Abstract
How to effectively transform the pro-oncogenic tumor microenvironments (TME) surrounding a tumor into an anti-tumoral never fails to attract people to study. Small interfering RNA (siRNA) is considered one of the most noteworthy research directions that can regulate gene expression following a process known as RNA interference (RNAi). The research about siRNA delivery targeting tumor cells and TME has been on the rise in recent years. Using siRNA drugs to silence critical proteins in TME was one of the most efficient solutions. However, the manufacture of a siRNA delivery system faces three major obstacles, i.e., appropriate cargo protection, accurately targeted delivery, and site-specific cargo release. In the following review, we summarized the pharmacological actions of siRNA drugs in remolding TME. In addition, the delivery strategies of siRNA drugs and combination therapy with siRNA drugs to remodel TME are thoroughly discussed. In the meanwhile, the most recent advancements in the development of all clinically investigated and commercialized siRNA delivery technologies are also presented. Ultimately, we propose that nanoparticle drug delivery siRNA may be the future research focus of oncogene therapy. This summary offers a thorough analysis and roadmap for general readers working in the field.
Keywords: Small interfering RNA, Tumor microenvironment, siRNA delivery, Cancer therapy, Co-delivery
Graphical abstract
1. Introduction
Cancer is one of the most frequent and deadly diseases in the world, adversely damaging people's health. The therapeutic effects of traditional drugs as well as treatment regimens are often lower than expected while having certain side effects. Therefore, given the unavoidable limitations of existing clinical drugs and treatments, seeking innovative treatment techniques along with directions remains a top priority [1]. Currently, regulating and controlling the human immune system is regarded as a significant focus of research for cancer treatment [2]. Studies have demonstrated that a special immune response occurs around the tumor, a series of complex reactions that are triggered via interaction between immune cells and tumor cells. This complicated chain of events created a unique tumor immune microenvironment (TME) that controls the development and spread of tumors [3].
TME is a dynamic, heterogeneous, and complex network that solely relies on three components: tumor cells, immune cells, and surrounding accessories. Among the auxiliary elements are blood vessels, fibroblasts, adipocytes, signaling molecules, and extracellular matrix components (Fig. 1). The function, applicability and molecular markers of infiltrating immune cells have previously been thoroughly examined. Some markers inhibitors, such as programmed cell death protein-ligand 1 (PD-L1), programmed cell death protein 1 (PD-1), and cytotoxic T lymphocyte-associated antigen 4 (CTLA-4) have the ability to treat various tumors by remodeling TME [4,5]. Clinical practice has further revealed that the effectiveness of tumor immunotherapy is greatly influenced by TME. Hence, it suggested that modulating TME through new advanced delivery systems and drugs could be one of the most important and successful treatments for malignant tumors [6].
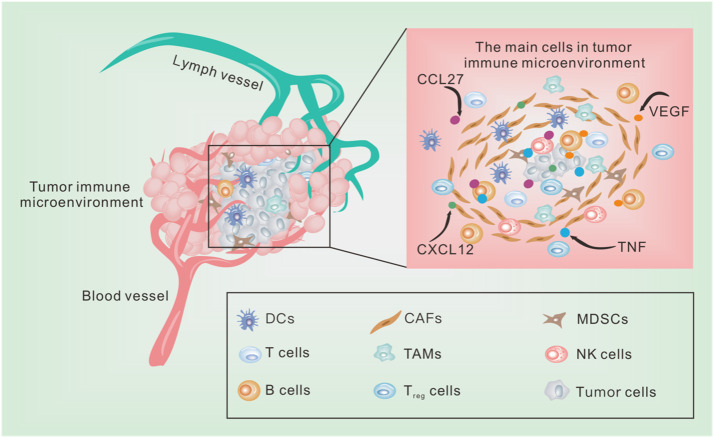
Fig. 1.
The main cells in TME. The stromal cells are closely related to fibroblasts and vascular endothelial cells, pericytes and platelets related to vascular composition, which are the main part of TME. Some cytokines such as TNF, VEGF and chemokines such as CXCL12, CCL27 also play a role in information transmission and energy transfer in TME. As the star component of TME, immune cells can be divided into two opposing categories: ones that inhibit tumor growth and the others that support tumor escape and invasion. The immune cells that inhibit tumor growth mainly refer to M1-type TAMs, tumor-associated Neutrophils (TANs)- N1 type, natural killer cells, B cells and T cells. The large number of immune cells that support tumor cell escape remains divided into many types, such as CAFs, M2-type TAMs, MDSCs, and regulatory cells (Treg) and tolerant DCs.
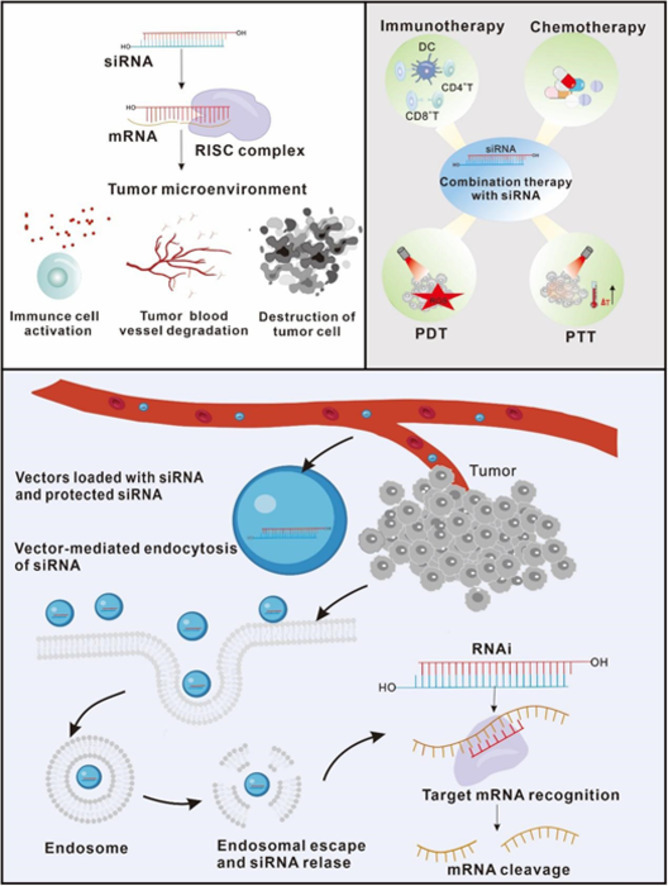
RNA interference (RNAi) is a natural biological mechanism while also being a unique innovative therapeutic approach to treating disease. This unique therapy strategy includes a number of variations, like short hairpin RNA (shRNA), siRNA, and micro RNA (miRNA), which can induce the degradation of a complementary mRNA sequence while lowering the expression of a protein (Fig. 2). The most widely publicized are siRNA medicines, which were discovered by David Baulcombe in 1998 [7]. Surprisingly, siRNAs, which are more highly targeted than conventional antibody drugs, can be targeted to complement the mRNA sequence of a single target gene, precisely blocking the expression of the target gene [8]. In addition to that, siRNA's post-transcriptional modulation of protein expression in vivo is more targeted as well as sustained than conventional chemical medicines. It can also effectively eliminate the interference of gene pathogenic mutations in traditional drug therapy [9]. Recently, some clinical trials using siRNA medicines have been conducted, with excellent outcomes in modulating TME, increasing the effectiveness of other immunotherapies, and killing tumor cells [10,11]. Therefore, the following novel nucleic acid delivery treatment through regulating TME can also effectively complement existing treatments, which raises the prospect of future tumor-specific gene therapy.
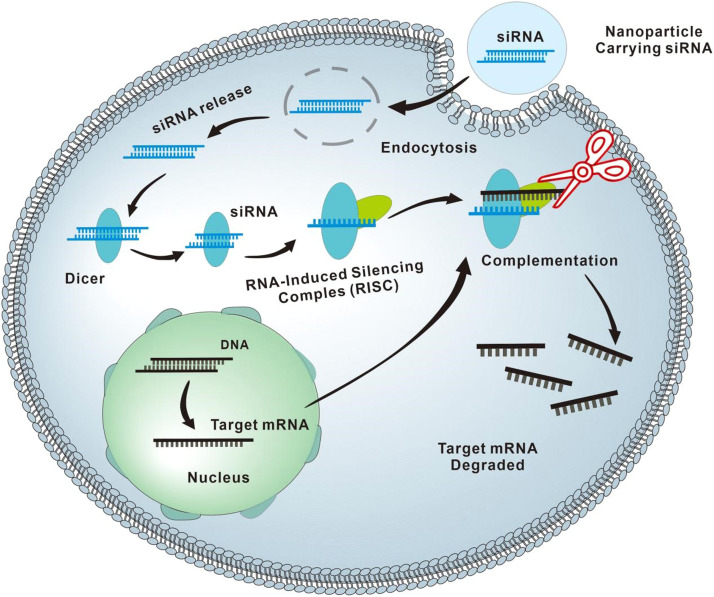
Fig. 2.
The working principle of RNAi. siRNAs are double-stranded RNA molecules (dsRNAs, with ≈20–25 nucleotides), which are generated by the cytoplasmic cleavage of long RNA, including mRNA and long noncoding RNA (lncRNA), with the RNase III enzyme Dicer. siRNAs specifically incorporate into the RNA-induced silencing complex (RISC) and then guide the RNAi machinery to degrade the target mRNAs containing the complementary sequences.
Compared with routine individual therapies, siRNA medicines showed considerable individual improvement, especially in cancer immunotherapy. Two fundamental factors determined the effectiveness of siRNA drugs for remodeling TME. Initially, the selection of target genes for siRNA in tumor therapy has distinctiveness in various circumstances [12]. From a therapeutic perspective, the first element that directly affects how well siRNA medicines work to alter TME is selecting typical gene targets in a certain tumor. Among them, the correct selection of appropriate siRNA fragments corresponding to target genes must acquire the following characteristics: it does not activate the innate immune system, efficiently and blatantly accomplishes its goal, while having no off-target or other toxic effects, and has a prolonged half-life and slow degradation in the systemic circulation and target cells [13,14]. Nonetheless, insufficient emphasis has been placed on the exact pharmacological mechanisms and significance of siRNA in modifying TME. Therefore, this review focuses on the pharmacological actions of siRNA drugs on the three different components of TME.
On the other hand, the correct selection as well as design of delivery systems is the factor determining the effectiveness of siRNA drugs in TME remodeling [15]. The following paper summarized and explained the nanoparticle (NP) design considerations and new advances in nucleic acid delivery to treat cancer. In addition to that, it was widely reported that siRNA drug delivery strategies could also enhance immune response and remodel TME. Therefore, we have explained various joint delivery strategies and discussed the applications and adverse effects in the current development of siRNA drugs.
2. The pharmacological actions of siRNA drugs for remodeling TME
According to previous literature, We discovered that the pharmacological effects of siRNA medicines on TME remodeling are primarily directed in the following direction: (1) siRNA drugs will directly inhibit the tumor activity by destroying the physiological activity of tumor cells in TME; (2) siRNA drugs enhance the body's autoimmunity by modifying the activity of immune cells in TME; (3) siRNA drugs mainly regulate the secretion of cell growth factors along with angiogenesis in TME to cause the destruction of the tumor matrix, thereby inhibiting the occurrence and development of tumors. The following process is mentioned below in detail.
2.1. Pharmacological mechanisms of siRNA for regulating tumor growth
2.1.1. The siRNA drugs regulate the physiological activities of tumor cells
In the complex network of TME, halting the physiological activities of tumor cells could be the core factor determining the therapeutic effect. Tumor cells' primary physiological traits include unrestricted proliferation, increased migration, and invasive ability, as well as the evasion of apoptosis programs [16,17]. The siRNA drugs can achieve their goal by efficiently silencing the overexpression of physiological-activity-related proteins in tumors. Due to the strong correlation between these physiological characteristics, siRNA interference often causes simultaneous changes in multiple physiological characteristics.
Zhang et al. [18] found that hypoxia-inducible factor-1α (HIF-1α) promoted the invasion of ovarian cancer cells via causing a change in the matrix metalloproteinase 13 (MMP13) protein. After silencing HIF-1α with siRNA and detecting by RT-PCR and Western blot analysis, the number of aggressive A2780 cells (human ovarian cancer cells) was drastically reduced by utilizing transwell invasion assays. Another investigation established that when HIF-1α in A2780 and SKOV3 (human ovarian adenocarcinoma cells) cells were destroyed by siRNA transfection, the phosphatidyl inositol3-kinase (PI3K)/protein kinase B (Akt)/mammalian target of rapamycin (mTOR) signaling pathway in the cells was inactivated, this results in decreased cell viability and increased cell apoptosis [19]. Survivin (encoded by surviving gene) is one of the inhibitory members of the apoptosis inhibitor protein family. It stimulates cell proliferation, inhibits apoptosis, and is intimately associated with tumor occurrence, development, diagnosis, therapy, and prognosis. Rmagnoli et al. [20] used siRNA to reduce the expression of survivin, it prevented the genesis and growth of tumors by slowing the growth rate of myeloma cells through the cell cycle and apoptosis destruction. Besides, Yang et al. [21] represented that the positive expression rate of survivin was significantly increased in patients with multiple refractory myelomas (MRM), and the patients with high expression of survivin had increased drug resistance and harsher chemotherapeutic effects. Using siRNA-survivin drugs would completely reverse vincristine resistance, which suggested that the siRNA-survivin drug was the potentially proper treatment of vincristine-resistant MRM [22]. Additionally, the signal transducer and activator of transcription 3 (STAT3) played an important influence in tumor cells and tumor-associated immune cells, further influencing tumorigenesis and progression. Zhang et al. [23] constructed a new human cell-specific unmethylated cytosine-guanine oligodeoxynucleotides CpG(A)-siRNA-STAT3 conjugate, which could effectively down-regulate oncoproteins like STAT3 and B-cell lymphoma-extra-large (BCL-XL), demonstrating that CpG(A)-siRNA treatment could prevent the growth of numerous bone marrow tumors.
2.1.2. siRNA drugs solve the dilemma of tumor drug resistance
Traditional chemotherapy drugs frequently lead to the emergence of tumor resistance in cancer patients, which has a detrimental impact on the efficacy of clinical tumor treatment. Significantly, the positive reaction of immunotherapy is typically based on the dynamic along with the effective interaction between tumor cells and the immune system in TME. Hence, solving the drug resistance of tumors is another important keynote to remodeling TME [24].
The siRNA drugs can contribute to the resolution of tumor drug resistance in treatment by modifying the expression of genes that induce drug resistance. In light of clinical practice, most patients with ovarian cancer receive paclitaxel (PTX) chemotherapy, yet, less than 50 % of them benefit from PTX when utilized alone. Therefore, using siRNA to target drug resistance pathways is a critical technique for addressing PTX's limited sensitivity to chemotherapy. Li et al. [25] found that the PTX-resistant phenotype was mainly attributed to the activation of the STAT3 signaling pathway. They utilized siRNA-ERp57 (recombinant Endoplasmic Reticulum Resident Protein 57) silencing and synergistic treatment with PTX to inhibit the STAT3 signaling pathway, which ultimately restored the drug-resistant ovaries' PTX sensitivity of cancer cells. Xu et al. [26] discovered that the JAK-STAT3 pathway enhanced the development of PTX resistance by up-regulating the expression of survival and anti-apoptotic genes.
Nevertheless, treatment of PTX-resistant human ovarian cancer cells with siRNA-JAK2 resulted in cell growth arrest while enhancing apoptosis in response to PTX. Furthermore, research has demonstrated that c-Met (cellular-mesenchymal to epithelial transition factor) protein is crucial for cisplatin resistance [27,28]. Cisplatin resistance can be reversed by inhibiting the expression of c-Met, and the expression of c-Met by siRNA can inhibit the proliferation and adhesion of ovarian cancer [27]. Zhao et al. [29] found that silencing EPEL with siRNA decreased the viability of carboplatin-resistant SNU-251 cells (human ovarian intimal cancer cells). Invasion and metastasis have gravely compromised the lives and health of ovarian cancer patients in clinical trials. Therefore, mastering its molecular and physiological mechanisms may provide new ideas and solutions for treating ovarian cancer. It is understood that BCL-2 (B-cell lymphoma-2) is an anti-apoptotic gene, and the MRP1 (Motility Related Protein 1) regulates the expression of P glycoprotein, which regulates drug efflux. Taratula et al. [30] highlighted that the use of siRNA to prevent the expression of BCL-2 and MRP1 genes could successfully overcome tumor cells' medication resistance.
2.1.3. siRNA drugs eliminate the interference of gene pathogenic mutations in traditional drug therapy
A significant challenge in the treatment of lung cancer has been the emergence of drug resistance, often caused by mutations in pathogenic genes. Taking TKI (tyrosine-kinase-inhibitor), which is a specific inhibitor of EGFR (epidermal growth factor receptor) tyrosine kinase, as an example [31]. The EGFR gene mutations, especially T790M and C797S, have been widely detected in some patients and re-activated some pathways. These pathogenic gene mutations have resulted in approximately 60 % of patients developing TKI resistance in advance, causing significant delays in the therapeutic process [32]. The siRNA therapy research showed that inhibiting the activation of related core pathways can make lung cancer cells re-sensitive to EGFR-TKI, making it a highly effective treatment strategy. It may aid in the advancement of treatment and serve as an effective supplement to traditional therapies as well. Concurrently, clinical practice often reveals that KRAS (kirsten ratsarcoma viral oncogene homolog) gene mutations might quickly promote the growth along with the spread of tumor cells when TKI treatments for individuals were going on [33]. There are already clinical trials underway targeting the following pathogenic gene mutation in lung cancer patients. In January 2019, Nitto BioPharma (Inc) began researching the clinical efficacy of a siRNA drug called NBF-006 for non-small cell lung cancer (NSCLC) patients with KRAS gene mutations [33]. Although the study is still ongoing, promising results have been observed in preclinical studies. There are numerous other harmful variants in NSCLC, and there have been many fundamental investigations based on the characteristic that siRNA can target many sites. These mutation sites have been used as targets to develop siRNA therapy, and remarkable achievements have been made. Henceforth, there may be more clinical trials of siRNA therapy shortly, promoting the clinical treatment of NSCLS.
2.2. Pharmacological mechanisms of remodeling TME by affecting immune cells
2.2.1. siRNA drugs regulate natural killer cell
The exact source of natural killer (NK) cells, innate immune core cells, is still unclear. NK cells can autonomously identify and attack cancer cells, hence they are important immune regulatory cells that have broad-spectrum anti-tumor effects [34]. Utilizing NK-92 cells (a human NK cell line) is an attractive next-generation cancer immunotherapy strategy, which has undergone clinical trial testing as a generic drug [35]. Recently research reported that the modulation of gene expression, like BCL-2 in NK-92 cells, enhanced the efficacy of NK-92 cell therapy [36]. Kaban et al. used the virus to transduce a siRNA drug, BCL-2 siRNA (siBCL-2), into NK-92 cells, causing NK-92 cells to generate substantially higher quantities of siRNA-BCL-2 into exosomes (NKExos). Because siBCL-2 was mostly located in exosomes rather than internals, the activity of NK-92 cells was not disturbed and could nevertheless have a lethal effect. The NKExos, when loaded with siBCL-2, could target endogenous apoptosis pathways within breast cancer cells, leading to enhanced therapeutic outcomes for the treatment of tumors [37].
