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Footnotes
Supplementary data to this article can be found online at https://doi.org/10.1016/j.ijcha.2023.101286.
Contributor Information
Min Sun, Email: sunmin@jsph.org.cn.
Mingfang Li, Email: mingfangli@njmu.edu.cn.
Appendix A. Supplementary data
The following are the Supplementary data to this article:
References
- 1.Kannel W.B., Wolf P.A., Benjamin E.J., Levy D. Prevalence, incidence, prognosis, and predisposing conditions for atrial fibrillation: population-based estimates. Am. J. Cardiol. 1998;82:2N–9N. doi: 10.1016/s0002-9149(98)00583-9. [DOI] [PubMed] [Google Scholar]
- 2.Santhanakrishnan R., Wang N., Larson M.G., Magnani J.W., McManus D.D., Lubitz S.A., et al. Atrial Fibrillation Begets Heart Failure and Vice Versa: Temporal Associations and Differences in Preserved Versus Reduced Ejection Fraction. Circulation. 2016;133:484–492. doi: 10.1161/CIRCULATIONAHA.115.018614. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Magnussen C., Niiranen T.J., Ojeda F.M., Gianfagna F., Blankenberg S., Njolstad I., et al. Sex Differences and Similarities in Atrial Fibrillation Epidemiology, Risk Factors, and Mortality in Community Cohorts: Results From the BiomarCaRE Consortium (Biomarker for Cardiovascular Risk Assessment in Europe) Circulation. 2017;136:1588–1597. doi: 10.1161/CIRCULATIONAHA.117.028981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Wyse D.G., Van Gelder I.C., Ellinor P.T., Go A.S., Kalman J.M., Narayan S.M., et al. Lone atrial fibrillation: does it exist? J. Am. Coll. Cardiol. 2014;63:1715–1723. doi: 10.1016/j.jacc.2014.01.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Weijs B., Schotten U., Crijns H.J. Pathophysiology of idiopathic atrial fibrillation - prognostic and treatment implications. Curr. Pharm. Des. 2015;21:551–572. doi: 10.2174/1381612820666140825150057. [DOI] [PubMed] [Google Scholar]
- 6.Hindricks G., Potpara T., Dagres N., Arbelo E., Bax J.J., Blomstrom-Lundqvist C., et al. 2020 ESC Guidelines for the diagnosis and management of atrial fibrillation developed in collaboration with the European Association for Cardio-Thoracic Surgery (EACTS): The Task Force for the diagnosis and management of atrial fibrillation of the European Society of Cardiology (ESC) Developed with the special contribution of the European Heart Rhythm Association (EHRA) of the ESC. Eur. Heart J. 2021;42:373–498. doi: 10.1093/eurheartj/ehaa612. [DOI] [PubMed] [Google Scholar]
- 7.Milliez P., Girerd X., Plouin P.F., Blacher J., Safar M.E., Mourad J.J. Evidence for an increased rate of cardiovascular events in patients with primary aldosteronism. J. Am. Coll. Cardiol. 2005;45:1243–1248. doi: 10.1016/j.jacc.2005.01.015. [DOI] [PubMed] [Google Scholar]
- 8.Neefs J., van den Berg N.W., Limpens J., Berger W.R., Boekholdt S.M., Sanders P., et al. Aldosterone Pathway Blockade to Prevent Atrial Fibrillation: A Systematic Review and Meta-Analysis. Int. J. Cardiol. 2017;231:155–161. doi: 10.1016/j.ijcard.2016.12.029. [DOI] [PubMed] [Google Scholar]
- 9.Swedberg K., Zannad F., McMurray J.J., Krum H., van Veldhuisen D.J., Shi H., et al. Eplerenone and atrial fibrillation in mild systolic heart failure: results from the EMPHASIS-HF (Eplerenone in Mild Patients Hospitalization And SurvIval Study in Heart Failure) study. J. Am. Coll. Cardiol. 2012;59:1598–1603. doi: 10.1016/j.jacc.2011.11.063. [DOI] [PubMed] [Google Scholar]