2.2.2. siRNA drugs regulate T cells
T cells can release a variety of cytokines in order to trigger an inflammatory response, trigger apoptosis, or after processing the information supplied by innate immune antigens, directly attack target cells [38,39]. Recent clinical successes of adoptive T cell therapy (CAR-T) and immune checkpoint inhibitors demonstrate the potential of modulating T cell function in anti-tumor effects. The interaction between PD-1 (released by T cell) and its ligand PD-L1 (located on the tumor) limited poor response to CAR-T cell therapy tumor efficacy [40]. Various studies have used siRNA to change targeting PD-1′s expression in T cells, resulting in significant silencing of PD-1 in CAR-T cells. The reduction of PD-1 expression on the surface of T cells strongly increases the production of cytokines in CAR-T cells and enhances the cytotoxicity of T cells to PD-L1-expressing cancer cells [41,42]. The following analysis demonstrated that silencing PD-1 in CAR-T cells significantly enhances their anti-tumor efficacy against several cancers. Huang and Liu et al. [41,42] also suggested that silencing other specific genes at immunological checkpoints may improve CAR-T cells' ability to treat human malignancies.
At the same time, numerous studies demonstrated that controlling T cell depletion and infiltration simultaneously was a good immune regulatory tactic to elicit a potent immune response [43]. Wu et al. modified tumor cells with siRNA to eliminate the expression of TOX (thymocyte selection-associated high mobility group box protein), which altered tumors, causing them to emit a “defense” signal, which in turn helped to alleviate the exhaustion of CD8+ T cells [44]. Dendritic cell (DC)-based vaccine is another measure for generating anti-tumor immune responses by improving specific T cell activation. According to the research which was performed previously, transfected with siRNA-BAK/BAX (recombinant BCL-2 antagonist/killer) gene in DC cells could prolong DC life while enhancing CD8+ activation of T cells [45].
2.2.3. siRNA drugs regulate B cells
For many years, investigations on the diverse functions of B cells in anti-tumor immunity have been ongoing, mostly based on mice studies. Clinical investigations have also disclosed that a high density of B cells in the TME often leads to better prognosis in patients [46]. B cells have been shown to promote the initial initiation and expansion of CD4+ T cells in the past [47]. In addition to that, they might promote neovascularization and perpetuate chronic inflammation, both of which have pro-tumor effects. For example, the treatment is severe because of the rapid development cycle of acute B lymphocytic leukemia (ALL), and conventional chemotherapy approaches are quite constrained. Thereby, the researchers focused on genes that regulate tumor formation in precursor B cells in ALL to investigate novel possibilities, and found that both MDX3 (MAX dimerization protein 3) and c-Rel (a member of the nuclear factor κB transcription factor family) were overexpressed in precursor B cells. Considering the same context, Satake et al. used nanomaterials to deliver siRNA-MDX3 and siRNA-c-Rel in B cells of ALL respectively, and found that they both successfully induced apoptosis of pre-B cells (precursor B-cell). Therefore, siRNA delivery may be a potential therapeutic option for treating ALL [48].
2.2.4. siRNA drugs regulate tumor-associated macrophages (TAMs)
TAMs play an essential role in tumor immunotherapy. Recoding TAMs can assist tumor immunotherapy by eliminating tumor cells and regulating adaptive immunity. As is well known, TAM has strong plasticity as well as is reprogrammed by siRNA to become a functional TAM to clear tumor cells while regulating the adaptive immune system. Hence, siRNA regulation of TAM can be used as an adjuvant therapy for cancer immunotherapy [49], [50], [51].
Through genetic screening, Dai et al. [49] discovered that the USP7 (ubiquitin specific peptidase 7) was highly expressed in the M2 type TAMs, and the implementation of siRNA suppression may result in changes to the M2 macrophages' phenotype and function. Nevertheless, they confirmed that the knockout of the USP7 in macrophage M2 could mediate the reprogramming process of macrophages via activating the p38-MAPK (p38 mitogen-activated protein kinase) pathway. This adjustment will successfully prevent the spread of lung cancer in mice.
Concurrently, the critically activated NF-kB (nuclear factor kappa-light-chain-enhancer of activated B cells) signaling pathway, which affected the depolarization process of TAM, was influenced by IKKβ (inhibitor kappa B kinase β). As a consequence of this, focused administration of siRNA-IKK to M2-type TAMs can improve anti-tumor efficacy and significantly activate M2-type TAMs' ability to repolarize [52].
2.2.5. siRNA drugs regulate myeloid-derived suppressor cells (MDSCs)
MDSCs are a heterogeneous population composed of multiple cells, chiefly including myeloid-derived cells composed of myeloid progenitor cells, immature granulocytes, immature monocytes/macrophages and immature DCs [53]. MDSCs are myeloid cells that have been activated in a pathological state. Notably, the number of MDSCs would expand significantly under pathological conditions like cancer, inflammation and infection [54]. The expansion of MDSCs was also a potent suppressor of T cell and NK cell responses [55]. Henceforth, reprogramming MDSC to remodel TME is the subject of study.
Rab7 GTPase (Ras-related proteins 7) can regulate the activity of MDSCs and enhance the growth of tumor cells. When knockdown of Rab7 GTPase in isolated bone marrow, MDSCs would reduce the hyperactivation of mTOR and regulated metabolic reprogramming. Ding et al. discovered that MDSCs with suppression of Rab7 GTPase may significantly reduce their suppressive effect on T cell growth in mice and their capacity to migrate across endothelial membranes [56]. According to a different analysis, A20 (ubiquitin-editing protein A20) was overexpressed in MDSCs, the infiltration of MDSCs was relevantly reduced, and also significantly inhibited tumor growth after siRNA-A20 treatment. Simultaneously, the numbers of DCs and macrophages were not affected [57].
2.3. Pharmacological mechanisms of regulating cytokines
Tumor-associated fibroblasts (CAFs) are one of the main components of breast cancer tumors, it was crucial in encouraging the recurrence of cancer, metastasis and chemoresistance by releasing pro-angiogenic mediators and proteins implicated in inflammatory pathways [58]. CAFs are also the most important stromal cells, and their secretion of SDF-1 (stromal-derived factor-1) can directly promote the development of many tumor cells. For instance, endothelial cell precursors (EPCs) which stimulated CXCR4+ (CXC chemokine receptor 4) can be recruited to participate in tumor angiogenesis. Activated fibroblasts attract CXCR4+ tumors for directed migration through SDF-1/CXCR4. Calvo et al. [59] discovered that considering the breast cancer biopsies from mice and people, YAP (yes-associated protein) was more expressed in CAFs than in normal and proliferative-related fibroblasts. On the other hand, when YAP was spliced by siRNA in tumor cells, CAFs' ability to decrease the collagen matrix was dramatically reduced. Additionally, it lessened the body's production of collagen fiber, angiogenesis, and local tumor adhesion, all of which lower the chance of breast cancer spread and recurrence.
2.4. Pharmacological mechanisms of regulating blood vessels
In comparison to healthy cells, cancer cells require more nutrients. They also necessitate greater resources for cell division, metastasis and invasion, all of which depend on angiogenesis. Whereas, if the tumor wants to generate blood vessels, it will secrete some angiogenesis-inducing factors, like interleukin-11 (IL-11) and interleukin-15 (IL-15). When the secretion of factors (like vascular endothelial growth factor (VEGF)) that block the body's angiogenesis creation is insufficient, new blood vessels will form. Du et al. [60] confirmed that the specific siRNA-mediated down-regulation of IL-11 and IL-15 will greatly prevent tumors of new blood vessels. In addition, the clinical application of siRNA-based therapy has many advantages in treating breast cancer. Chen et al. [61] used NPs to deliver siRNA in vivo, and the outcomes demonstrated that siRNA mediated by them might decrease in vitro VEGF expression by 60 % to 80 % in MCF-7 (human breast carcinoma cells). When using siRNA-VEGF to treat tumors in mice, it effectively observed that tumor growth and angiogenesis were effectively inhibited. In addition, Gu et al. [62] used a dual gene targeting siRNA conjugate composed of VEGF and survivin siRNA to explore the proliferation and angiogenesis of two human osteosarcoma cell lines. The results showed that siRNA medicines greatly reduced osteosarcoma cell migration, proliferation, and angiogenesis while increasing cell death. It was projected to become a popular option for the development of human osteosarcoma treatment strategies. All of above mentioned are represented in Fig. 3.
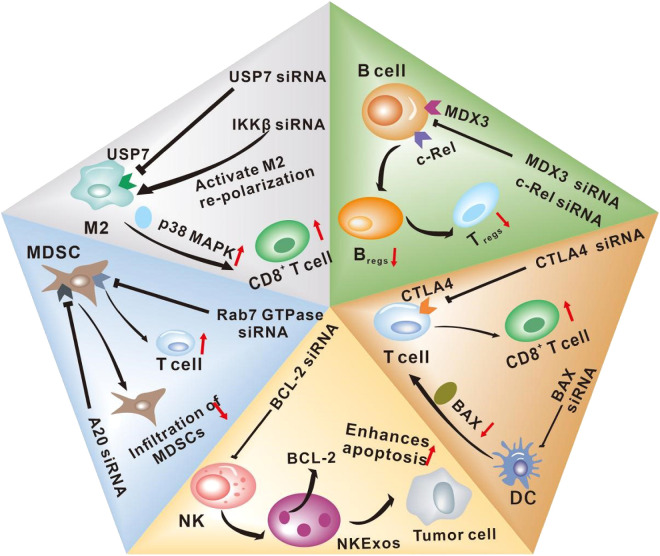
Fig. 3.
siRNA therapy modulates immune cell function in the TME. The siRNA system recognizes specific ligands on immune cells, such as the USP7 ligand on M2, MDX3 ligand on B cells, and CTLA-4 ligand on T cells, so as to achieve precise targeting of specific immune cells. Subsequently, siRNA enters the cell and interferes with the expression of related proteins, regulating TME, thereby increasing the number of killer immune cells in the TME and further inhibiting the occurrence and development of tumors.
3. Delivery vector-assisted siRNA drugs for TME modulation
3.1. Barriers to siRNA delivery
Regardless of the enormous potential of siRNA in modulating the TME, the secure and efficient delivery of siRNA to target cells remains a great challenge. The administration routes for siRNA include intravenous drip, subcutaneous injection, or local administration. Compared to systemic delivery, local administration of siRNA encounters fewer barriers. Nevertheless, the treatment of most cancers with siRNA typically requires systemic administration. Currently, except for a few, such as Patisiran, which is injected intravenously, most of the siRNA drugs available in the clinical market are administered subcutaneously [63]. From the injection/ingestion site to the cytoplasm in target cells, siRNA must surmount a variety of physiological obstacles to perform their role in silencing or knocking down disease-causing genes. Several obstacles at the extracellular and intracellular levels are depicted in Fig. 4.
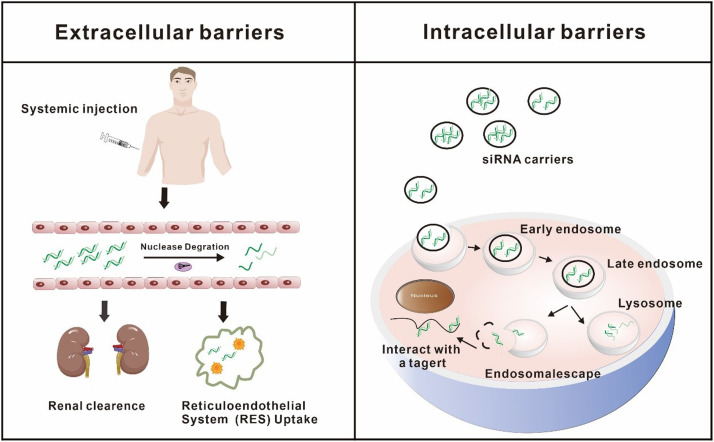
Fig. 4.
Barriers of siRNA delivery after systemic administration. Due to extracellular and intracellular barriers, the arrival of siRNA at the target site is complicated. siRNA may be degraded by nucleases in the blood, cleared by kidneys, and taken up by RES, which makes it difficult for siRNA to reach the target site. Even if siRNA reaches the target site, the gene silencing effect may not be exerted due to limited cellular uptake and poor lysosome escape.
Numerous nucleases present in the blood and tissues break the intra-nucleotide bonds of naked siRNA, rendering it unstable. In addition to that, due to the size of naked siRNA is about only 7 nm, it is easily removed by glomerular filtration (the filtered pore size is about 8 nm) [64]. Considering the other side, the non-specific uptake of siRNA by the reticuloendothelial system (RES) is another major obstacle to siRNA distribution [64]. The RES is composed of phagocytic cells with the capacity to capture and rapidly eliminate siRNA. These factors result in the short half-life of siRNA and pose challenges in achieving its delivery to the target area. Even if it is transported to the target tissues, siRNA needs to be taken up by the target cells. Somehow, all of the physical properties of siRNA, including high hydrophilicity, large molecule (13 kDa) as well as negative charge, collectively impede its ability to traverse the negatively charged cell membrane structure [65]. According to several lines of evidence, it is apparent that only a few cell types, such as neurons and retinal ganglion cells (RGCs), possess the capacity to internalize bare siRNA [66]. This suggests that siRNA necessitates the assistance of vectors for cellular entrance. Furthermore, the challenge of achieving endo/lysosomal escape following cellular internalization represents another notable constraint on the therapeutic efficacy of siRNA [67]. A membrane organelle in cells called an endosome transfers substances taken in through endocytosis to lysosomes. After internalization by endocytosis, siRNA initially confronted the early endosomes. Then early endosomes fuse with late endosomes to combine with lysosomes which are acidified while also containing nucleases capable of degrading siRNA [68,69].
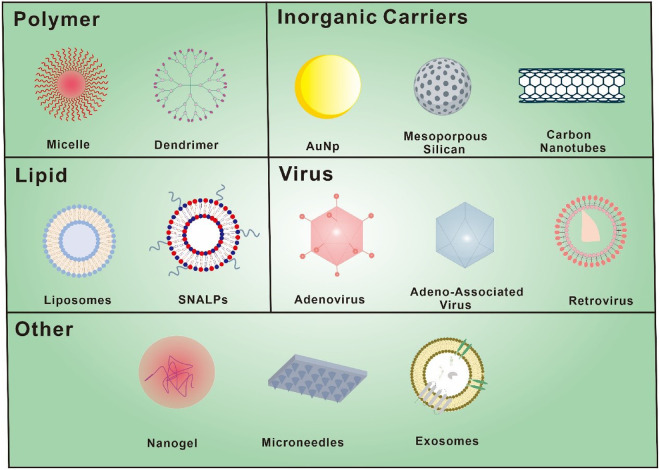
Lastly, siRNA as a cancer treatment drug still has certain safety risks. First of all, siRNA drugs always are synthetic in vitro. They exhibit immunogenicity due to their foreign nature and can activate toll-like receptors, including TLR3, TLR7 and TLR8, triggering the innate immune system and initiating an immune response [70,71]. Secondly, siRNA drugs might face the dilemma of “off-target” effects [72,73]. Off-target transcription can cause undesirable transcription outcomes, including toxicities, by silencing mistake genes that share sequence homology with the target genes. To resolve the aforementioned issues that may affect the therapeutic efficacy of siRNA, there are two standard techniques. One is to improve the inherent properties of siRNA through chemical modification of the ribonucleotide skeleton or base of siRNA. Chemical modification can increase siRNA's nuclease resistance, enhance serum stability, lessen immunogenicity, and diminish off-target effects [74]. On the other hand, encapsulation of siRNA into vectors is considered to be another successful systemic delivery technique [75]. Excellent drug delivery vectors must acquire biocompatibility, biodegradability and non-immunogenicity. In addition, they should serve the purpose of safeguarding siRNA from nuclease degradation and facilitating its cellular internalization. In order to execute RNAi impact, they should play a pivotal role in assisting siRNA in escaping from lysosomes and gaining access to the cytoplasm [76]. The several sophisticated siRNA delivery vectors are demonstrated in Fig. 5 and delivery systems for siRNA-mediated cancer therapy are summarized in the following Table 1.
Fig. 5.
Various vectors are used for the delivery of siRNA. siRNA delivery vectors can be divided into two main categories, viral and non-viral vectors. Common non-viral vectors include liposomes, polymers and inorganic nanomaterials.
Table 1.
Examples of different delivery systems for cancer treatment.
| Delivery system | Component excipients for preparing nanocarriers | Target genes | Treated Cancer (Target cells) | Main outcomes of the research | Ref. |
|---|---|---|---|---|---|
| Liposomes | Chol: DOPE: MAL-PEG-DOPE = 1:1:0.1 | BRAF | Melanoma (A375) | Significantly inhibition of cancer growth was observed due to silencing BRAF | [199] |
| AtuFECT01: Chol: mPEG 2000-DSPE=70:29:1 | CD31 | Lung cancer (LLC) | Reduced formation of lung metastases and increased life span | [200] | |
| DOPC | JNK-1 | Ovary cancer (HeyA8 or SKOV3ip1) | Growth inhibition and apoptosis of cance rwas observed due to down-regulation of the JNK-1 | [201] | |
| DOTAP: DOPE=1:1 | BCL-XL | Breast cancer (MCF-7) | Formulation efficiently deliveredsiRNA and inhibited the expression of BCL-XL in cells | [202] | |
| c-RGD conjugated DSPE-PEG2000; Chol: DOTAP: DSPC | STAT3 | Melanoma (B16F10) | Conjugation of c-RGD on the liposomes increased the efficiency of siRNA delivery to cancer | [203] | |
| polymer | PAMAM | BCL-2 | Cervical cancer (HeLa) | The use of PAMAM loaded siRNA-BCL-2 and Cur showed more effective cellular uptake, and higher inhibition of tumor cell proliferation. | [112] |
| PEI-PEG | CD44 | GBM multiforme (U87MG) | The delivery of siRNA-CD44 using the polymeric NPs significantly downregulated CD44 gene and attenuated the cell cycle | [204] | |
| Carboxymethyl chitosan modified with histidine, cholesterol, and anti-EGFR antibody | VEGFA | Breast cancer (MDA-MB-231) | Formulation could effectively silence the VEGFA to cause cell apoptosis and inhibit proliferation. | [205] | |
| Arg-Gly-Asp (RGD) peptide-labeled chitosan NP | POSTN, FAK, and PLXDC1 | Ovarian cancer (SKOV3ip1, HeyA8, and A2780) | siRNA successfully delivered by chitosan nanoparticle against ovarian cancer | [206] | |
| FA-targeted PEGylated cyclodextrin | RelA | CRC (CT26) | Formulation enhanced the apoptotic effect of DTX with thedownregulation of RelA expression | [119] | |
| Inorganic NPs | AuNP | EGFR | Lung cancer (A549) | Systemic delivery of AuNP significantly inhibits tumor growth in mics | [207] |
| AuNPs-PEI-FA | RelA | Prostate cancer (LNCaP) | The functionalized AuNPs efficiently delivered siRNA at targeted site | [208] | |
| Magnetic nanocarriers | Yap | Hepatocellular carcinoma (HCC) | Formulation could decrease the YAP1 expression down to approximately 35 %, reducing the cell proliferation and migration | [209] | |
| Graphene | Survivin | Breast cancer (MCF-7) | Graphene could deliver siRNA to MCF-7 cells and prevented tumor development | [210] | |
| Carbon nanotubes and DOTAP | CXCR4 | Ovarian cancer (SW626 MG and SKOV-3) | The co-delivery of Gemcitabine and siRNA significantly repressed the tumor growth of ovarian | [211] | |
| Mesoporous silica NPs(surface-modified with APTES and chitosan) | STAT3 | Breast cancer (MCF7) | The co-delivery of methotrexate and siRNA-STAT3 significantly decreased the viability of breast cancer cells | [212] | |
| Biological | Exosomes | ITGB6 | Prostate cancer (LNCaP) | The delivery of siRNA-ITGB6 significantly downregulated expression of the β6 subunit genes, had remarkable suppressive effects on migration and adhesion | [213] |
| KRAS | Pancreatic cancer (PANC-1,BxPC-3 and KPC689) | Treatment with iExosomes suppressed cancer in multiple mouse models of pancreatic cancer and significantly increased overall survival | [214] | ||
| Cancer cell membrane, PLGA | PD-L1 | Breast/Cervical cancer (MDA-MB-231 and Hela) | Effectively transported siRNA to cancer cells and enhanced the cellular uptake | [132] | |
| Hydrogels | Platelet membrane–coated metal-organic framework | Survivin | Breast cancer (SK-BR-3) | High silencing efficiency could be achieved in vitro against multiple target genes | [215] |
| PDLLA-PEG-PDLLA, cholesterol‐modified antimicrobial peptide DP7 | Pin1 | Hepatocellular carcinoma (Hep3B) | DP7‐C NPs and hydrogel‐assisted siRNA delivery prolonged the silencing effects of Pin1 siRNA and inhibited tumor progression | [216] | |
| Collagen hydrogel, PEI | Id1 | gastric cancer (SGC-7901) | Hydrogel significantly prolonged siRNA action time and enhanced its efficacy on target gene | [217] |
3.2. Strategies for overcoming the delivery barriers of siRNA
3.2.1. Naked siRNA delivery
Chemical modification can enhance the resistance of siRNA to nucleases, improve its serum stability, and reduce both immunogenicity and off-target effects [74]. In this way, employing siRNA's physicochemical features can also achieve targeted disease treatment in the lack of protective vectors. For example, because of the small size of siRNA, most siRNA administered intravenously can be filtered through the kidney, providing a possibility to treat kidney diseases. Additionally, the low content of nucleases in particular organs, such as the eye and lung, may allow naked siRNA to exert its gene-silencing effect through local administration [77]. Shen et al. [78] substantiated that intravitreous or periocular injection of naked siRNA-VEGFR1 could specifically reduce the levels of VEGFR1 (vascular endothelial growth factor receptor 1) in mouse models of retinal and choroidal neovascularization. On top of that, it has already been demonstrated the feasibility of directly delivering naked siRNA to lung tissues for the treatment of lung disorders [77], [78], [79], [80].
Although the delivery of naked siRNA has been quite successful, it still confronts several difficulties, such as limited effectiveness in treating multi-organ diseases and unintended toxicological consequences. Henceforth, carrier-based delivery of siRNA has been researched more and more extensively.
3.2.2. Viral vector
Viruses are naturally evolved delivery vectors. Through bioengineering, viruses that naturally convey their genomes into cells may be altered to function as gene delivery vectors, efficiently delivering siRNA without being pathogenic. The primary advantage of viral vectors is that they can put forward genes to various types of mammalian cells, including hard-to-transfect, primary, and even non-dividing cell types [81]. Therapeutic gene expression is maintained throughout the cell cycle once they have been integrated into the genome [82]. Viral vectors derived from adenovirus, adeno-associated virus, lentivirus, retrovirus, poxvirus, herpes simplex virus, and human foamy virus are available for gene delivery [83]. Adenovirus (Ad) is a non-enveloped double-stranded DNA virus known for its efficient transfection capabilities. By manipulating the Ad capsid, targeting properties also be transmitted [84]. Nevertheless, the sensitivity of Ad to chemical or physical factors renders it unable to survive in vitro for a long time. Additionally, its high immunogenicity imposes limitations on repeated administration. Adeno-associated virus (AAV) is a non-pathogenic enveloped single-stranded DNA virus with good biological characteristics [85]. Despite this, the amount of the insertable foreign gene section is limited to less than 5 kb due to the AVV capsid's size of around 22 nanometers and membership in the parvovirus family [86]. Although virus-related drugs have been used in the clinic and offer a crucial advantage with their superior transduction efficiency than non-viral vectors, there still exist safety concerns in the utilization of viral vectors, such as immunogenicity and carcinogenic risk.
A novel virus-derived vector called virosomes has been developed by merging viral and non-viral components to address these concerns [87]. The virosomes composed of a phospholipid bilayer and viral envelope proteins preserve the high transfection efficiency of viral vectors and improve their safety for the fact that they contain no genetic material. Currently, the influenza virus, Sendai virus, human immunodeficiency virus, Newcastle disease virus, and vesicular stomatitis virus G protein have been used to construct virosomes [88], [89], [90]. These virosomes have shown versatile potential in cancer treatment as drug delivery vectors, adjuvants, anti-cancer immune activators, etc. Wang et al. [89] first proposed synthesizing influenza virus hemagglutinin 2 (HA2) virosomes by cell-free protein synthesis. Particularly, they engineered virosomes characterized by a membrane made of lecithin and cholesterol along with a stiff core consisting of chitosan. Chitosan was used to adsorb siRNA-VEGF, thereby enhancing the encapsulation efficiency of siRNA. Liposomes offered a hydrophobic lipid bilayer framework for HA2 protein multimer assembly. The silencing efficiency of HA2 virosomes against VEGF in A549 cells was 19.02 %, demonstrating the virosomes' capacity to trasnsport siRNA.
3.2.3. Nano vehicle delivery
Nanoparticles (NPs) are particles with diameters ranging from 1 to 1000 nm, frequently utilized in drug delivery to improve the bioavailability and pharmacokinetics of drugs for cancer therapy. Without any doubt, the physicochemical properties of NPs, including size, shape, charge and surface chemistry, have a notable impact on their in vivo distribution and effectiveness in siRNA delivery [91]. The size of NPs is a key factor in determining their absorption, distribution, organ accumulation and elimination in vivo [92]. In general, NPs with particle diameters ranging from 10 to 200 nm can effectively deliver siRNA to the target area [93]. NPs with particle sizes less than 10 nm are easily cleared by the kidney, and those larger than 200 nm are easily taken up by phagocytes [94]. The geometry also has an impact on NP distribution and cellular uptake. According to various analyses, spherical particles have the highest absorption rate [95]. However, the discoidal and elongated NPs attained higher tumor accretion than spherical NPs [96]. Positively charged NPs have a higher cellular absorption capacity than negatively charged NPs due to electrostatic interactions between them and the cell membrane [97]. However, the positively charged NPs also tended to exhibit higher cytotoxicity. Therefore, the deliberate design of NPs plays a pivotal role in facilitating the delivery of siRNA to the tumor site while realizing therapeutic efficacy.
3.2.3.1. Lipid-based delivery
Lipid-based systems for delivering anticancer siRNAs embody cationic liposomes, neutral liposomes, stable nucleic acid-lipid particles, and solid lipid NPs. Liposomes are spherical, monolayer or multilayered vesicles made of phospholipids that are either natural or synthetic [98]. There are various advantages of using liposomes as a siRNA delivery. (1) Liposomes are straightforward to prepare and can be produced in substantial quantities, which is beneficial to clinical applications. Adriamycin, the first liposomal drug approved by the FDA in 1995, has been employed in cancer treatment with notable therapeutic efficacy; (2) They can protect siRNA from endonuclease degradation; (3) Liposomes can ensure efficient delivery of siRNA to tumor tissues through the permeation retention effect (passive targeting) or/and the use of ligands (active targeting). The number of layers, size, and surface charge of the liposomes dictate the delivery efficiencies of siRNA [66]. Positively charged cationic liposomes can form complexes with oppositely charged siRNA through electrostatic interaction and subsequently bind to negatively charged cell membranes, so as to improve the transfection efficiency. Consequently, cationic lipids have emerged as the most widely utilized liposomal carriers in gene therapy among all lipid-based systems.
Cationic liposomes: Cationic liposomes are usually composed of cationic lipids and helper lipids. Various cationic lipids, including N-[1-(2,3-dioleoyloxy) propyl]-N, N, N- trimethylammonium chloride (DOTMA), [1,2-dioleoyloxy)-3- (Trimethylammonium) propane] (DOTAP) and 3β [N-(N', N'- dimethylaminoethane)-carbamoyl] cholesterol (DC-Chol), are conducive to facilitating transmembrane transport for siRNA delivery. Whereas, helper lipids, like cholesterol (Chol) and 1,2-Dioleoyl-sn-glycero-3-phosphoethanolamine (DOPE), are often incorporated into the liposome structure to improve the stability of cationic liposomes or endosomal release. Although cationic liposomes can be prepared using a wide range of materials as well as the complexes which are formed by liposomes and siRNA are easily endocytosed by cells, their in vivo application is constrained by cytotoxicity like cell membrane rupture and the generation of reactive oxygen species (ROS) [99]. On top of that, positively charged cationic liposomes come into contact with proteins in the blood and are promptly removed. Surface modification of liposomes with polyethylene glycol (PEG) provides an exterior polymeric layer to hinder the adsorption of serum proteins and can reduce the excretion by kidneys and scavenging in the liver, ultimately enhancing circulation time and biocompatibility [100]. Nevertheless, using PEG is not always advantageous. The presence of PEG not only reduces the uptake of complexes by tumor cells but also has the ability to interfere with endosomal escape [101]. The efficacy of PEGy-lated NPs can be increased by linking liposomes and PEG using pH-sensitive, redox-sensitive, or enzyme-sensitive bonds. Zang et al. [102] constructed a pH-sensitive cholesterol-Schiff base-polyethylene glycol (Chol-SIB-PEG)-modified cationic liposome that conjugated with the recombinant humanized anti-EphA10 antibody (Eph) incorporating siRNA against MDR1 gene (EPSLR). Eph binds precisely to anti-EphA10 expressed in breast cancer cells, improving liposomes' capacity to target cancer cells preferentially. The pH-sensitive PEG-lipid can be degraded under acidic conditions in favor of endosomal escape. The results explained that PEGylation and anti-EphA10 antibody modification increased tumor targeting in vivo and reduced MDR1 protein expression. In addition, cationic liposomes can be used as carriers for the co-delivery of siRNA and chemotherapy drugs to synergistically enhance the anticancer effect by way of the combination of gene therapy and chemotherapy [103].
Neutral liposomes: Anionic or neutral liposomes exhibit superior biocompatibility and pharmacokinetic characteristics than cationic lipids. However, the electrostatic repulsion between anionic liposomes and siRNA causes them to struggle to form stable complexes, resulting in low delivery efficiency. This limitation is also a reason for the relatively limited studies on using anionic liposomes as siRNA delivery carriers. Since charged liposomes have numerous drawbacks, neutral liposomes have emerged as desirable siRNA complexation biomaterials. Sahu et al. [104] developed a neutral nanoliposome based on 1,2-dioleoyl-Sn-glycerol-3-phosphatidylcholine (DOPC) containing siRNA targeting the mTOR gene (NL-siRNA-mTOR) to treat breast cancer. The pharmacokinetic study of NL-siRNA-mTOR outcomes represented that neutral liposomes could significantly prolong blood circulation by protecting siRNA from degradation. The biotoxicity assessment of NL-siRNA-mTOR demonstrated that it was free of toxicity. Significantly, NL-siRNA-mTOR could down-regulate the expression of mTOR, leading to a profound reduction in tumor growth, proliferation and invasion. Likewise, Halder et al. [105] successfully co-delivered siRNA-FAK (focal adhesion kinase) and the chemotherapy agents docetaxel (DTX) or cisplatin to ovarian tumors employing DOPC depending on neutral liposome. Various studies highlight that the combined treatment of chemotherapy and siRNA-FAK had a significant impact on slowing the spread of ovarian cancer through both direct and indirect anti-vascular mechanisms.
Stable nucleic acid-lipid particles (SNALPs): SNALPs, another lipid-based siRNA delivery vector, have been used in medical experiments. SNALPs contain a lipid bilayer with a mixture of cationic and fused lipids, which has a high encapsulation efficiency for siRNA [106]. The bilayer is additionally coated with PEG to improve retention in systemic circulation and lessen NP aggregation. At the same time, targeting ligands can be conjugated to the lipids of liposomes to enhance their specificity towards the target.
3.2.3.2. Polymer-mediated delivery
Polymer-mediated siRNA delivery systems have been extensively investigated because of their promising qualities such as simplicity of manufacture, cost-effectiveness, biocompatibility, biodegradability and high surface functionality. Like cationic liposomes, polymers with high positive charge density can form stable complexes. Cationic polymers and the negatively charged phosphate groups of siRNA self-assemble into microparticles through electrostatic adsorption, boosting intracellular gene transport via the proton sponge and shielding nucleic acids from enzyme destruction [107]. Depending on the material utilized, polymers used for gene delivery can be categorized into synthetic polymers, dendrimers, and natural polymers [108].
Synthetic polymers: Polyethyleneimine (PEI) has been widely employed as a cationic polymer carrier to deliver anticancer siRNA. Under healthy settings, many of the secondary and tertiary amine groups in the chemical structure of PEI carry positive charges, which can interact electrostatically with negatively charged siRNA to condense the nucleic acid into a complex. The amine groups also enable PEI to have buffering capacity in a wide pH range, which can trigger connotation escape via proton sponge action and promote the intracellular release of siRNA [109]. Klein et al. [110] used PEI as a carrier for siRNA-HER-2 (human epidermal growth factor receptor-2) delivery inducing effective gene knockdown. According to the information gathered the siRNA/PEI complex could down-regulate HER-2 mRNA by about 50 % in SKOV-3 ovarian cancer cells. The siRNA/PEI complex demonstrated greater significant transfection efficiency and greater gene silencing effectiveness in vitro and in vivo when compared to using siRNA alone. This enhanced performance can be traced back to PEI's protective role in protecting siRNA from nuclease degradation and promoting its intracellular release. Nevertheless, PEI's low targeting and molecular weight-dependent cytotoxicity limit its application. To overcome these barriers, targeted ligands and chemical alterations are employed. Hou et al. [111] synthesized folic acid-modified leucine polyethyleneimine (NPF) and formed NPs through self-assembly, which could effectively load siRNA-PLK1 (polo-like kinase 1) and deliver to SGC-7901 gastric cancer cells overexpressed folate (FA) receptor. As per research findings, the NPF/siPLK1 complex decreased the SGC-7901 cell's capacity for migration and invasion while increasing the anti-tumor proliferative effect.
Dendrimers: In addition to the cationic polymers, cationic dendrimers are being considered as viable candidates for siRNA delivery. Cationic dendrimers are macromolecular 3D spherical entities with well-defined molecular weight, molecular size, stable structure and high branching. Compared to other various linear polymers, dendrimers feature a high density of surface groups, rendering them amenable to facile modifications [83]. Poly(amidoamine) (PAMAM), poly (propylene imine) (PPI) and poly-l-lysine (PLL) are the most frequently utilized cationic dendrimers for gene delivery. Within the endosomal environment, protonation of the amine group on the dendrimer molecule occurs, generating a proton sponge effect. This effect induces an influx of chloride ions, which raises osmotic pressure. Finally, the endosomal membrane ruptures and siRNA release occurs [112]. PAMAM is particularly suitable as a vehicle for the co-delivery of drugs and genes due to its controllable size and excellent uniformity [113]. Ghaffari et al. [114] co-delivered a siRNA molecule targeting the BCL-2 anti-apoptotic gene and curcumin (Cur) into human cervical cancer (HeLa) cells using the fourth-generation PAMAM dendrimer (G4). Cur was trapped within the PAMAM dendrimer cavity, and then it formed a complex with siRNA-BCL-2 via electrostatic interactions. In comparison to the individual delivery of Cur or siRNA-BCL-2, the co-delivery approach of both substances exhibited enhanced cell death. Similarly, Gaballu et al. [115] fabricated methotrexate (MTX)-modified polyamidoamine dendritic molecule 4 (G4) (G4/MTX) to deliver siRNA-HMGA2. MTX endowed the nanocapsules to target human breast cancer cell lines (MCF-7, MDA-MB-231) expressing folate receptor (FR). Consequently, G4/MTX-siRNA demonstrated strong impacts on cellular internalization and gene silencing.
Natural polymers: Cyclodextrin (CD) and chitosan are promising natural polymers widely used in siRNA delivery for targeted cancer therapy [116]. CDs are cyclic oligosaccharides with a hydrophobic cavity and a hydrophilic outer surface [117,118]. The cationic polyamine skeleton of CDs enables electrostatic interactions with siRNA to form complexes. Concomitantly, the internal cavity structure can be loaded with hydrophobic drugs. It is suggested that CDs hold the potential for synergistic drug delivery. Zou et al. [119] constructed a CDNP for DTX and siRNA-NF-kB delivery while modified with PEGylated folate to find a solution to the problem of target specificity. The design demonstrated decreased tumor growth and toxicity, achieving a synergistic treatment for colorectal cancer (CRC). Chitosan is a class of natural cationic polymers acquiring a positive charge, which is beneficial for the loading of genetic material. However, chitosan's free amine protonation can only successfully complex siRNA under acidic pH conditions. Owing to its poor solubility under physiological conditions, modified derivatives of chitosan are frequently utilized as gene delivery carriers and for the treatment of CRC [120], osteosarcoma [121], glioblastoma (GBM) [122], gastric cancer [123], breast cancer [124], etc. Zhong et al. [125] synthesized a novel gene delivery carrier (MixNCH) by hybridizing chitosan with 2-chloroethylamine hydrochloride and N, N-dimethyl-2-chloroethylamine hydrochloride. The vector delivered siRNA of midkine (MK) overexpressed in malignant tumors. All of the experimental observations represented that MixNCH/siRNA-MK NPs had favorable gene delivery physicochemical properties and had the potential to drastically reduce MK's mRNA and protein levels. Furthermore, they effectively inhibited the proliferation of HepG2 cells (human hepatocarcinoma cells).
3.2.3.3. Hydrogels
Three-dimensional networks made of natural or artificial polymers are called hydrogels [126]. Both the physical and chemical properties of hydrogels, particularly their electrified properties, vary depending on the types of polymers used, which have been closely linked to the design of hydrogels for siRNA delivery. For instance, the hydrogels prepared from dextran are neutral, while those derived from cationic polymers such as PEI and chitosan carry a positive charge. Neutral hydrogels are frequently employed to deliver siRNA-NP complexes or siRNA conjugates, facilitating cellular uptake as well as gene silencing. Conversely, positively charged hydrogels can be harnessed as transfection agents to promote the release of siRNA and the continuous induction of gene silencing. Liechty et al. [127] used 2-diethyl aminoethyl methacrylate and tert-butyl methacrylate copolymers to fabricate two nanogels, one of which was engineered with a disulfide crosslinker. The disulfide crosslinker made the nanogels degradable in a reducing environment. The results demonstrated that functional siRNA could be delivered into Caco-2 cells (human CRC cells) by biodegradable and non-degradable nanogels, achieving 47% and 83% gene silencing, respectively. These findings emphasize the potential of nanogels as efficient siRNA delivery vehicles.
3.2.3.4. EV-based delivery NPs
Exosomes: Exosomes are extracellular vesicles with a size of 30–150 nm that are actively secreted by the majority of cells in the human body [128]. Similar in structure to liposomes, exosomes also have a lipid bilayer structure which protects their payload from destruction during circulation inside the bloodstream. Additionally, exosomes encapsulate a diverse array of cellular components, encompassing lipids, proteins and nucleic acid molecules that mediate information transfer between cells. Valad et al. [129] discovered that mRNA and miRNA contained in exosomes secreted by mast cells could be transported to other cells and subsequently translated. It proved that exosomes could mediate the horizontal transfer of genetic material. Notably, endogenous exosomes are nontoxic and nonimmunogenic compared to synthetic NPs, making them good candidates for siRNA delivery. Erviti et al. [130] initially proposed using exosomes for siRNA delivery and confirmed the therapeutic potential of exosome-mediated siRNA delivery. In another study, Rehman et al. [131] isolated exosomes from bone marrow mesenchymal stem cells (BMSCExo) as dual therapy containing temozolomide (TMZ) as well as siRNA for treating TMZ-resistant GBM. Concurrently, BMSCExo decorated with a specific short peptide targeting heme oxygenase-1 (HSSP) exhibited the ability to target GBM cells due to the overexpression of heme oxygenase-1 in TMZ-resistant GBM cells. HSSP-decorated BMSCExo could deliver the siRNA against STAT3 to the GBM, thereby reinstating TMZ sensitivity and successfully restraining GBM growth.