- 10.Tsai C.T., Lai L.P., Hwang J.J., Chen W.P., Chiang F.T., Hsu K.L., et al. Renin-angiotensin system component expression in the HL-1 atrial cell line and in a pig model of atrial fibrillation. J. Hypertens. 2008;26:570–582. doi: 10.1097/HJH.0b013e3282f34a4a. [DOI] [PubMed] [Google Scholar]
- 11.Fuster V., Ryden L.E., Cannom D.S., Crijns H.J., Curtis A.B., Ellenbogen K.A., et al. 2011 ACCF/AHA/HRS focused updates incorporated into the ACC/AHA/ESC 2006 Guidelines for the management of patients with atrial fibrillation: a report of the American College of Cardiology Foundation/American Heart Association Task Force on Practice Guidelines developed in partnership with the European Society of Cardiology and in collaboration with the European Heart Rhythm Association and the Heart Rhythm Society. J. Am. Coll. Cardiol. 2011;57:e101–e198. doi: 10.1016/j.jacc.2010.09.013. [DOI] [PubMed] [Google Scholar]
- 12.Yang G., Yang B., Wei Y., Zhang F., Ju W., Chen H., et al. Catheter Ablation of Nonparoxysmal Atrial Fibrillation Using Electrophysiologically Guided Substrate Modification During Sinus Rhythm After Pulmonary Vein Isolation. Circ. Arrhythm. Electrophysiol. 2016;9:e003382. doi: 10.1161/CIRCEP.115.003382. [DOI] [PubMed] [Google Scholar]
- 13.Stefanska A., Kenyon C., Christian H.C., Buckley C., Shaw I., Mullins J.J., et al. Human kidney pericytes produce renin. Kidney Int. 2016;90:1251–1261. doi: 10.1016/j.kint.2016.07.035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Fu S., Ping P., Wang F., Luo L. Synthesis, secretion, function, metabolism and application of natriuretic peptides in heart failure. J. Biol. Eng. 2018;12:2. doi: 10.1186/s13036-017-0093-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Theilig F., Wu Q. ANP-induced signaling cascade and its implications in renal pathophysiology. Am. J. Physiol. Renal Physiol. 2015;308:F1047–F1055. doi: 10.1152/ajprenal.00164.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Sezai A., Hata M., Niino T., Yoshitake I., Unosawa S., Wakui S., et al. Continuous low-dose infusion of human atrial natriuretic peptide in patients with left ventricular dysfunction undergoing coronary artery bypass grafting: the NU-HIT (Nihon University working group study of low-dose Human ANP Infusion Therapy during cardiac surgery) for left ventricular dysfunction. J. Am. Coll. Cardiol. 2010;55:1844–1851. doi: 10.1016/j.jacc.2009.11.085. [DOI] [PubMed] [Google Scholar]
- 17.Scriven T.A., Burnett J.C., Jr. Effects of synthetic atrial natriuretic peptide on renal function and renin release in acute experimental heart failure. Circulation. 1985;72:892–897. doi: 10.1161/01.cir.72.4.892. [DOI] [PubMed] [Google Scholar]
- 18.Opgenorth T.J., Burnett J.C., Jr., Granger J.P., Scriven T.A. Effects of atrial natriuretic peptide on renin secretion in nonfiltering kidney. Am. J. Phys. Anthropol. 1986;250:F798–F801. doi: 10.1152/ajprenal.1986.250.5.F798. [DOI] [PubMed] [Google Scholar]
- 19.Thosar S.S., Rueda J.F., Berman A.M., Lasarev M.R., Herzig M.X., Clemons N.A., et al. Separate and interacting effects of the endogenous circadian system and behaviors on plasma aldosterone in humans. Am. J. Physiol. Regul. Integr. Comp. Physiol. 2019;316:R157–R164. doi: 10.1152/ajpregu.00314.2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Takemoto Y., Ramirez R.J., Kaur K., Salvador-Montanes O., Ponce-Balbuena D., Ramos-Mondragon R., et al. Eplerenone Reduces Atrial Fibrillation Burden Without Preventing Atrial Electrical Remodeling. J. Am. Coll. Cardiol. 2017;70:2893–2905. doi: 10.1016/j.jacc.2017.10.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Dabrowski R., Borowiec A., Smolis-Bak E., Kowalik I., Sosnowski C., Kraska A., et al. Effect of combined spironolactone-beta-blocker +/- enalapril treatment on occurrence of symptomatic atrial fibrillation episodes in patients with a history of paroxysmal atrial fibrillation (SPIR-AF study) Am. J. Cardiol. 2010;106:1609–1614. doi: 10.1016/j.amjcard.2010.07.037. [DOI] [PubMed] [Google Scholar]