Cell membrane: Exploiting the advantages inherent in natural materials, cell-derived biomimetic drug delivery vehicles have garnered substantial attention in the research community. They have demonstrated utility in mitigating immunogenic responses and improving biocompatibility. Cell membrane vesicles derived from tumor cells can target tumor tissues for precise treatment due to the presence of functional membrane proteins on their surfaces. Chen et al. [132] prepared poly(lactic-co-glycolic acid) (PLGA) NPs loaded with doxorubicin (DOX) and siRNA-PD-L1, which were then enveloped with the membrane of HeLa cells (CCMNPs). The results demonstrated that, when compared to MDA-MB-231 cells, CCMNPs induced higher levels of cytotoxicity in HeLa cells and exhibited increased cellular uptake. CCMNPs exhibited their source-targeting capability through specific self-recognition within homologous cancer cells.
The majority of NPs altered by vesicles produced from cell membranes have a conventional core-shell configuration. Nevertheless, if the liposome is modified with vesicles, the core-shell structure may not be observed. On the contrary, the cell membrane tends to fuse with the liposome to form a mixed membrane lipid, which might be attributed to the structural similarities between liposomes and cell membranes [133].
3.2.3.5. Microneedles
Microneedles(MNs)are microarrays with a height of 150–1,500 µm and a width of 50–250 µm [134]. MNs may offer a novel transdermal medication delivery pathway, allowing for non-invasive and painless drug release into the dermis by overcoming the skin's primary barrier, the cuticle. According to the mechanism of drug release, MNs can be divided into five types: solid MNs, hollow MNs, coated MNs, dissolving MNs, and hydrogel-forming MNs [135]. The materials and methods employed in MNs' fabrication to determine their physical properties and geometry. Solid, hollow and coated MNs are mainly made of inorganic materials such as silicon, ceramics, glass and stainless steel. However, there have been numerous drawbacks to MNs made of inorganic materials, such as limited drug loading capacity, intricate manufacturing processes, and especially the potential safety hazard of the needle body remaining in the skin after breakage [135]. Organic components used to make MNs, such as polymers and polysaccharides, are often soluble. After insertion into the skin, soluble MNs could be dissolved in contact with the skin interstitial fluid, avoiding the residue of sharp objects.
There is a burgeoning body of research on the delivery of macromolecules (nucleic acids, proteins and vaccines) via MNs for the treatment of cancer since the delivery method utilizing MNs) imposes no limitations on the molecular weight and hydrophilicity of medications. MNs are commonly employed to administer siRNA for the treatment of melanoma. Ruan et al. [136] fabricated a stainless steel-based microneedle as an octaarginine/siRNA-BRAF (V-raf murine sarcoma viral oncogene homolog B) nano-complexes carrier for cutaneous melanoma therapy. Octaarginine is an arginine-rich cell-penetrating peptide and has been reported as a vector to improve cellular absorption of siRNA [137]. In vivo, experiments demonstrated that MNs could deliver octaarginine/siRNA-BRAF nanocomposites through the skin to the melanoma site. Then octaarginine delivered siRNA to melanoma cells to successfully silence the BRAF gene and arrest tumor development.
3.2.3.6. Inorganic NPs
As a result of the easily modifiable surface, higher stability, and controllable size, shape, structure, and composition of inorganic NPs, they become potential siRNA delivery systems for cancer therapy. Gold NPs, magnetic NPs, carbon NPs, silicon NPs, quantum dots, and other inorganic NPs are often employed in this context [138].
Gold NPs: The unique physical and chemical properties of Gold NPs (AuNPs) endow them with tunable size and elastic surface characteristics. AuNPs have both covalent and non-covalent bonding properties [139]. On the one hand, their surface can be rendered positively charged by selecting a suitable stabilizer and then electrostatically bound to siRNA. On the other hand, AuNPs can also bind siRNA through chemical interactions involving functional groups like gold-thiol, amine, and carboxylate [140]. Leveraging the photothermal effect of AuNPs, siRNA-AuNP complexes can release siRNA upon exposure to near-infrared (NIR) laser light [141]. These characteristics endow AuNPs with significant potential for applications in gene delivery. AuNPs have anti-angiogenic products, which provide great potential for cancer therapy. Furthermore, AuNPs can serve as fluorescence quenchers based on the principle of fluorescence resonance energy transfer and can also be used as a contrast agent for computed tomography (CT) due to their high X-ray absorption ability. Sun et al. [142] coupled Cy5-modified survivin siRNA to AuNPs and then coated with lipid NPs (Au-DR-siRNA@LNPs). Au-DR-siRNA@LNPs not only successfully inhibited tumor cell survival protein expression, but also enabled fluorescence imaging and CT imaging to monitor the behavior of siRNA in vivo.
Carbon NPs: Different types of reagents, such as Carbon nanofibers, carbon nanotubes, nanodiamonds, graphene, fullerenes and carbon dots, have been created based on the fact that carbon exists in a range of allotropes [143]. Graphene, a two-dimensional nanomaterial with excellent optical and chemical properties, has been considered an ideal gene delivery system. Yin et al. [144] prepared functionalized graphene oxide (GO) for siRNA delivery by conjugating FA, NH2-mPEG-NH2 and poly-allylamine hydrochloride (PAH) onto GO nanosheets. The conjugation of FA and GO was designed to target cancer cells expressing the folate receptors, allowing for specific attachment to these cells. This approach led to apoptosis, proliferation inhibition, and cell cycle arrest in pancreatic cancer cells MIA PaCa-2 by silencing the expression of HDAC1 and K-Ras. Moreover, photothermal therapy exhibited by the following GO nanosheets under NIR light synergistically suppresses tumor growth by more than 80% in vivo. Furthermore, it also had no adverse effects on the mice's body weight, learning ability, memory management or voluntary movement.
Magnetic NPs: Because of their superparamagnetic properties and biocompatibility, magnetic NPs have attracted a lot of attention in biomedical applications. They are often used as MRI contrast agents, so magnetic NPs as siRNA delivery systems offer the potential not only for gene therapy but also for imaging and real-TME monitoring in solid tumors [145]. To enhance the siRNA loading efficiency and the cellular uptake efficacy of NPs, their surfaces are often coated with cationic polymers. Wang et al. [146] used PEI-modified magnetic NPs (PEI-MNPs) for in vitro treatment of GBM multiforme U251 through the combination of bioimaging and siRNA delivery. Intracellular imaging displayed that the efficient uptake of PEI-MNPs by tumor cells, and the siRNA-PEI-MNPs complex effectively inhibited cell proliferation and induced apoptosis and autophagy.
Mesoporous silica NPs: Mesoporous silica NPs (MSNPs) have a large surface area, a high pore volume, homogeneous porous channels, good biocompatibility, and biodegradability, making them a viable siRNA delivery vehicle. Generally, there have been two strategies for preparing nucleic acid-MSN complexes: (1) Coating MSN with cationic polymers to create a positively charged surface suitable for complexing with siRNA [147]; (2) Loading siRNA in the unique pore structure of MSN. Therefore, it is essential to fabricate MSNs with larger pore sizes to accommodate bigger siRNA molecules, as smaller pores may restrict their passage. Meka et al. [148] prepared MSNPs with a large pore size (9 nm) and small particle size (200–400 nm) which could be loaded with siRNA. After modification with an octadecyl (C18), siRNA-survivin was successfully introduced into HCT-116 (human colon cancer cells) and the survivin protein expression was inhibited. Moreover, multiple nucleic acid-MSN complexes with different functionalities could also be generated through loading or modification strategies.
Quantum dots (QDs): Due to the distinctive electrical and optical characteristics of QDs, the use of QDs as drug delivery carriers can monitor the distribution of drugs in tissues through diagnostic imaging. In addition, QDs have a tunable size and shape. Hence, many studies have investigated the use of QDs as effective therapeutic diagnostic agents to deliver siRNA [149]. Lin et al. [150] developed cadmium sulphoselenide/Zinc sulfide quantum dots (CdSSe/ZnS QDs)-based nanocarriers. These CdSSe/ZnS QDs were conjugated with PEI (QD-PEI) to enhance the siRNA loading efficiency. The delivery of siRNA-TERT by QD-PEI was adequate, and the gene expression of TERT was successfully suppressed in U87 (human malignant GBM cells,) and U251 cells (human astrocytoma cells) without significant cytotoxicity. This study demonstrates the QD nanocarriers' enormous potential as versatile delivery platforms.
Delivery vectors could enhance the immune response in addition to helping siRNA drugs penetrate the targeted organs or tissues. In actuality, the immune system may be impacted by the majority of materials. For example, since iron, calcium, and zinc can promote the secretion of pro-inflammatory cytokines, inorganic NPs prepared with them have a beneficial effect on the M1 polarization of tumor macrophages [151]. In particular, the US FDA-approved iron oxide NPs can polarize M2-type TAMs to M1-type TAMs in vitro and in vivo and activate anti-tumor immune responses in TME [152]. As a commonly used material for photothermal therapy, the thermal effect of AuNPs can promote local infiltration of immune cells and maturation of DCs to remodel TME [153]. In addition, Feray et al. found that synthetic amorphous silica NPs also could induce the maturation of human monocyte-derived DCs [154]. Some cell membranes, aside from inorganic substances, can also act as distinct factors influencing macrophage polarization. Deng et al. reported that various proteins (e.g., RANKL or CB1) expressed on NK cell membranes can induce proinflammatory M1-macrophage polarization to produce anti-tumor immunity via an interaction with macrophage surface receptors [155].
4. Combination therapy improves the role of siRNA in remodeling TME
4.1. siRNA combined with immunotherapy
Immunotherapy, which eliminates tumors by activating the body's immune system to restore the normal anti-tumor immune response, has recently been the principal focus of cancer treatment. Nowadays, representative cancer immunotherapy involves adoptive immune cell therapy (ACT), chimeric antigen receptor (CAR)-T cells, targeted immune checkpoint therapy, tumor vaccines, oncolytic virotherapy, etc. [156]. It has been demonstrated that adoptive immune cells may be primed for cancer treatment using NPs carrying siRNA. For instance, Cheng et al. [157] prepared adoptive macrophages with DNA nanomedicine containing siRNA-CSF-1R (Colony stimulating factor-1)and immune adjuvant CpG. The synergistic effect of CpG and CSF-1R reprograms M2-type TAMs to M1-type TAMs to secrete anti-tumor cytokines. The data showed that these adoptive macrophages demonstrated effectiveness against tumors both in vitro and in vivo.
Blocking tumor immune checkpoints is among the most effective immunotherapy strategies, such as PD-1, PD-L1 and CTLA-4. Although immune checkpoint inhibitors (ICIs) have shown therapeutic benefits in cancer patients, the fact that they have a highly effective therapeutic effect in a small population of patients [158]. This was interconnected to the immunosuppressive microenvironment. siRNA has great potential to regulate genes that can inhibit various immunosuppressive pathways. Therefore, improving TME through siRNA-based approaches could alleviate the problem of low response rates to ICI treatments. For example, Ngamcherdtrakul et al. [159] developed an MSNP to co-deliver CpG and siRNA-STAT3 (AIRIST-02). CpG was a toll-like receptor 9 (TLR9) agonist that triggered the TLR9 pathway to activate antigen-presenting cells (APCs). STAT3 played an immunosuppressive role in APCs through a variety of mechanisms, limiting the immunological stimulatory effects of CpG. Hence, AIRIST-02 showed a robust anti-tumor immune response, characterized by an increase in systemic CD8+anti-tumor T cells. This enabled the treatment of distant tumors in addition to the original tumor's shrinkage. Significantly, AIRISE-02 improved the efficacy of ICIs. Compared with ICIs alone, the combination of the three (CpG, siRNA-STAT3 and ICIs) exerted a more pronounced influence on the inhibition of tumor growth. Another study [160] reported a light-responsive conjugate of αPD-L1 with siRNA-PD-L1 (PARC). PARC exhibited a specific targeting capability towards cancer cells and caused reactivation of immune cell activity through the interaction of αPD-L1 and membrane PD-L1. Following internalization through endocytosis, siRNA-PD-L1 could be released from PARC to inhibit intracellular PD-L1 expression. This dual-action approach synergistically contributed to the augmentation of anti-cancer immunity.
4.2. siRNA combined with chemotherapy
Chemotherapy is widely regarded as a successful kind of treatment since it uses cytotoxic medicines to eliminate cancer cells. The traditional perspective is that chemotherapy drugs can suppress the body's immune function while killing tumor cells. This immunosuppression might be linked to the irreversible exposure of phosphatidylserine (PS) located in the inner phospholipid bilayer of the cell membrane to the cell surface when drugs induce apoptosis in tumor cells [161]. PS that faces outward binds to immune cell receptors, severely suppressing the immune system [162]. Hence, blocking PS overturn has become a new strategy to ameliorate the TME. The creation of a novel nanocarrier was accomplished by Chen et al. [163], where they used amphiphilic PMBOP polymer to prepare the nanocarrier-loaded siRNA-Xkr8 and FuOXP, a prodrug conjugate of 5-fluorouracil and oxaliplatin. Xkr8 was an important scramblase that promoted exposure of PS on the surface of apoptotic cells. Therefore, the downregulation of Xkr8 expression could reduce the PS version. Importantly, it abolished the rise in the level of surface PS caused by the FuOXP treatment in CT26 tumor cells. Compared to the treatment with FuOXP NPs alone, the combined utilization of these two drugs resulted in a notable increase in the infiltration of anti-tumor immune cells, thereby significantly improving the anti-tumor activity.
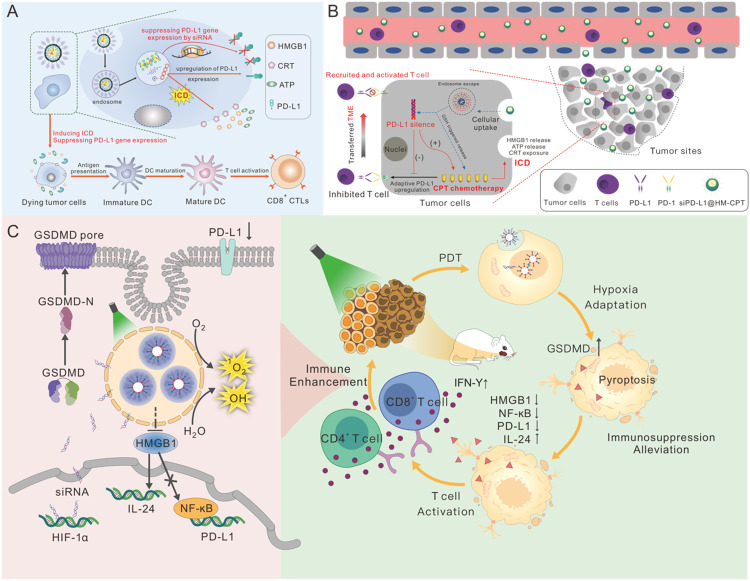
In recent years, some studies have found that low-dose chemotherapy drugs, such as anthracyclines, oxaliplatin and taxanes, can induce immunogenic cell death (ICD) in tumor cells as well as stimulate tumor-specific immune response [164], [165], [166]. Nevertheless, chemotherapy drugs also can trigger the up-regulation of various immunosuppressive genes (such as PD-L1, CD47) to offset the ICD effect [167]. Many scientists believe that reducing the expression of these associated immunosuppressive genes with siRNA will help to resolve this dispute. Chen et al. [168] developed a carrier-free nano-assembly of acid-activatable DOX prodrug and siRNA (PEG@D:siRNA). siRNA-PD-L1 suppressed the upregulation of PD-L1 of tumor cells induced by DOX-mediated ICD which achieved the combinational ICD induction and reversion of immunosuppression for cancer treatment (Fig. 6A). In a separate study, Tan et al. [169] created a new camptothecin (CPT) prodrug NP that was biodegradable, glutathione (GSH) sensitive, and capable of delivering siRNA-PD-L1. This approach of knocking down PD-L1 enhanced cancer cell sensitivity to CPT and alleviated the intensive immunosuppression caused by up-regulation of PD-L1 following CPT therapy (Fig. 6B).
Fig. 6.
Combination therapy remodels TME. (A) Schematic diagram mechanism of the combinational ICD induction and reversion of immunosuppression by PEG@D:siRNA promoted the activation and infiltration of CTLs (Reprinted with permission from [168]. Copyright 2020 Elsevier); (B)Schematic diagram of the combinational antitumor chemo-immunotherapy mechanism of siPD-L1@HM-CPT (Reprinted with permission from [169]. Copyright 2023 SpringerLink); (C) Schematic diagram of action mechanism of TiO2@Ru@siRNA (Reprinted with permission from [176].Copyright 2022 Elsevier.).
4.3. siRNA combined with photodynamic therapy
Photodynamic therapy (PDT) is a non-invasive treatment based on photosensitizer (PS). When exposed to the proper wavelength of light, the cytotoxic ROS produced by PS can cause the death of cancer cells [170]. Additionally, PDT has the ability to damage blood vessels as well as has immunomodulatory effects in tumor tissues [171]. Numerous researches have demonstrated that PDT can elicit ICD and promote the maturation of DCs, which then activate effector T cells and induce anti-tumor immunity [172]. Therefore, the combination of PDT and gene immunotherapy can synergistically augment the immune-mediated anti-tumor effect. Guo et al. [173] designed a nucleic acid nanogel (siRNA/PPA-NG) for the simultaneous delivery of siRNA-PD-L1 and pheophytin A (PPA) for melanoma treatment. Upon laser irradiation, siRNA/PPA-NG could greatly stimulate cancer cell death. Moreover, in contrast to the negative group treated with PBS, nanogel co-packaged with PPA and siRNA-PD-L1 without irradiation treatments could promote the frequencies of mature DCs and infiltration of cytotoxic T cells to 18.3%±1.6% and 44.9%±5.0%, respectively. Notably, these values were lower than those obtained with nanogel co-packaged with PPA and siRNA-PD-L1 along with irradiation treatments, which resulted in maturation of DCs at 62.3%±10.4% and infiltration of cytotoxic T cells at 49.0%±3.5%.
Although PDT has a high potential for cancer treatment, hypoxia remains a significant hurdle in PDT therapy [174,175]. To address this issue, Zhou et al. [176] designed a novel ruthenium-based photosensitizer (Ru) modified-TiO2 NPs loaded with siRNA-HIF-1α. The inclusion of HIF-1α siRNA is aimed at ameliorating the localized hypoxic conditions. Under visible light irradiation, the NPs exhibited a remarkable capability to elevate intracellular l levels of ROS and induce pyroptosis. Concurrently, the NPs down-regulated the expression of PD-L1 in tumor cells through the HMGB1 (high-mobility group protein B1)-NF-kB-PD-L1 axis, promoted the secretion of anti-tumor cytokine IL-24 and activated CD4+ and CD8+ T cells (Fig. 6C).
4.4. siRNA combined with photothermal therapy
Photothermal therapy (PTT) is a promising method for cancer treatment. In contrast to PDT, PTT employs localized heat produced by photothermal nanomaterials excited by near-infrared light (in the range of 650–900 nm) to eliminate tumor cells. Commonly used photothermal conversion materials such as precious metal nanomaterials, carbon nanomaterials, polymers (polyaniline NPs, Dopamine), and near-infrared fluorescent dyes, etc. [177]. Aside from direct heat ablation of tumors, the thermal effects generated during PTT treatment can also induce ICD, release tumor-associated antigens (TAAs), and damage-associated molecular patterns (DAMPs) [178]. DAMPs, as a "danger" signal of immune stimulation, can increase the infiltration and activation of antigen-presenting cells and intensify their phagocytosis as well as the processing of TAAs. These actions can activate T cells and start anti-tumor immune responses. Nevertheless, studies have demonstrated that PTT treatment increases the expression of indoleamine 2,3-dioxygenase 1 (IDO). IDO restrains the proliferation of T cells and induces the expression of regulatory T cells by converting tryptophan to kynurenine, inhibiting tumor cell apoptosis and impairing anti-tumor immunity [179]. Thus, Zhang et al. [180] developed a folate-targeting gold nanorod system for combining siRNA-IDO delivery and PTT. Their results revealed that IDO suppression by siRNA-IDO boosted T cell infiltration and the production of anti-tumor inflammatory cytokines (TNF and IFN) in TME, decreased T cell death, and improved treatment outcomes.
5. Clinical applications of siRNA-based anti-tumor drugs
5.1. Marketed siRNA-based anti-tumor drugs
As a novel anticancer drug, siRNA has been used in the treatment of several malignancies. In order to improve the therapeutic effect, researchers have developed siRNA therapeutic drugs from the perspectives of synergistic effect to remodel TME, overcoming tumor multi-drug resistance or minimizing chemotherapy drug side effects, hoping to increase the efficacy and reduce side effects. We have summarized all of the siRNA-based drugs in clinical trials due in 2022 as shown in Table 2.
Table 2.
siRNA-based drugs in clinical trials (As of 1st May 2023, surveys are available at clinicaltrials.gov.).
| Drugs | First posted | Target | Delivery system | Cancer type | Status | Company | ClinicalTrials.gov identifier |
|---|---|---|---|---|---|---|---|
| SY | 2018–08–01 | KRAS G12D | Exosomes | Metastatic Pancreatic; Adenocarcinoma Pancreatic Ductal Adenocarcinoma Stage IV; Pancreatic Cancer AJCC v8 | Phase 1 Recruiting | M.D. Anderson Cancer Center | NCT03608631 |
| siRNA-EphA2-DOPC | 2012–05–04 | EphA2 | LNP | Advanced cancers | Phase 1 Recruiting | M.D. Anderson Cancer Center | NCT01591356 |
| Atu027 | 2009–07–14 | PKN3 | LNP | Solid tumours | Phase 1 Completed | Silence Therapeutics | NCT00938574 |
| CALAA-01 | 2008–06–03 | RRM2 | Cyclodextrin NP | Solid tumours | Phase 1 Terminated | Calando Pharmaceuticals | NCT00689065 |
| Unknown | 2008–05–06 | Proteasome | Naked siRNA | Metastatic Melanoma | Phase 1 Completed | Scott Pruitt | NCT00672542 |
| APN401 | 2014–06–18 | siRNA-transfected | Naked siRNA | Solid Tumors | Phase 1 Completed | Wake Forest University Health Sciences | NCT0216625 |
| APN401 | 2017–03–22 | siRNA-transfected | Naked siRNA | Solid Tumors | Phase 1 Completed | Wake Forest University Health Sciences | NCT03087591 |
| PSCT19 | 2015–08–19 | PD-L1/PD-L2 | Naked siRNA | Hematological Malignancies | Phase 2 Completed | Radboud University Medical Center | NCT02528682 |
| siG12D LODER | 2012–08–30 | KRAS | LODER polymer | Pancreatic Ductal AdenocarcinomaPancreatic Cancer | Phase 2 Recruiting | Silenseed | NCT01676259 |
| SXL01 | 2016–08–15 | androgen receptor | Naked siRNA | Prostatic Cancer | Phase 1 Withdrawn | Institut Claudius Regaud | NCT02866916 |
| Unknown | 2005–11–23 | Unknown | SV40 vectors | Chronic Myeloid Leukemia | Completed | Hadassah Medical Organization | NCT00257647 |
| CAS3/SS3 | 2021–08–09 | TLR9 receptor; STAT3 | CpG conjugated | Relapsed/Refractory B-Cell NHL | Phase 1 Recruiting | City of Hope Medical Center | NCT04995536 |
| Unknown | 2011–01–05 | BCL-B | Unknown | Multiple Myeloma | unknown | Center hospitalier universitaire de nice | NCT01270009 |
| STP705 | 2021–04–14 | TGF-β1; COX-2 | Unknown | Squamous Cell Carcinoma in Situ | Phase 2 Recruiting | Sirnaomics | NCT04844983 |
| Atu027 | 2013–03–11 | PKN3 | Cationic lipoplex | Carcinoma, Pancreatic Ductal | Phase 1/2 Completed | Silence Therapeutics | NCT01808638 |
| Unknown | 2010–02–25 | E2F1; MYCN | Unknown | Neuroblastoma | Completed | National Taiwan University Hospital | NCT01075360 |
| Unknown | 2010–01–29 | B4GALNT3 | Unknown | Neuroblastoma | Completed | National Taiwan University Hospital | NCT01058798 |
| SLN124 | 2019–11–25 | TMPRSS6 | GalNAc conjugated | Beta-thalassaemia; Myelodysplastic Syndrome | Phase 1 Withdrawn | Silence Therapeutics | NCT04176653 |
| SLN124 | 2022–08–12 | TMPRSS6 | GalNAc conjugated | Polycythemia Vera | Phase 1/2 Not yet recruiting | Silence Therapeutics | NCT05499013 |
| NU-0129 | 2017–01–13 | BCL-2 L12 | AuNPs | Gliosarcoma | Phase 1 Completed | Northwestern University | NCT03020017 |
| NBF-006 | 2019–01–28 | GSTP | LNP | NSCLC; Pancreatic Cancer; CRC | Phase 1 Recruiting | Nitto BioPharma | NCT03819387 |
| STP705 | 2021–04–14 | TGF-β1; COX-2 | Copolymer PNPs | Keloid | Phase 2 Recruiting | Sirnaomics | NCT04844840 |
| ALN-VSP02 | 2010–07–08 | KSP; VEGF | SNALP | Solid Tumors | Phase 1 Completed | Alnylam Pharmaceuticals | NCT01158079 |
| TKM-080301 | 2011–09–20 | PLK1 | LNP | Liver Cancer | Phase 1 Completed | National Cancer Institute | NCT01437007 |
| CALAA-01 | 2008–06–03 | R2 | Cationic lipoplex | Solid Tumors | Phase 1 Terminated | Calando Pharmaceuticals | NCT00689065 |
| DCR-MYC | 2014–04–10 | MYC | LNP | Solid Tumors, Multiple Myeloma, Lymphoma | Phase 1 Terminated | Dicerna Pharmaceuticals | NCT02110563 |
| NBF-006 | 2019–01–28 | KRAS | LNP | NSCLC, Pancreatic, CRC | Phase 1 Recruiting | Nitto BioPharma | NCT03819387 |
According to the study, PKN3 has a significant role in regulating how quickly lung cancer cells spread. Furthermore, the siRNA drug Atu027, which is the target, has completed a phase I clinical study. The findings indicate potential anti-metastatic lung cancer activities as well as lymph node metastasis development [181]. The most prominent family of receptor-type tyrosine kinases is the Eph [182], of which EphA2 is highly expressed in solid tumors while being closely interconnected to tumor cell proliferation, survival and drug resistance. EphA2-targeting DOPC-encapsulated siRNA is a drug in phase I clinical trials for the treatment of advanced or recurrent solid tumors. The main goal of the study is to determine the safety, tolerability and maximum tolerated dosage, or delivered dose. The medication was well tolerated at all test doses, according to safety tests in primate and mouse animal models [183], and might be employed in phase 1 trials.
SiG12D LODER [184], a siRNA drug targeting the KRAS gene, has entered phase 1 clinical trials in combination with chemotherapy. The RAS gene is involved in regulating cell life activities. KRAS [185] is a member of the RAS family and is linked to cell growth, proliferation, differentiation and other life activities. Mutation of KRAS will lead to abnormal downstream signaling pathways to promote malignant cell proliferation and carcinogenesis. Because of its high mutation rate, KRAS has been chosen as a therapeutic target for the development of anti-tumor drugs. SiG12D LODER is a micro biodegradable implant with KRAS-targeting siRNA injected into patients with advanced pancreatic cancer (LAPC) along with chemotherapy. Negative incidents occurred in 5 of the 15 patients treated, and the remainder had a 70 % reduction in tumor marker CA19–9, with a median overall survival of 15.12 months and 18-month survival of 38.5 %. This hints at the possible efficacy of SiG12D LODER combined with chemotherapy in patients with LAPC.
Atu027 is a siRNA coated liposome drug that can target the expression of PKN3, a protein kinase N3, that is expressed in vascular and lymphatic endothelial cells [186], is the effector protein of RAS homologous in order to enhance the proliferation and migration of tumor cells, among other things. As a result, PKN3 may be employed as a therapeutic target for solid tumors. In the phase 1 clinical trial [187], 34 patients with advanced solid tumors received ten incremental doses of Atu027 without prior medication, and 41 % of patients remained stable without further disease progression within eight weeks.
5.2. Adverse reactions
As previously mentioned, many barriers still exist to the delivery of platforms of siRNA drugs in cancer therapy. When siRNA is provided to patients, double-stranded RNA may cause innate immune responses and have negative side effects. Some patients have severe hypersensitivity reactions to oligonucleotides or lipid and phospholipid products. In addition to that, it must be said that among the delivery vectors for siRNA drugs, Viruses have emerged as the most thoroughly researched and effective delivery vehicle. Nowadays, there are various AAV viruses developed for the specificity of different tissue structures, which can greatly enhance tissue targeting and specificity. When using viral vectors to deliver siRNA, the viral vectors have problems such as immunotoxicity and cytotoxicity, which may cause adverse reactions in clinical applications. In SiG12D LODER's phase I clinical study, three subjects reported neutropenia and two subjects reported cholangitis, a grade 3–4 serious adverse event [184]. During the phase 1 study of Atu027, four patients reported serious adverse events whereas 13 cases of elevated lipase were reported [187]. Hence, perhaps more research can focus on exploring safer and more biocompatible nanomaterials, in order to discover safer medications that are better suited for human clinical application. Additionally, there are many problems of drug resistance induced by pathogenic gene mutations in treating lung cancer. Therefore, while utilizing treatment techniques like siRNA internal gene interference, it is recommended that patients screen for some common pathogenic mutations in advance.
6. Future outlook and summary
Recently, the incidence rate of tumors has increased. Finding an effective approach for treating tumors is a typical difficulty for global scientific research and clinical treatment specialists. In the 21st century, the rapid development of biology has gradually clarified the molecular mechanism of tumors. Different tumor therapeutic targets have also been discovered, laying the foundation for the research of tumor-targeted drugs.
Multiple simple nano-delivery traditional therapeutic medications have been approved for cancer patients, however, their efficacy is typically inferior to that of other recognized treatment techniques. Therefore, people were searching for new treatments [188]. It is worth noting that the recent clinical advancements in immunotherapy have resulted in a substantial shift in the treatment of advanced malignant tumors. Nevertheless, this treatment is not perfect either, as there are still many patients who have not benefited from the currently available immunotherapy, and many people have had immune-related side effects [189]. Patient-specific genetic mutations frequently contribute to these adverse events. This also proposes that tumor immunotherapy could be coupled with gene therapy as another more precise and efficient treatment. Meanwhile, TME is the main cause of failure in nanomedicine and immunotherapy, which limits the delivery while also affecting efficacy, even if the drug accumulates in TME. Coincidentally, disrupting the TME characteristics of nanomedicine delivery can also lead to immune suppression. siRNA drugs have significant advantages in addressing the aforementioned issues [190]. Compared it to other therapeutic approaches, siRNA provides substantial therapeutic benefits. It can accurately inhibit the expression of target genes, promote tumor cell apoptosis, and have fewer side effects than traditional treatment methods. The ability of siRNA medicines to remodel TME has also proven to be extremely effective and could interfere with the core proteins in all components of TME. However, the development of drug delivery is relatively slow now. Virus is the most frequently utilized carrier, while NP drug delivery is the most famous and promising direction. With more and more rare disease siRNA delivery platforms gaining recognition, such as “Patisiran” and “ASO inotersen”. As a result, increasing amounts of funding are being directed in this manner [66,191,192].
Many novel delivery solutions have been continuously explored, among which the following three main strategies to optimize delivery technology are the most influential trendiest: chemical modification [193,194], biological coupling [195,196], and carrier-mediated vesicles [193,197]. The following strategies have been used to optimize the safety, efficacy and stability of siRNA therapy. The development of siRNA therapeutics is ongoing, and selecting alternative drug carriers and target genes for siRNA will alter its dosing system [198]. Therefore, how to establish an efficient and standard delivery dose for this drug is worth further research and discussion. It should be stated that the mechanism of target gene selection and delivery vectors for siRNA drugs often requires different researchers with different backgrounds, which always creates an undeniable knowledge gap or missing link. We believe that we are able to benefit from the study methodologies of uncommon genetic illnesses (http://www.orpha.net) and establish a mechanisms database for pharmaceutical researchers to choose target genes. Concurrently, it can also set up a complete delivery vectors database of nucleic acid drugs for molecular genetics researchers to learn. Generally speaking, customizing siRNA to control tumor microenvironment to acquire precise cancer treatment is a promising new strategy for tumor prevention and treatment.
Conflicts of interest
The authors declare that they have no conflict of interest.
Acknowledgments
This work was supported by the Natural Science Foundation of China (U2230123, 81870683, 82121003 and 82201234); CAMS Innovation Fund for Medical Sciences (2019-I2M-5-032); and the Department of Science and Technology of Sichuan Province, China (22ZYZYTS0159, 2022YFS0606, 2023YFS0125, 2023YFS0131, 2023NSFSC0033, and 22ZYZYTS0151).
Contributor Information
Yi Shi, Email: yshi@uestc.edu.cn.
Lulu Cai, Email: cailulu@med.uestc.edu.cn.
References
- 1.Wu W., Pu Y., Shi J. Nanomedicine-enabled chemotherapy-based synergetic cancer treatments. J Nanobiotechnology. 2022;20(1):4. doi: 10.1186/s12951-021-01181-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Carlisle J.W., Steuer C.E., Owonikoko T.K., Saba N.F. An update on the immune landscape in lung and head and neck cancers. CA Cancer J Clin. 2020;70(6):505–517. doi: 10.3322/caac.21630. [DOI] [PubMed] [Google Scholar]
- 3.Zhu P.F., Wang M.X., Chen Z.L., Yang L. Targeting the tumor microenvironment: a literature review of the novel anti-tumor mechanism of statins. Front Oncol. 2021;11 doi: 10.3389/fonc.2021.761107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Carlino M.S., Larkin J., Long G.V. Immune checkpoint inhibitors in melanoma. Lancet. 2021;398(10304):1002–1014. doi: 10.1016/S0140-6736(21)01206-X. [DOI] [PubMed] [Google Scholar]
- 5.Liu Y., Zheng M., Ma Z., Zhou Y., Huo J., Zhang W., et al. Design, synthesis, and evaluation of PD-L1 degraders to enhance T cell killing activity against melanoma. Chin Chem Lett. 2023;34(5) [Google Scholar]
- 6.Chen J., Zhu Y., Wu C., Shi J. Nanoplatform-based cascade engineering for cancer therapy. Chem Soc Rev. 2020;49(24):9057–9094. doi: 10.1039/d0cs00607f. [DOI] [PubMed] [Google Scholar]
- 7.Baulcombe D.C. Molecular biology-unwinding RNA silencing. Science. 2000;290(5494):1108–1109. doi: 10.1126/science.290.5494.1108. [DOI] [PubMed] [Google Scholar]
- 8.Zuckerman J.E., Davis M.E. Clinical experiences with systemically administered siRNA-based therapeutics in cancer. Nat Rev Drug Discov. 2015;14(12):843–856. doi: 10.1038/nrd4685. [DOI] [PubMed] [Google Scholar]
- 9.Wang Q., Xue Y., Zhang L., Zhong Z., Feng S., Wang C., et al. Mechanism of siRNA production by a plant Dicer-RNA complex in dicing-competent conformation. Science. 2021;374(6571):1152–1157. doi: 10.1126/science.abl4546. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Shen J., Zhang W., Qi R., Mao Z.W., Shen H. Engineering functional inorganic-organic hybrid systems: advances in siRNA therapeutics. Chem Soc Rev. 2018;47(6):1969–1995. doi: 10.1039/c7cs00479f. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Chen X., Yang H., Song X., Liang H., Wei Y., Lu J., et al. Integrated and dual-responsive lipopeptide nanovector with parallel effect to tumor and micro-environment regulation by efficient gene and drug co-delivery. Chin Chem Lett. 2023;34(5) [Google Scholar]
- 12.Akhtar S., Benter I. Toxicogenomics of non-viral drug delivery systems for RNAi: potential impact on siRNA-mediated gene silencing activity and specificity. Adv Drug Deliv Rev. 2007;59(2–3):164–182. doi: 10.1016/j.addr.2007.03.010. [DOI] [PubMed] [Google Scholar]
- 13.Kumar V., Turnbull W.B. Targeted delivery of oligonucleotides using multivalent protein-carbohydrate interactions. Chem Soc Rev. 2023;52(4):1273–1287. doi: 10.1039/d2cs00788f. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Qiu M., Li Y., Bloomer H., Xu Q. Developing biodegradable lipid nanoparticles for intracellular mRNA delivery and genome editing. Acc Chem Res. 2021;54(21):4001–4011. doi: 10.1021/acs.accounts.1c00500. [DOI] [PubMed] [Google Scholar]
- 15.Deng K., Yang D., Zhou Y. Nanotechnology-based siRNA delivery systems to overcome tumor immune evasion in cancer immunotherapy. Pharmaceutics. 2022;14(7):1344. doi: 10.3390/pharmaceutics14071344. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Wei X., Dombkowski D., Meirelles K., Pieretti-Vanmarcke R., Szotek P.P., Chang H.L., et al. Mullerian inhibiting substance preferentially inhibits stem/progenitors in human ovarian cancer cell lines compared with chemotherapeutics. Proc Natl Acad Sci USA. 2010;107(44):18874–18879. doi: 10.1073/pnas.1012667107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Rossi M., Altea-Manzano P., Demicco M., Doglioni G., Bornes L., Fukano M., et al. PHGDH heterogeneity potentiates cancer cell dissemination and metastasis. Nature. 2022;605(7911):747–753. doi: 10.1038/s41586-022-04758-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Zhang H., Yang Q., Lian X., Jiang P., Cui J. Hypoxia-Inducible Factor-1alpha (HIF-1alpha) promotes hypoxia-induced invasion and metastasis in ovarian cancer by targeting matrix metallopeptidase 13 (MMP13) Med Sci Monit. 2019;25:7202–7208. doi: 10.12659/MSM.916886. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Chen L., Liu T., Zhang S., Zhou J., Wang Y., Di W. Succinate dehydrogenase subunit B inhibits the AMPK-HIF-1alpha pathway in human ovarian cancer in vitro. J Ovarian Res. 2014;7:115. doi: 10.1186/s13048-014-0115-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Romagnoli M., Trichet V., David C., Clement M., Moreau P., Bataille R., et al. Significant impact of survivin on myeloma cell growth. Leukemia. 2007;21(5):1070–1078. doi: 10.1038/sj.leu.2404602. [DOI] [PubMed] [Google Scholar]