- 22.Zhao L.Q., Zhang G.B., Wen Z.J., Huang C.K., Wu H.Q., Xu J., et al. Common variants predict recurrence after nonfamilial atrial fibrillation ablation in Chinese Han population. Int. J. Cardiol. 2017;227:360–366. doi: 10.1016/j.ijcard.2016.11.057. [DOI] [PubMed] [Google Scholar]
- 23.Buiatti A., Kaess B., Reents T., Semmler V., Telishveska M., Bourier F., et al. Catheter Ablation for “Lone” Atrial Fibrillation: Efficacy and Predictors of Recurrence. J. Cardiovasc. Electrophysiol. 2016;27:536–541. doi: 10.1111/jce.12936. [DOI] [PubMed] [Google Scholar]
- 24.Uemura T., Kondo H., Sato H., Takahashi M., Shinohara T., Mitarai K., et al. Predictors of outcome after catheter ablation for atrial fibrillation: Group analysis categorized by age and type of atrial fibrillation. Ann. Noninvasive Electrocardiol. 2023;28:e13020. doi: 10.1111/anec.13020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Schneider M.P., Hua T.A., Bohm M., Wachtell K., Kjeldsen S.E., Schmieder R.E. Prevention of atrial fibrillation by Renin-Angiotensin system inhibition a meta-analysis. J. Am. Coll. Cardiol. 2010;55:2299–2307. doi: 10.1016/j.jacc.2010.01.043. [DOI] [PubMed] [Google Scholar]
- 26.Okin P.M., Wachtell K., Devereux R.B., Harris K.E., Jern S., Kjeldsen S.E., et al. Regression of electrocardiographic left ventricular hypertrophy and decreased incidence of new-onset atrial fibrillation in patients with hypertension. J. Am. Med. Assoc. 2006;296:1242–1248. doi: 10.1001/jama.296.10.1242. [DOI] [PubMed] [Google Scholar]
- 27.Ducharme A., Swedberg K., Pfeffer M.A., Cohen-Solal A., Granger C.B., Maggioni A.P., et al. Prevention of atrial fibrillation in patients with symptomatic chronic heart failure by candesartan in the Candesartan in Heart failure: Assessment of Reduction in Mortality and morbidity (CHARM) program. Am. Heart J. 2006;152:86–92. [PubMed] [Google Scholar]
- 28.Wachtell K., Lehto M., Gerdts E., Olsen M.H., Hornestam B., Dahlof B., et al. Angiotensin II receptor blockade reduces new-onset atrial fibrillation and subsequent stroke compared to atenolol: the Losartan Intervention For End Point Reduction in Hypertension (LIFE) study. J. Am. Coll. Cardiol. 2005;45:712–719. doi: 10.1016/j.jacc.2004.10.068. [DOI] [PubMed] [Google Scholar]
- 29.Olsson L.G., Swedberg K., Ducharme A., Granger C.B., Michelson E.L., McMurray J.J., et al. Atrial fibrillation and risk of clinical events in chronic heart failure with and without left ventricular systolic dysfunction: results from the Candesartan in Heart failure-Assessment of Reduction in Mortality and morbidity (CHARM) program. J. Am. Coll. Cardiol. 2006;47:1997–2004. doi: 10.1016/j.jacc.2006.01.060. [DOI] [PubMed] [Google Scholar]
- 30.Erne P., Muller A., Rossi G.P., Seifert B., Stehlin F., Redondo M., et al. Aldosterone and renin in cardiac patients referred for catheterization. Medicine (Baltimore) 2017;96:e7282. doi: 10.1097/MD.0000000000007282. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.