- 21.Yang H., Du X., Xi Y. Effects of survivin on FVADT chemotherapy for refractory multiple myeloma. Exp Ther Med. 2016;12(2):771–776. doi: 10.3892/etm.2016.3401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Tsubaki M., Takeda T., Ogawa N., Sakamoto K., Shimaoka H., Fujita A., et al. Overexpression of survivin via activation of ERK1/2, Akt, and NF-kappaB plays a central role in vincristine resistance in multiple myeloma cells. Leuk Res. 2015;39(4):445–452. doi: 10.1016/j.leukres.2015.01.016. [DOI] [PubMed] [Google Scholar]
- 23.Zhang Q., Hossain D.M., Nechaev S., Kozlowska A., Zhang W., Liu Y., et al. TLR9-mediated siRNA delivery for targeting of normal and malignant human hematopoietic cells in vivo. Blood. 2013;121(8):1304–1315. doi: 10.1182/blood-2012-07-442590. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Craig M., Jenner A.L., Namgung B., Lee L.P., Goldman A. Engineering in medicine to address the challenge of cancer drug resistance: from micro- and nanotechnologies to computational and mathematical modeling. Chem Rev. 2021;121(6):3352–3389. doi: 10.1021/acs.chemrev.0c00356. [DOI] [PubMed] [Google Scholar]
- 25.Li S., Zhao X., Chang S., Li Y., Guo M., Guan Y. ERp57-small interfering RNA silencing can enhance the sensitivity of drug-resistant human ovarian cancer cells to paclitaxel. Int J Oncol. 2019;54(1):249–260. doi: 10.3892/ijo.2018.4628. [DOI] [PubMed] [Google Scholar]
- 26.Xu Y., Zhang J., Wu J., Zhong S., Li H. Inhibition of JAK2 reverses paclitaxel resistance in human ovarian cancer cells. Int J Gynecol Cancer. 2015;25(9):1557–1564. doi: 10.1097/IGC.0000000000000550. [DOI] [PubMed] [Google Scholar]
- 27.Zhang Q., Zhang H., Ning T., Liu D., Deng T., Liu R., et al. Exosome-delivered c-Met siRNA could reverse chemoresistance to cisplatin in gastric cancer. Int J Nanomedicine. 2020;15:2323–2335. doi: 10.2147/IJN.S231214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Li E., Hu Z., Sun Y., Zhou Q., Yang B., Zhang Z., et al. Small molecule inhibitor of c-Met (PHA665752) suppresses the growth of ovarian cancer cells and reverses cisplatin resistance. Tumour Biol. 2016;37(6):7843–7852. doi: 10.1007/s13277-015-4318-x. [DOI] [PubMed] [Google Scholar]
- 29.Zhao L., Yang J., Liu X., Fu X., Ding Y., Wang C. Overexpression of long noncoding RNA E2F-mediated cell proliferation enhancing long noncoding RNA is involved in the development of chemoresistance of cancer cells to carboplatin in ovarian endometrioid adenocarcinoma. Cancer Biother Radiopharm. 2019;34(9):566–571. doi: 10.1089/cbr.2019.2851. [DOI] [PubMed] [Google Scholar]
- 30.Taratula O., Kuzmov A., Shah M., Garbuzenko O.B., Minko T. Nanostructured lipid carriers as multifunctional nanomedicine platform for pulmonary co-delivery of anticancer drugs and siRNA. J Control Release. 2013;171(3):349–357. doi: 10.1016/j.jconrel.2013.04.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Zhang Y.L., Yuan J.Q., Wang K.F., Fu X.H., Han X.R., Threapleton D., et al. The prevalence of EGFR mutation in patients with non-small cell lung cancer: a systematic review and meta-analysis. Oncotarget. 2016;7(48):78985–78993. doi: 10.18632/oncotarget.12587. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Johnson M., Garassino M.C., Mok T., Mitsudomi T. Treatment strategies and outcomes for patients with EGFR-mutant non-small cell lung cancer resistant to EGFR tyrosine kinase inhibitors: focus on novel therapies. Lung Cancer. 2022;170:41–51. doi: 10.1016/j.lungcan.2022.05.011. [DOI] [PubMed] [Google Scholar]
- 33.Zarredar H., Pashapour S., Ansarin K., Khalili M., Baghban R., Farajnia S. Combination therapy with KRAS siRNA and EGFR inhibitor AZD8931 suppresses lung cancer cell growth in vitro. J Cell Physiol. 2019;234(2):1560–1566. doi: 10.1002/jcp.27021. [DOI] [PubMed] [Google Scholar]
- 34.Shimasaki N., Jain A., Campana D. NK cells for cancer immunotherapy. Nat Rev Drug Discov. 2020;19(3):200–218. doi: 10.1038/s41573-019-0052-1. [DOI] [PubMed] [Google Scholar]
- 35.Wang F., Wu L., Yin L., Shi H., Gu Y., Xing N. Combined treatment with anti-PSMA CAR NK-92 cell and anti-PD-L1 monoclonal antibody enhances the antitumour efficacy against castration-resistant prostate cancer. Clin Transl Med. 2022;12(6):e901. doi: 10.1002/ctm2.901. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Nakamura T., Nakade T., Yamada K., Sato Y., Harashima H. The hydrophobic tail of a pH-sensitive cationic lipid influences siRNA transfection activity and toxicity in human NK cell lines. Int J Pharm. 2021;609 doi: 10.1016/j.ijpharm.2021.121140. [DOI] [PubMed] [Google Scholar]
- 37.Kaban K., Hinterleitner C., Zhou Y., Salva E., Kantarci A.G., Salih H.R., et al. Therapeutic silencing of BCL-2 using NK cell-derived exosomes as a novel therapeutic approach in breast cancer. Cancers. 2021;13(10):2397. doi: 10.3390/cancers13102397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Kishton R.J., Sukumar M., Restifo N.P. Metabolic regulation of T cell longevity and function in tumor immunotherapy. Cell Metab. 2017;26(1):94–109. doi: 10.1016/j.cmet.2017.06.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Schwartz RH. T cell anergy. Annu Rev Immunol. 2003;21:305–334. doi: 10.1146/annurev.immunol.21.120601.141110. [DOI] [PubMed] [Google Scholar]
- 40.Rafiq S., Yeku O.O., Jackson H.J., Purdon T.J., van Leeuwen D.G., Drakes D.J., et al. Targeted delivery of a PD-1-blocking scFv by CAR-T cells enhances anti-tumor efficacy in vivo. Nat Biotechnol. 2018;36(9):847–856. doi: 10.1038/nbt.4195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Huang X., Yang Y. Driving an improved CAR for cancer immunotherapy. J Clin Invest. 2016;126(8):2795–2798. doi: 10.1172/JCI88959. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Liu G., Zhang Q., Li D., Zhang L., Gu Z., Liu J., et al. PD-1 silencing improves anti-tumor activities of human mesothelin-targeted CAR T cells. Hum Immunol. 2021;82(2):130–138. doi: 10.1016/j.humimm.2020.12.002. [DOI] [PubMed] [Google Scholar]
- 43.Lokugamage M.P., Sago C.D., Gan Z., Krupczak B.R., Dahlman J.E. Constrained nanoparticles deliver siRNA and sgRNA to T cells in vivo without targeting ligands. Adv Mater. 2019;31(41) doi: 10.1002/adma.201902251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Wu P., Zhang H., Sun M., Mao S., He Q., Shi Y., et al. Manipulating offense and defense signaling to fight cold tumors with carrier-free nanoassembly of fluorinated prodrug and siRNA. Adv Mater. 2022;34(38) doi: 10.1002/adma.202203019. [DOI] [PubMed] [Google Scholar]
- 45.Kang T.H., Lee J.H., Noh K.H., Han H.D., Shin B.C., Choi E.Y., et al. Enhancing dendritic cell vaccine potency by combining a BAK/BAX siRNA-mediated antiapoptotic strategy to prolong dendritic cell life with an intracellular strategy to target antigen to lysosomal compartments. Int J Cancer. 2007;120(8):1696–1703. doi: 10.1002/ijc.22377. [DOI] [PubMed] [Google Scholar]
- 46.He L., Valignat M.P., Zhang L., Gelard L., Zhang F., Le Guen V., et al. ARHGAP45 controls naive T- and B-cell entry into lymph nodes and T-cell progenitor thymus seeding. EMBO Rep. 2021;22(4):e52196. doi: 10.15252/embr.202052196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Renand A., Cervera-Marzal I., Gil L., Dong C., Garcia A., Kervagoret E., et al. Integrative molecular profiling of autoreactive CD4 T cells in autoimmune hepatitis. J Hepatol. 2020;73(6):1379–1390. doi: 10.1016/j.jhep.2020.05.053. [DOI] [PubMed] [Google Scholar]
- 48.Satake N., Duong C., Chen C., Barisone G.A., Diaz E., Tuscano J., et al. Targeted therapy with MXD3 siRNA, anti-CD22 antibody and nanoparticles for precursor B-cell acute lymphoblastic leukaemia. Br J Haematol. 2014;167(4):487–499. doi: 10.1111/bjh.13066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Dai X., Lu L., Deng S., Meng J., Wan C., Huang J., et al. USP7 targeting modulates anti-tumor immune response by reprogramming tumor-associated macrophages in lung cancer. Theranostics. 2020;10(20):9332–9347. doi: 10.7150/thno.47137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Li M., Li M., Yang Y., Liu Y., Xie H., Yu Q., et al. Remodeling tumor immune microenvironment via targeted blockade of PI3K-gamma and CSF-1/CSF-1R pathways in tumor associated macrophages for pancreatic cancer therapy. J Control Release. 2020;321:23–35. doi: 10.1016/j.jconrel.2020.02.011. [DOI] [PubMed] [Google Scholar]
- 51.Zhou W., Zhou Y., Chen X., Ning T., Chen H., Guo Q., et al. Pancreatic cancer-targeting exosomes for enhancing immunotherapy and reprogramming tumor microenvironment. Biomaterials. 2021;268 doi: 10.1016/j.biomaterials.2020.120546. [DOI] [PubMed] [Google Scholar]
- 52.Wang T., Mu W., Li F., Zhang J., Hou T., Pang X., et al. Layer peeling" co-delivery system for enhanced RNA interference-based tumor associated macrophages-specific chemoimmunotherapy. Nanoscale. 2020;12(32):16851–16863. doi: 10.1039/d0nr04025h. [DOI] [PubMed] [Google Scholar]
- 53.Mucha J., Majchrzak K., Taciak B., Hellmen E., Krol M. MDSCs mediate angiogenesis and predispose canine mammary tumor cells for metastasis via IL-28/IL-28RA (IFN-lambda) signaling. PLoS One. 2014;9(7) doi: 10.1371/journal.pone.0103249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Guven Maiorov E., Keskin O., Gursoy A., Nussinov R. The structural network of inflammation and cancer: merits and challenges. Semin Cancer Biol. 2013;23(4):243–251. doi: 10.1016/j.semcancer.2013.05.003. [DOI] [PubMed] [Google Scholar]
- 55.Parihar R., Rivas C., Huynh M., Omer B., Lapteva N., Metelitsa L.S., et al. NK Cells Expressing a chimeric activating receptor eliminate MDSCs and rescue impaired CAR-T cell activity against solid tumors. Cancer Immunol Res. 2019;7(3):363–375. doi: 10.1158/2326-6066.CIR-18-0572. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Ding X., Zhang W., Zhao T., Yan C., Du H. Rab7 GTPase controls lipid metabolic signaling in myeloid-derived suppressor cells. Oncotarget. 2017;8(18):30123–30137. doi: 10.18632/oncotarget.16280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Shao B., Wei X., Luo M., Yu J., Tong A., Ma X., et al. Inhibition of A20 expression in tumor microenvironment exerts anti-tumor effect through inducing myeloid-derived suppressor cells apoptosis. Sci Rep. 2015;5:16437. doi: 10.1038/srep16437. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Kalluri R., Zeisberg M. Fibroblasts in cancer. Nat Rev Cancer. 2006;6(5):392–401. doi: 10.1038/nrc1877. [DOI] [PubMed] [Google Scholar]
- 59.Calvo F., Ege N., Grande-Garcia A., Hooper S., Jenkins R.P., Chaudhry S.I., et al. Mechanotransduction and YAP-dependent matrix remodelling is required for the generation and maintenance of cancer-associated fibroblasts. Nat Cell Biol. 2013;15(6):637–646. doi: 10.1038/ncb2756. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Du Y.E., Tu G., Yang G., Li G., Yang D., Lang L., et al. MiR-205/YAP1 in activated fibroblasts of breast tumor promotes VEGF-independent angiogenesis through STAT3 signaling. Theranostics. 2017;7(16):3972–3988. doi: 10.7150/thno.18990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Chen J., Sun X., Shao R., Xu Y., Gao J., Liang W. VEGF siRNA delivered by polycation liposome-encapsulated calcium phosphate nanoparticles for tumor angiogenesis inhibition in breast cancer. Int J Nanomedicine. 2017;12:6075–6088. doi: 10.2147/IJN.S142739. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Gu J., Ji Z., Li D., Dong Q. Proliferation inhibition and apoptosis promotion by dual silencing of VEGF and Survivin in human osteosarcoma. Acta Biochim Biophys Sin. 2019;51(1):59–67. doi: 10.1093/abbs/gmy146. [DOI] [PubMed] [Google Scholar]
- 63.Zhang M.M., Bahal R., Rasmussen T.P., Manautou J.E., Zhong X.B. The growth of siRNA-based therapeutics: updated clinical studies. Biochem Pharmacol. 2021;189 doi: 10.1016/j.bcp.2021.114432. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Li S.D., Huang L. Nanoparticles evading the reticuloendothelial system: role of the supported bilayer. Biochim Biophys Acta. 2009;1788(10):2259–2266. doi: 10.1016/j.bbamem.2009.06.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Wang J., Lu Z., Wientjes M.G., Au J.L. Delivery of siRNA therapeutics: barriers and carriers. AAPS J. 2010;12(4):492–503. doi: 10.1208/s12248-010-9210-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Hu B., Zhong L., Weng Y., Peng L., Huang Y., Zhao Y., et al. Therapeutic siRNA: state of the art. Signal Transduct Target Ther. 2020;5(1):101. doi: 10.1038/s41392-020-0207-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Whitehead K.A., Langer R., Anderson D.G. Knocking down barriers: advances in siRNA delivery. Nat Rev Drug Discov. 2009;8(2):129–138. doi: 10.1038/nrd2742. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Kara G., Calin G.A., Ozpolat B. RNAi-based therapeutics and tumor targeted delivery in cancer. Adv Drug Deliv Rev. 2022;182 doi: 10.1016/j.addr.2022.114113. [DOI] [PubMed] [Google Scholar]
- 69.Paul A., Muralidharan A., Biswas A., Kamath B.V., Joseph A., Alex A.T. siRNA therapeutics and its challenges: recent advances in effective delivery for cancer therapy. OpenNano. 2022;7 [Google Scholar]
- 70.Judge A.D., Sood V., Shaw J.R., Fang D., McClintock K., MacLachlan I. Sequence-dependent stimulation of the mammalian innate immune response by synthetic siRNA. Nat Biotechnol. 2005;23(4):457–462. doi: 10.1038/nbt1081. [DOI] [PubMed] [Google Scholar]
- 71.Hornung V., Guenthner-Biller M., Bourquin C., Ablasser A., Schlee M., Uematsu S., et al. Sequence-specific potent induction of IFN-alpha by short interfering RNA in plasmacytoid dendritic cells through TLR7. Nat Med. 2005;11(3):263–270. doi: 10.1038/nm1191. [DOI] [PubMed] [Google Scholar]
- 72.Jackson A.L., Linsley P.S. Recognizing and avoiding siRNA off-target effects for target identification and therapeutic application. Nat Rev Drug Discov. 2010;9(1):57–67. doi: 10.1038/nrd3010. [DOI] [PubMed] [Google Scholar]
- 73.Kumari A., Kaur A., Aggarwal G. The emerging potential of siRNA nanotherapeutics in treatment of arthritis. Asian J Pharm Sci. 2023 doi: 10.1016/j.ajps.2023.100845. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Watts J.K., Deleavey G.F., Damha M.J. Chemically modified siRNA: tools and applications. Drug Discov Today. 2008;13(19–20):842–855. doi: 10.1016/j.drudis.2008.05.007. [DOI] [PubMed] [Google Scholar]
- 75.Zeinali M., Abbaspour-Ravasjani S., Ghorbani M., Babazadeh A., Soltanfam T., Santos A.C., et al. Nanovehicles for co-delivery of anticancer agents. Drug Discov Today. 2020;25(8):1416–1430. doi: 10.1016/j.drudis.2020.06.027. [DOI] [PubMed] [Google Scholar]
- 76.Dong Y., Siegwart D.J., Anderson D.G. Strategies, design, and chemistry in siRNA delivery systems. Adv Drug Deliv Rev. 2019;144:133–147. doi: 10.1016/j.addr.2019.05.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Chow M.Y.T., Qiu Y., Lo F.F.K., Lin H.H.S., Chan H.K., Kwok P.C.L., et al. Inhaled powder formulation of naked siRNA using spray drying technology with l-leucine as dispersion enhancer. Int J Pharm. 2017;530(1–2):40–52. doi: 10.1016/j.ijpharm.2017.07.013. [DOI] [PubMed] [Google Scholar]
- 78.Shen J., Samul R., Silva R.L., Akiyama H., Liu H., Saishin Y., et al. Suppression of ocular neovascularization with siRNA targeting VEGF receptor 1. Gene Ther. 2006;13(3):225–234. doi: 10.1038/sj.gt.3302641. [DOI] [PubMed] [Google Scholar]
- 79.Li N., Song Y., Zhao W., Han T., Lin S., Ramirez O., et al. Small interfering RNA targeting NF-kappaB attenuates lipopolysaccharide-induced acute lung injury in rats. BMC Physiol. 2016;16(1):7. doi: 10.1186/s12899-016-0027-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Bitko V., Musiyenko A., Shulyayeva O., Barik S. Inhibition of respiratory viruses by nasally administered siRNA. Nat Med. 2005;11(1):50–55. doi: 10.1038/nm1164. [DOI] [PubMed] [Google Scholar]
- 81.Reischl D., Zimmer A. Drug delivery of siRNA therapeutics: potentials and limits of nanosystems. Nanomedicine. 2009;5(1):8–20. doi: 10.1016/j.nano.2008.06.001. [DOI] [PubMed] [Google Scholar]
- 82.Barati M., Mirzavi F., Atabaki M., Bibak B., Mohammadi M., Jaafari M.R. A review of PD-1/PD-L1 siRNA delivery systems in immune T cells and cancer cells. Int Immunopharmacol. 2022;111 doi: 10.1016/j.intimp.2022.109022. [DOI] [PubMed] [Google Scholar]
- 83.Sayed N., Allawadhi P., Khurana A., Singh V., Navik U., Pasumarthi S.K., et al. Gene therapy: comprehensive overview and therapeutic applications. Life Sci. 2022;294 doi: 10.1016/j.lfs.2022.120375. [DOI] [PubMed] [Google Scholar]
- 84.Kaiser J. How safe is a popular gene therapy vector? Science. 2020;367(6474):131. doi: 10.1126/science.367.6474.131. [DOI] [PubMed] [Google Scholar]
- 85.Pan X., Veroniaina H., Su N., Sha K., Jiang F., Wu Z., et al. Applications and developments of gene therapy drug delivery systems for genetic diseases. Asian J Pharm Sci. 2021;16(6):687–703. doi: 10.1016/j.ajps.2021.05.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Stanek L.M., Sardi S.P., Mastis B., Richards A.R., Treleaven C.M., Taksir T., et al. Silencing mutant huntingtin by adeno-associated virus-mediated RNA interference ameliorates disease manifestations in the YAC128 mouse model of Huntington's disease. Hum Gene Ther. 2014;25(5):461–474. doi: 10.1089/hum.2013.200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Daemen T., de Mare A., Bungener L., de Jonge J., Huckriede A., Wilschut J. Virosomes for antigen and DNA delivery. Adv Drug Deliv Rev. 2005;57(3):451–463. doi: 10.1016/j.addr.2004.09.005. [DOI] [PubMed] [Google Scholar]
- 88.Adamina M., Guller U., Bracci L., Heberer M., Spagnoli G.C., Schumacher R. Clinical applications of virosomes in cancer immunotherapy. Expert Opin Biol Ther. 2006;6(11):1113–1121. doi: 10.1517/14712598.6.11.1113. [DOI] [PubMed] [Google Scholar]
- 89.Wang Y., Jin B., Li B., Luo Y., Ma M., Chen Y., et al. Cell-free protein synthesis of influenza virus hemagglutinin HA2-integrated virosomes for siRNA delivery. Int J Pharm. 2022;623 doi: 10.1016/j.ijpharm.2022.121890. [DOI] [PubMed] [Google Scholar]
- 90.Blom R.A.M., Amacker M., Moser C., van Dijk R.M., Bonetti R., Seydoux E., et al. Virosome-bound antigen enhances DC-dependent specific CD4(+) T cell stimulation, inducing a Th1 and Treg profile in vitro. Nanomedicine. 2017;13(5):1725–1737. doi: 10.1016/j.nano.2017.02.004. [DOI] [PubMed] [Google Scholar]
- 91.Zhang A., Meng K., Liu Y., Pan Y., Qu W., Chen D., et al. Absorption, distribution, metabolism, and excretion of nanocarriers in vivo and their influences. Adv Colloid Interface Sci. 2020;284 doi: 10.1016/j.cis.2020.102261. [DOI] [PubMed] [Google Scholar]
- 92.Ding J., Chen J., Gao L., Jiang Z., Zhang Y., Li M., et al. Engineered nanomedicines with enhanced tumor penetration. Nano Today. 2019;29 [Google Scholar]
- 93.Jiang W., Kim B.Y., Rutka J.T., Chan W.C. Nanoparticle-mediated cellular response is size-dependent. Nat Nanotechnol. 2008;3(3):145–150. doi: 10.1038/nnano.2008.30. [DOI] [PubMed] [Google Scholar]
- 94.Zhao Z., Ukidve A., Krishnan V., Mitragotri S. Effect of physicochemical and surface properties on in vivo fate of drug nanocarriers. Adv Drug Deliv Rev. 2019;143:3–21. doi: 10.1016/j.addr.2019.01.002. [DOI] [PubMed] [Google Scholar]
- 95.Li Y., Kroger M., Liu W.K. Shape effect in cellular uptake of PEGylated nanoparticles: comparison between sphere, rod, cube and disk. Nanoscale. 2015;7(40):16631–16646. doi: 10.1039/c5nr02970h. [DOI] [PubMed] [Google Scholar]
- 96.Sharma G., Valenta D.T., Altman Y., Harvey S., Xie H., Mitragotri S., et al. Polymer particle shape independently influences binding and internalization by macrophages. J Control Release. 2010;147(3):408–412. doi: 10.1016/j.jconrel.2010.07.116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Feng T., Ai X., An G., Yang P., Zhao Y. Charge-convertible carbon dots for imaging-guided drug delivery with enhanced in vivo cancer therapeutic efficiency. ACS Nano. 2016;10(4):4410–4420. doi: 10.1021/acsnano.6b00043. [DOI] [PubMed] [Google Scholar]
- 98.Shah S., Dhawan V., Holm R., Nagarsenker M.S., Perrie Y. Liposomes: advancements and innovation in the manufacturing process. Adv Drug Deliv Rev. 2020;154-155:102–122. doi: 10.1016/j.addr.2020.07.002. [DOI] [PubMed] [Google Scholar]
- 99.Dokka S., Toledo D., Shi X., Castranova V., Rojanasakul Y. Oxygen radical-mediated pulmonary toxicity induced by some cationic liposomes. Pharm Res. 2000;17(5):521–525. doi: 10.1023/a:1007504613351. [DOI] [PubMed] [Google Scholar]
- 100.Lee J., Ahn H.J. PEGylated DC-Chol/DOPE cationic liposomes containing KSP siRNA as a systemic siRNA delivery carrier for ovarian cancer therapy. Biochem Biophys Res Commun. 2018;503(3):1716–1722. doi: 10.1016/j.bbrc.2018.07.104. [DOI] [PubMed] [Google Scholar]
- 101.Xia Y., Tian J., Chen X. Effect of surface properties on liposomal siRNA delivery. Biomaterials. 2016;79:56–68. doi: 10.1016/j.biomaterials.2015.11.056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Zang X., Ding H., Zhao X., Li X., Du Z., Hu H., et al. Anti-EphA10 antibody-conjugated pH-sensitive liposomes for specific intracellular delivery of siRNA. Int J Nanomedicine. 2016;11:3951–3967. doi: 10.2147/IJN.S107952. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Li Y., Tan X., Liu X., Liu L., Fang Y., Rao R., et al. Enhanced anticancer effect of doxorubicin by TPGS-coated liposomes with Bcl-2 siRNA-corona for dual suppression of drug resistance. Asian J Pharm Sci. 2020;15(5):646–660. doi: 10.1016/j.ajps.2019.10.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Sahu R., Jha S., Pattanayak S.P. Therapeutic silencing of mTOR by systemically administered siRNA-loaded neutral liposomal nanoparticles inhibits DMBA-induced mammary carcinogenesis. Br J Cancer. 2022;127(12):2207–2219. doi: 10.1038/s41416-022-02011-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Halder J., Kamat A.A., Landen C.N., Jr., Han L.Y., Lutgendorf S.K., Lin Y.G., et al. Correction: focal adhesion kinase targeting using in vivo short interfering RNA delivery in neutral liposomes for ovarian carcinoma therapy. Clin Cancer Res. 2019;25(10):3194. doi: 10.1158/1078-0432.CCR-19-1132. [DOI] [PubMed] [Google Scholar]
- 106.Tysnes O.B., Verhoeven A.J., Holmsen H. Rates of production and consumption of phosphatidic acid upon thrombin stimulation of human platelets. Eur J Biochem. 1988;174(1):75–79. doi: 10.1111/j.1432-1033.1988.tb14064.x. [DOI] [PubMed] [Google Scholar]
- 107.Rehman U., Parveen N., Sheikh A., Abourehab M.A.S., Sahebkar A., Kesharwani P. Polymeric nanoparticles-siRNA as an emerging nano-polyplexes against ovarian cancer. Colloids Surf B Biointerfaces. 2022;218 doi: 10.1016/j.colsurfb.2022.112766. [DOI] [PubMed] [Google Scholar]
- 108.Barua S., Ramos J., Potta T., Taylor D., Huang H.C., Montanez G., et al. Discovery of cationic polymers for non-viral gene delivery using combinatorial approaches. Comb Chem High Throughput Screen. 2011;14(10):908–924. doi: 10.2174/138620711797537076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Neuberg P., Kichler A. Recent Developments in nucleic acid delivery with polyethylenimines. Adv Genet. 2014;88:263–288. doi: 10.1016/B978-0-12-800148-6.00009-2. [DOI] [PubMed] [Google Scholar]
- 110.Urban-Klein B., Werth S., Abuharbeid S., Czubayko F., Aigner A. RNAi-mediated gene-targeting through systemic application of polyethylenimine (PEI)-complexed siRNA in vivo. Gene Ther. 2005;12(5):461–466. doi: 10.1038/sj.gt.3302425. [DOI] [PubMed] [Google Scholar]
- 111.Hou L., Song Z., Xu Z., Wu Y., Shi W. Folate-mediated targeted delivery of siPLK1 by leucine-bearing polyethylenimine. Int J Nanomedicine. 2020;15:1397–1408. doi: 10.2147/IJN.S227289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Palmerston Mendes L., Pan J., Torchilin V.P. Dendrimers as nanocarriers for nucleic acid and drug delivery in cancer therapy. Molecules. 2017;22(9):1401. doi: 10.3390/molecules22091401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Abedi-Gaballu F., Dehghan G., Ghaffari M., Yekta R., Abbaspour-Ravasjani S., Baradaran B., et al. PAMAM dendrimers as efficient drug and gene delivery nanosystems for cancer therapy. Appl Mater Today. 2018;12:177–190. doi: 10.1016/j.apmt.2018.05.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Ghaffari M., Dehghan G., Baradaran B., Zarebkohan A., Mansoori B., Soleymani J., et al. Co-delivery of curcumin and Bcl-2 siRNA by PAMAM dendrimers for enhancement of the therapeutic efficacy in HeLa cancer cells. Colloids Surf B Biointerfaces. 2020;188 doi: 10.1016/j.colsurfb.2019.110762. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Abedi Gaballu F., Cho W.C., Dehghan G., Zarebkohan A., Baradaran B., Mansoori B., et al. Silencing of HMGA2 by siRNA loaded methotrexate functionalized polyamidoamine dendrimer for human breast cancer cell therapy. Genes. 2021;12(7):1102. doi: 10.3390/genes12071102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Morales-Becerril A., Aranda-Lara L., Isaac-Olive K., Ocampo-Garcia BE, Morales-Avila E. Nanocarriers for delivery of siRNA as gene silencing mediator. EXCLI J. 2022;21:1028–1052. doi: 10.17179/excli2022-4975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Jansook P., Ogawa N., Loftsson T. Cyclodextrins: structure, physicochemical properties and pharmaceutical applications. Int J Pharm. 2018;535(1–2):272–284. doi: 10.1016/j.ijpharm.2017.11.018. [DOI] [PubMed] [Google Scholar]
- 118.Arima H., Motoyama K. Recent findings concerning PAMAM dendrimer conjugates with cyclodextrins as carriers of DNA and RNA. Sensors. 2009;9(8):6346–6361. doi: 10.3390/s90806346. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Zou Y., Xiao F., Song L., Sun B., Sun D., Chu D., et al. A folate-targeted PEGylated cyclodextrin-based nanoformulation achieves co-delivery of docetaxel and siRNA for colorectal cancer. Int J Pharm. 2021;606 doi: 10.1016/j.ijpharm.2021.120888. [DOI] [PubMed] [Google Scholar]
- 120.Heichler C., Scheibe K., Schmied A., Geppert C.I., Schmid B., Wirtz S., et al. STAT3 activation through IL-6/IL-11 in cancer-associated fibroblasts promotes colorectal tumour development and correlates with poor prognosis. Gut. 2020;69(7):1269–1282. doi: 10.1136/gutjnl-2019-319200. [DOI] [PubMed] [Google Scholar]
- 121.Journal of Cancer. Retraction: IL-6 promotes cancer stemness and oncogenicity in U2OS and MG-63 osteosarcoma cells by upregulating the OPN-STAT3 pathway. J Cancer. 2021;12(23):6948. doi: 10.7150/jca.67290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Agemy L., Friedmann-Morvinski D., Kotamraju V.R., Roth L., Sugahara K.N., Girard O.M., et al. Targeted nanoparticle enhanced proapoptotic peptide as potential therapy for glioblastoma. Proc Natl Acad Sci USA. 2011;108(42):17450–17455. doi: 10.1073/pnas.1114518108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Huo J. Effects of chitosan nanoparticle-mediated BRAF siRNA interference on invasion and metastasis of gastric cancer cells. Artif Cells Nanomed Biotechnol. 2016;44(5):1232–1235. doi: 10.3109/21691401.2015.1019666. [DOI] [PubMed] [Google Scholar]
- 124.Sun P., Huang W., Kang L., Jin M., Fan B., Jin H., et al. siRNA-loaded poly(histidine-arginine)(6)-modified chitosan nanoparticle with enhanced cell-penetrating and endosomal escape capacities for suppressing breast tumor metastasis. Int J Nanomedicine. 2017;12:3221–3234. doi: 10.2147/IJN.S129436. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 125.Zhong J., Huang H.L., Li J., Qian F.C., Li L.Q., Niu P.P., et al. Development of hybrid-type modified chitosan derivative nanoparticles for the intracellular delivery of midkine-siRNA in hepatocellular carcinoma cells. Hepatobiliary Pancreat Dis Int. 2015;14(1):82–89. doi: 10.1016/s1499-3872(15)60336-8. [DOI] [PubMed] [Google Scholar]
- 126.Oliva N., Conde J., Wang K., Artzi N. Designing hydrogels for on-demand therapy. Acc Chem Res. 2017;50(4):669–679. doi: 10.1021/acs.accounts.6b00536. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Liechty W.B., Scheuerle R.L., Vela Ramirez J.E., Peppas N.A. Cytoplasmic delivery of functional siRNA using pH-responsive nanoscale hydrogels. Int J Pharm. 2019;562:249–257. doi: 10.1016/j.ijpharm.2019.03.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Pegtel D.M., Gould SJ. Exosomes. Annu Rev Biochem. 2019;88:487–514. doi: 10.1146/annurev-biochem-013118-111902. [DOI] [PubMed] [Google Scholar]
- 129.Valadi H., Ekstrom K., Bossios A., Sjostrand M., Lee J.J., Lotvall J.O. Exosome-mediated transfer of mRNAs and microRNAs is a novel mechanism of genetic exchange between cells. Nat Cell Biol. 2007;9(6):654–659. doi: 10.1038/ncb1596. [DOI] [PubMed] [Google Scholar]
- 130.Alvarez-Erviti L., Seow Y., Yin H., Betts C., Lakhal S., Wood M.J. Delivery of siRNA to the mouse brain by systemic injection of targeted exosomes. Nat Biotechnol. 2011;29(4):341–345. doi: 10.1038/nbt.1807. [DOI] [PubMed] [Google Scholar]
- 131.Rehman F.U., Liu Y., Yang Q., Yang H., Liu R., Zhang D., et al. Heme oxygenase-1 targeting exosomes for temozolomide resistant glioblastoma synergistic therapy. J Control Release. 2022;345:696–708. doi: 10.1016/j.jconrel.2022.03.036. [DOI] [PubMed] [Google Scholar]
- 132.Chen M., Chen M., He J. Cancer cell membrane cloaking nanoparticles for targeted co-delivery of doxorubicin and PD-L1 siRNA. Artif Cells, Nanomedicine, Biotechnol. 2019;47(1):1635–1641. doi: 10.1080/21691401.2019.1608219. [DOI] [PubMed] [Google Scholar]
- 133.Qiu C., Han H.H., Sun J., Zhang H.T., Wei W., Cui S.H., et al. Regulating intracellular fate of siRNA by endoplasmic reticulum membrane-decorated hybrid nanoplexes. Nat Commun. 2019;10(1):2702. doi: 10.1038/s41467-019-10562-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Chen M., Quan G., Sun Y., Yang D., Pan X., Wu C. Nanoparticles-encapsulated polymeric microneedles for transdermal drug delivery. J Control Release. 2020;325:163–175. doi: 10.1016/j.jconrel.2020.06.039. [DOI] [PubMed] [Google Scholar]
- 135.Ahmed Saeed Al-Japairai K., Mahmood S., Hamed Almurisi S., Reddy Venugopal J., Rebhi Hilles A., Azmana M., et al. Current trends in polymer microneedle for transdermal drug delivery. Int J Pharm. 2020;587 doi: 10.1016/j.ijpharm.2020.119673. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Ruan W., Zhai Y., Yu K., Wu C., Xu Y. Coated microneedles mediated intradermal delivery of octaarginine/BRAF siRNA nanocomplexes for anti-melanoma treatment. Int J Pharm. 2018;553(1–2):298–309. doi: 10.1016/j.ijpharm.2018.10.043. [DOI] [PubMed] [Google Scholar]
- 137.Khalil I.A., Kogure K., Futaki S., Harashima H. Octaarginine-modified liposomes: enhanced cellular uptake and controlled intracellular trafficking. Int J Pharm. 2008;354(1–2):39–48. doi: 10.1016/j.ijpharm.2007.12.003. [DOI] [PubMed] [Google Scholar]
- 138.Jiang Y., Huo S., Hardie J., Liang X.J., Rotello V.M. Progress and perspective of inorganic nanoparticle-based siRNA delivery systems. Expert Opin Drug Deliv. 2016;13(4):547–559. doi: 10.1517/17425247.2016.1134486. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Khan A.K., Rashid R., Murtaza G., Zahra A. Gold nanoparticles: synthesis and applications in drug delivery. Trop J Pharm Res. 2014;13:7. [Google Scholar]
- 140.Veselov V.V., Nosyrev A.E., Jicsinszky L., Alyautdin R.N., Cravotto G. Targeted delivery methods for anticancer drugs. Cancers. 2022;14(3):622. doi: 10.3390/cancers14030622. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.Lu W., Zhang G., Zhang R., Flores L.G., 2nd, Huang Q., Gelovani J.G., et al. Tumor site-specific silencing of NF-kappaB p65 by targeted hollow gold nanosphere-mediated photothermal transfection. Cancer Res. 2010;70(8):3177–3188. doi: 10.1158/0008-5472.CAN-09-3379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.Sun L., Zhang J., Zhou J.E., Wang J., Wang Z., Luo S., et al. Monitoring the in vivo siRNA release from lipid nanoparticles based on the fluorescence resonance energy transfer principle. Asian J Pharm Sci. 2023;18(1) doi: 10.1016/j.ajps.2022.11.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143.Jaleel J.A., Pramod K. Artful and multifaceted applications of carbon dot in biomedicine. J Control Release. 2018;269:302–321. doi: 10.1016/j.jconrel.2017.11.027. [DOI] [PubMed] [Google Scholar]
- 144.Yin F., Hu K., Chen Y., Yu M., Wang D., Wang Q., et al. SiRNA delivery with PEGylated graphene oxide nanosheets for combined photothermal and genetherapy for pancreatic cancer. Theranostics. 2017;7(5):1133–1148. doi: 10.7150/thno.17841. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145.Ku S.H., Kim K., Choi K., Kim S.H., Kwon I.C. Tumor-targeting multifunctional nanoparticles for siRNA delivery: recent advances in cancer therapy. Adv Healthc Mater. 2014;3(8):1182–1193. doi: 10.1002/adhm.201300607. [DOI] [PubMed] [Google Scholar]
- 146.Wang X., Zhu L., Hou X., Wang L., Yin S. Polyethylenimine mediated magnetic nanoparticles for combined intracellular imaging, siRNA delivery and anti-tumor therapy. RSC Adv. 2015;5(123):101569–101581. [Google Scholar]
- 147.Heidari R., Khosravian P., Mirzaei S.A., Elahian F. siRNA delivery using intelligent chitosan-capped mesoporous silica nanoparticles for overcoming multidrug resistance in malignant carcinoma cells. Sci Rep. 2021;11(1):20531. doi: 10.1038/s41598-021-00085-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148.Meka A.K., Niu Y., Karmakar S., Hartono S.B., Zhang J., Lin C.X.C., et al. Facile synthesis of large-pore bicontinuous cubic mesoporous silica nanoparticles for intracellular gene delivery. ChemNanoMat. 2016;2(3):220–225. [Google Scholar]
- 149.He Z.Y., Jin Z.H., Zhan M., Qin Z., Li Z., Xu T. Advances in quantum dot-mediated siRNA delivery. Chin Chem Lett. 2017;28(9):1851–1856. [Google Scholar]
- 150.Lin G., Chen T., Zou J., Wang Y., Wang X., Li J., et al. Quantum dots-siRNA nanoplexes for gene silencing in central nervous system tumor cells. Front Pharmacol. 2017;8:182. doi: 10.3389/fphar.2017.00182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 151.Zhang Y., Guo C., Liu L., Xu J., Jiang H., Li D., et al. ZnO-based multifunctional nanocomposites to inhibit progression and metastasis of melanoma by eliciting antitumor immunity via immunogenic cell death. Theranostics. 2020;10(24):11197–11214. doi: 10.7150/thno.44920. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 152.Zanganeh S., Hutter G., Spitler R., Lenkov O., Mahmoudi M., Shaw A., et al. Iron oxide nanoparticles inhibit tumour growth by inducing pro-inflammatory macrophage polarization in tumour tissues. Nat Nanotechnol. 2016;11(11):986–994. doi: 10.1038/nnano.2016.168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 153.Liu Y., Crawford B.M., Vo-Dinh T. Gold nanoparticles-mediated photothermal therapy and immunotherapy. Immunotherapy. 2018;10(13):1175–1188. doi: 10.2217/imt-2018-0029. [DOI] [PubMed] [Google Scholar]
- 154.Feray A., Guillet E., Szely N., Hullo M., Legrand F.X., Brun E., et al. Synthetic amorphous silica nanoparticles promote human dendritic cell maturation and CD4+ T-lymphocyte activation. Toxicol Sci. 2021;185(1):105–116. doi: 10.1093/toxsci/kfab120. [DOI] [PubMed] [Google Scholar]
- 155.Deng G., Sun Z., Li S., Peng X., Li W., Zhou L., et al. Cell-Membrane immunotherapy based on natural killer cell membrane coated nanoparticles for the effective inhibition of primary and abscopal tumor growth. ACS Nano. 2018;12(12):12096–12108. doi: 10.1021/acsnano.8b05292. [DOI] [PubMed] [Google Scholar]
- 156.Meng X., Lei Y., Zhang X., Sun K., Zhang L., Wang Z. Cancer immunotherapy: classification, therapeutic mechanisms, and nanomaterial-based synergistic therapy. Appl Mater Today. 2021;24 [Google Scholar]
- 157.Cheng J., Wang S., Min Q., Song J., Tian Y. Reconstructed adoptive-macrophages with DNA-tetrahedron-CpG/siRNA for synergistic solid tumor immunotherapy. Colloid Surface A. 2022;637 [Google Scholar]
- 158.Darvin P., Toor S.M., Sasidharan Nair V., Elkord E. Immune checkpoint inhibitors: recent progress and potential biomarkers. Exp Mol Med. 2018;50(12):1–11. doi: 10.1038/s12276-018-0191-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 159.Ngamcherdtrakul W., Reda M., Nelson M.A., Wang R., Zaidan H.Y., Bejan D.S., et al. In situ tumor vaccination with nanoparticle co-delivering CpG and STAT3 siRNA to effectively induce whole-body antitumor immune response. Adv Mater. 2021;33(31) doi: 10.1002/adma.202100628. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 160.Wang X., Xiao X., Feng Y., Li J., Zhang Y. A photoresponsive antibody-siRNA conjugate for activatable immunogene therapy of cancer. Chem Sci. 2022;13(18):5345–5352. doi: 10.1039/d2sc01672a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 161.Kumar S., Calianese D., Birge R.B. Efferocytosis of dying cells differentially modulate immunological outcomes in tumor microenvironment. Immunol Rev. 2017;280(1):149–164. doi: 10.1111/imr.12587. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 162.Hochreiter-Hufford A., Ravichandran K.S. Clearing the dead: apoptotic cell sensing, recognition, engulfment, and digestion. Cold Spring Harb Perspect Biol. 2013;5(1) doi: 10.1101/cshperspect.a008748. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 163.Chen Y., Huang Y., Li Q., Luo Z., Zhang Z., Huang H., et al. Targeting Xkr8 via nanoparticle-mediated in situ co-delivery of siRNA and chemotherapy drugs for cancer immunochemotherapy. Nat Nanotechnol. 2023;18:193–204. doi: 10.1038/s41565-022-01266-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 164.Galluzzi L., Humeau J., Buque A., Zitvogel L., Kroemer G. Immunostimulation with chemotherapy in the era of immune checkpoint inhibitors. Nat Rev Clin Oncol. 2020;17(12):725–741. doi: 10.1038/s41571-020-0413-z. [DOI] [PubMed] [Google Scholar]
- 165.Salas-Benito D., Perez-Gracia J.L., Ponz-Sarvise M., Rodriguez-Ruiz M.E., Martinez-Forero I., Castanon E., et al. Paradigms on immunotherapy combinations with chemotherapy. Cancer Discov. 2021;11(6):1353–1367. doi: 10.1158/2159-8290.CD-20-1312. [DOI] [PubMed] [Google Scholar]
- 166.Liu J., Li Z., Zhao D., Feng X., Wang C., Li D., et al. Immunogenic cell death-inducing chemotherapeutic nanoformulations potentiate combination chemoimmunotherapy. Mater Des. 2021;202 [Google Scholar]
- 167.Cai S., Chen Z., Wang Y., Wang M., Wu J., Tong Y., et al. Reducing PD-L1 expression with a self-assembled nanodrug: an alternative to PD-L1 antibody for enhanced chemo-immunotherapy. Theranostics. 2021;11(4):1970–1981. doi: 10.7150/thno.45777. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 168.Chen S., Li D., Du X., He X., Huang M., Wang Y., et al. Carrier-free nanoassembly of doxorubicin prodrug and siRNA for combinationally inducing immunogenic cell death and reversing immunosuppression. Nano Today. 2020;35 [Google Scholar]
- 169.Tan X., Zhou H., Wang C., Liu X., Yang X., Liu W. GSH-responsive camptothecin prodrug-based hybrid micellar nanoparticles enable antitumor chemo-immunotherapy by PD-L1 knockdown. Nano Res. 2022;16(1):834–848. [Google Scholar]
- 170.Lin L., Pang W., Jiang X., Ding S., Wei X., Gu B. Light amplified oxidative stress in tumor microenvironment by carbonized hemin nanoparticles for boosting photodynamic anticancer therapy. Light Sci Appl. 2022;11(1):47. doi: 10.1038/s41377-021-00704-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 171.Zhang S., Wang J., Kong Z., Sun X., He Z., Sun B., et al. Emerging photodynamic nanotherapeutics for inducing immunogenic cell death and potentiating cancer immunotherapy. Biomaterials. 2022;282 doi: 10.1016/j.biomaterials.2022.121433. [DOI] [PubMed] [Google Scholar]
- 172.Donohoe C., Senge M.O., Arnaut L.G., Gomes-da-Silva L.C. Cell death in photodynamic therapy: from oxidative stress to anti-tumor immunity. Biochim Biophys Acta Rev Cancer. 2019;1872(2) doi: 10.1016/j.bbcan.2019.07.003. [DOI] [PubMed] [Google Scholar]
- 173.Guo Y., Zhang Q., Zhu Q., Gao J., Zhu X., Yu H., et al. Copackaging photosensitizer and PD-L1 siRNA in a nucleic acid nanogel for synergistic cancer photoimmunotherapy. Sci Adv. 2022;8(16):eabn2941. doi: 10.1126/sciadv.abn2941. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 174.Li M., Xu Y., Peng X., Kim J.S. From Low to No O(2)-dependent hypoxia photodynamic therapy (hPDT): a new perspective. Acc Chem Res. 2022;55(22):3253–3264. doi: 10.1021/acs.accounts.2c00531. [DOI] [PubMed] [Google Scholar]
- 175.Li L., Shao C., Liu T., Chao Z., Chen H., Xiao F., et al. An NIR-II-emissive photosensitizer for hypoxia-tolerant photodynamic theranostics. Adv Mater. 2020;32(45) doi: 10.1002/adma.202003471. [DOI] [PubMed] [Google Scholar]
- 176.Zhou J.Y., Wang W.J., Zhang C.Y., Ling Y.Y., Hong X.J., Su Q., et al. Ru(II)-modified TiO(2) nanoparticles for hypoxia-adaptive photo-immunotherapy of oral squamous cell carcinoma. Biomaterials. 2022;289 doi: 10.1016/j.biomaterials.2022.121757. [DOI] [PubMed] [Google Scholar]
- 177.Li K., Lu M., Xia X., Huang Y. Recent advances in photothermal and RNA interfering synergistic therapy. Chin Chem Lett. 2021;32(3):1010–1016. [Google Scholar]
- 178.Zhou S., Li D., Lee C., Xie J. Nanoparticle phototherapy in the era of cancer immunotherapy. Trends Chem. 2020;2(12):1082–1095. doi: 10.1016/j.trechm.2020.09.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 179.Brandacher G., Perathoner A., Ladurner R., Schneeberger S., Obrist P., Winkler C., et al. Prognostic value of indoleamine 2,3-dioxygenase expression in colorectal cancer: effect on tumor-infiltrating T cells. Clin Cancer Res. 2006;12(4):1144–1151. doi: 10.1158/1078-0432.CCR-05-1966. [DOI] [PubMed] [Google Scholar]
- 180.Zhang Y., Feng Y., Huang Y., Wang Y., Qiu L., Liu Y., et al. Tumor-targeted gene silencing IDO synergizes PTT-induced apoptosis and enhances anti-tumor immunity. Front Immunol. 2020;11:968. doi: 10.3389/fimmu.2020.00968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 181.Aleku M., Schulz P., Keil O., Santel A., Schaeper U., Dieckhoff B., et al. Atu027, a liposomal small interfering RNA formulation targeting protein kinase N3, inhibits cancer progression. Cancer Res. 2008;68(23):9788–9798. doi: 10.1158/0008-5472.CAN-08-2428. [DOI] [PubMed] [Google Scholar]
- 182.Huang C., Chen Z., He Y., He Z., Ban Z., Zhu Y., et al. EphA2 promotes tumorigenicity of cervical cancer by up-regulating CDK6. J Cell Mol Med. 2021;25(6):2967–2975. doi: 10.1111/jcmm.16337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 183.Wagner M.J., Mitra R., McArthur M.J., Baze W., Barnhart K., Wu S.Y., et al. Preclinical mammalian safety studies of EPHARNA (DOPC nanoliposomal EphA2-targeted siRNA) Mol Cancer Ther. 2017;16(6):1114–1123. doi: 10.1158/1535-7163.MCT-16-0541. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 184.Golan T., Khvalevsky E.Z., Hubert A., Gabai R.M., Hen N., Segal A., et al. RNAi therapy targeting KRAS in combination with chemotherapy for locally advanced pancreatic cancer patients. Oncotarget. 2015;6(27):24560–24570. doi: 10.18632/oncotarget.4183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 185.Liu P., Wang Y., Li X. Targeting the untargetable KRAS in cancer therapy. Acta Pharm Sin B. 2019;9(5):871–879. doi: 10.1016/j.apsb.2019.03.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 186.Hattori Y., Kikuchi T., Nakamura M., Ozaki K.I., Onishi H. Therapeutic effects of protein kinase N3 small interfering RNA and doxorubicin combination therapy on liver and lung metastases. Oncol Lett. 2017;14(5):5157–5166. doi: 10.3892/ol.2017.6830. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 187.Schultheis B., Strumberg D., Santel A., Vank C., Gebhardt F., Keil O., et al. First-in-human phase I study of the liposomal RNA interference therapeutic Atu027 in patients with advanced solid tumors. J Clin Oncol. 2014;32(36):4141–4148. doi: 10.1200/JCO.2013.55.0376. [DOI] [PubMed] [Google Scholar]
- 188.Foster J.M., Zhang C., Rehman S., Sharma P., HR Alexander. The contemporary management of peritoneal metastasis: a journey from the cold past of treatment futility to a warm present and a bright future. CA Cancer J Clin. 2023;73(1):49–71. doi: 10.3322/caac.21749. [DOI] [PubMed] [Google Scholar]
- 189.Kamdar M., Solomon S.R., Arnason J., Johnston P.B., Glass B., Bachanova V., et al. Lisocabtagene maraleucel versus standard of care with salvage chemotherapy followed by autologous stem cell transplantation as second-line treatment in patients with relapsed or refractory large B-cell lymphoma (TRANSFORM): results from an interim analysis of an open-label, randomised, phase 3 trial. Lancet. 2022;399(10343):2294–2308. doi: 10.1016/S0140-6736(22)00662-6. [DOI] [PubMed] [Google Scholar]
- 190.Liu Z., Parveen N., Rehman U., Aziz A., Sheikh A., Abourehab M.A.S., et al. Unravelling the enigma of siRNA and aptamer mediated therapies against pancreatic cancer. Mol Cancer. 2023;22(1):8. doi: 10.1186/s12943-022-01696-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 191.Aimo A., Castiglione V., Rapezzi C., Franzini M., Panichella G., Vergaro G., et al. RNA-targeting and gene editing therapies for transthyretin amyloidosis. Nat Rev Cardiol. 2022;19(10):655–667. doi: 10.1038/s41569-022-00683-z. [DOI] [PubMed] [Google Scholar]
- 192.Kulkarni J.A., Witzigmann D., Chen S., Cullis P.R., van der Meel R. Lipid nanoparticle technology for clinical translation of siRNA therapeutics. Acc Chem Res. 2019;52(9):2435–2444. doi: 10.1021/acs.accounts.9b00368. [DOI] [PubMed] [Google Scholar]
- 193.Rong Y., Wang Z., Tang P., Wang J., Ji C., Chang J., et al. Engineered extracellular vesicles for delivery of siRNA promoting targeted repair of traumatic spinal cord injury. Bioact Mater. 2023;23:328–342. doi: 10.1016/j.bioactmat.2022.11.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 194.Wang J., Chen G., Liu N., Han X., Zhao F., Zhang L., et al. Strategies for improving the safety and RNAi efficacy of noncovalent peptide/siRNA nanocomplexes. Adv Colloid Interface Sci. 2022;302 doi: 10.1016/j.cis.2022.102638. [DOI] [PubMed] [Google Scholar]
- 195.Takashima A., English B., Chen Z., Cao J., Cui R., Williams R.M., et al. Protein kinase Cδ is a therapeutic target in malignant melanoma with NRAS mutation. ACS Chem Biol. 2014;9(4):1003–1014. doi: 10.1021/cb400837t. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 196.Ha S.H., Kang S.K., Choi H., Kwak C.H., Abekura F., Park J.Y., et al. Induction of GD3/alpha1-adrenergic receptor/transglutaminase 2-mediated erythroid differentiation in chronic myelogenous leukemic K562 cells. Oncotarget. 2017;8(42):72205–72219. doi: 10.18632/oncotarget.20080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 197.Zhou X., Yu M., Ma L., Fu J., Guo J., Lei J., et al. In vivo self-assembled siRNA as a modality for combination therapy of ulcerative colitis. Nat Commun. 2022;13(1):5700. doi: 10.1038/s41467-022-33436-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 198.Hossen M.N., Wang L., Chinthalapally H.R., Robertson J.D., Fung K.M., Wilhelm S., et al. Switching the intracellular pathway and enhancing the therapeutic efficacy of small interfering RNA by auroliposome. Sci Adv. 2020;6(30):eaba5379. doi: 10.1126/sciadv.aba5379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 199.Li L., Hou J., Liu X., Guo Y., Wu Y., Zhang L., et al. Nucleolin-targeting liposomes guided by aptamer AS1411 for the delivery of siRNA for the treatment of malignant melanomas. Biomaterials. 2014;35(12):3840–3850. doi: 10.1016/j.biomaterials.2014.01.019. [DOI] [PubMed] [Google Scholar]
- 200.Fehring V., Schaeper U., Ahrens K., Santel A., Keil O., Eisermann M., et al. Delivery of therapeutic siRNA to the lung endothelium via novel Lipoplex formulation DACC. Mol Ther. 2014;22(4):811–820. doi: 10.1038/mt.2013.291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 201.Vivas-Mejia P., Benito J.M., Fernandez A., Han H.D., Mangala L., Rodriguez-Aguayo C., et al. c-Jun-NH2-kinase-1 inhibition leads to antitumor activity in ovarian cancer. Clin Cancer Res. 2010;16(1):184–194. doi: 10.1158/1078-0432.CCR-09-1180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 202.Li M., Li S., Li Y., Li X., Yang G., Li M., et al. Cationic liposomes co-deliver chemotherapeutics and siRNA for the treatment of breast cancer. Eur J Med Chem. 2022;233 doi: 10.1016/j.ejmech.2022.114198. [DOI] [PubMed] [Google Scholar]
- 203.Khabazian E., Vakhshiteh F., Norouzi P., Fatahi Y., Dinarvand R., Atyabi F. Cationic liposome decorated with cyclic RGD peptide for targeted delivery of anti-STAT3 siRNA to melanoma cancer cells. J Drug Target. 2022;30(5):522–533. doi: 10.1080/1061186X.2021.1973481. [DOI] [PubMed] [Google Scholar]
- 204.Mahinfar P., Mokhtarzadeh A., Baradaran B., Siasi Torbati .E.. Antiproliferative activity of CD44 siRNA-PEI-PEG nanoparticles in glioblastoma: involvement of AKT signaling. Res Pharm Sci. 2022;17(1):78–85. doi: 10.4103/1735-5362.329928. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 205.Zhang X., Qin B., Wang M., Feng J., Zhang C., Zhu C., et al. Dual pH-responsive and tumor-targeted nanoparticle-mediated anti-angiogenesis siRNA delivery for tumor treatment. Int J Nanomedicine. 2022;17:953–967. doi: 10.2147/IJN.S340926. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 206.Han H.D., Mangala L.S., Lee J.W., Shahzad M.M., Kim H.S., Shen D., et al. Targeted gene silencing using RGD-labeled chitosan nanoparticles. Clin Cancer Res. 2010;16(15):3910–3922. doi: 10.1158/1078-0432.CCR-10-0005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 207.Yu A.Y.H., Fu R.H., Hsu S.H., Chiu C.F., Fang W.H., Yeh C.A., et al. Epidermal growth factor receptors siRNA-conjugated collagen modified gold nanoparticles for targeted imaging and therapy of lung cancer. Mater Today Adv. 2021;12 [Google Scholar]
- 208.Rahme K., Guo J., Holmes J.D. Bioconjugated gold nanoparticles enhance siRNA delivery in prostate cancer cells. Methods Mol Biol. 2019;1974:291–301. doi: 10.1007/978-1-4939-9220-1_21. [DOI] [PubMed] [Google Scholar]
- 209.Zhang Q., Zhao W., Cheng J., Deng Z., Zhang P., Zhang A., et al. Heat-induced manganese-doped magnetic nanocarriers combined with Yap-siRNA for MRI/NIR-guided mild photothermal and gene therapy of hepatocellular carcinoma. Chem Eng J. 2021;426 [Google Scholar]
- 210.Chen S., Zhang S., Wang Y., Yang X., Yang H., Cui C. Anti-EpCAM functionalized graphene oxide vector for tumor targeted siRNA delivery and cancer therapy. Asian J Pharm Sci. 2021;16(5):598–611. doi: 10.1016/j.ajps.2021.04.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 211.Yin W., Qian S.M. CD44v6-O-MWNTS-Loaded Gemcitabine and CXCR4 siRNA improves the anti-tumor effectiveness of ovarian cancer. Front Cell Dev Biol. 2021;9 doi: 10.3389/fcell.2021.687322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 212.Shakeran Z., Varshosaz J., Keyhanfar M., Mohammad-Beigi H., Rahimi K., Sutherland D.S. Co-delivery of STAT3 siRNA and methotrexate in breast cancer cells. Artif Cells Nanomed Biotechnol. 2022;50(1):29–39. doi: 10.1080/21691401.2022.2030746. [DOI] [PubMed] [Google Scholar]
- 213.Krishn S.R., Garcia V., Naranjo N.M., Quaglia F., Shields C.D., Harris M.A., et al. Small extracellular vesicle-mediated ITGB6 siRNA delivery downregulates the alphaVbeta6 integrin and inhibits adhesion and migration of recipient prostate cancer cells. Cancer Biol Ther. 2022;23(1):173–185. doi: 10.1080/15384047.2022.2030622. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 214.Kamerkar S., LeBleu V.S., Sugimoto H., Yang S., Ruivo C.F., Melo S.A., et al. Exosomes facilitate therapeutic targeting of oncogenic KRAS in pancreatic cancer. Nature. 2017;546(7659):498–503. doi: 10.1038/nature22341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 215.Zhuang J., Gong H., Zhou J., Zhang Q., Gao W., Fang R.H., et al. Targeted gene silencing in vivo by platelet membrane-coated metal-organic framework nanoparticles. Sci Adv. 2020;6(13):eaaz6108. doi: 10.1126/sciadv.aaz6108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 216.Zhao B., Zhou B., Shi K., Zhang R., Dong C., Xie D., et al. Sustained and targeted delivery of siRNA/DP7-C nanoparticles from injectable thermosensitive hydrogel for hepatocellular carcinoma therapy. Cancer Sci. 2021;112(6):2481–2492. doi: 10.1111/cas.14903. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 217.Peng H., Yang H., Song L., Zhou Z., Sun J., Du Y., et al. Sustained delivery of siRNA/PEI complex from in situ forming hydrogels potently inhibits the proliferation of gastric cancer. J Exp Clin Cancer Res. 2016;35:57. doi: 10.1186/s13046-016-0334-y. [DOI] [PMC free article] [PubMed] [Google Scholar]