Abstract
Salmonella spp. is a prevalent pathogen that causes great public health concern worldwide. Bacteriophage-based cocktails have arisen as an alternative to antibiotics to inhibit the growth of Salmonella. However, the bactericidal effect of bacteriophage cocktails in vivo largely differs from their observed effect in vitro. This is partly because in vitro developments of cocktails do not always consider the bacterial diversity nor the environmental conditions where bacteriophages will have to replicate. Here, we isolated and sequenced 47 bacteriophages that showed variable degrees of lytic activity against 258 Salmonella isolates from a commercial broiler company in Brazil. Three of these bacteriophages were characterized and selected to assemble a cocktail. In vitro quantitative assays determined the cocktail to be highly effective against multiple serovars of Salmonella, including Minnesota and Heidelberg. Remarkably, the in vitro lytic activity of the cocktail was retained or improved in conditions that more closely resembled the chicken gut, such as anaerobiosis, 42°C, and Salmonella mono-strain biofilms. Analysis of bacterial cross-resistance between the 3 bacteriophages composing the cocktail revealed limited or no generation of cross-resistance. Our results highlight the relevance of an optimized flux of work to develop bacteriophage cocktails against Salmonella with high lytic efficacy and strong potential to be applied in vivo in commercial broiler farms.
Key words: Salmonella, bacteriophage, cocktail, poultry
INTRODUCTION
Salmonella is one of the major foodborne pathogens that can cause a variety of illnesses ranging from self-limiting gastroenteritis to severe systemic infection and death in humans and animals. Outbreaks of foodborne diseases associated to Salmonella are estimated to cause 8.76 million cases annually (Kirk et al., 2015), of which 535,000 can end up in salmonellosis and more than 77,000 in deaths worldwide (Stanaway et al., 2019; Ikuta et al., 2022). The genus Salmonella comprises 2 species: S. enterica and S. bongori. Salmonella enterica includes at least 2,600 serovars and 6 subspecies: enterica, salamae, arizonae, diarizonae, houtenae, and indica (Popoff et al., 2004; Ferrari et al., 2019). There are specific serovars restricted to a very narrow range of hosts, such as S. Typhi and S. Gallinarum in humans and poultry, respectively. Conversely, generalist serovars, including nontyphoidal Salmonella such as S. Typhimurium and S. Enteritidis are able to infect and colonize any warm-blooded animal (Gut et al., 2018). Recently, the overall incidence per 100,000 population associated with Salmonella infections was 17.1% in the United States, being the 6 most common serovars: S. Enteritidis (2.6%); S. Newport (1.4%); S. Typhimurium (1.3%); S. Javiana (1.1%); monophasic S. Typhimurium (1,4,[5],12:i:) (0.7%) and S. Infantis (0.5%) (Tack et al., 2020).
Salmonella spends an important part of its life cycle in the environment, ready to spread and colonize different niches. The major route of infection is through fecal-oral transmission, and a variety of food matrices have been reported as sources and vehicles of Salmonella spp. including eggs, poultry meat, pork, beef, dairy products, nuts, and vegetables. The most frequent meat involved in salmonellosis has been associated with poultry (chicken and turkeys) (Pui et al., 2011; EFSA and ECDC, 2022). These animals can acquire a variety of Salmonella strains through vertical and horizontal transmission, causing symptoms in the gastrointestinal tract or chronic asymptomatic infections. When young infected birds survive to clinical disease, they may become carriers of Salmonella in the adult stage. Infected ovaries, oviducts, and eggs can spread the bacteria to the environment due to the excretion of contaminated feces (Kabir, 2010). In recent years, a high prevalence of serovar S. Infantis (80%) was found in samples of chicken meat and carcasses, followed by S. Kentucky (10%) and S. Agona (5%) (Castello et al., 2023). According to an EFSA report, the highest prevalence of Salmonella in isolates from laying hens samples corresponds to S. Enteritidis (35.5%) and S. Typhimurium (9.4%). In broiler chickens, the most prevalent reported serovars were S. Infantis, S. Enteritidis, S. Livingstone and S. Mbandaka (EFSA and ECDC, 2022). These serovars and others in different stages of poultry production are a public health concern because they increase the risk of acquired salmonellosis in poultry industry workers and consumers.
Currently, antimicrobials are not used as a primary tool to control Salmonella in poultry in Europe (Official Journal of the European Union, 2006). Control programs have been developed to maintain or decrease the prevalence of Salmonella in productive systems, relying on the principles of good manufacturing practices (EFSA and ECDC, 2019). In a few particular cases outside the EU, some antibiotics may be used to control salmonellosis, such as chloramphenicol, neomycin, polymyxin-B, nitrofurazone, amoxicillin, and tetracycline, but their use must be informed and authorized (Tariq et al., 2022). On the other hand, US regulations in the use of antimicrobials in poultry are transitioning towards standardized management practices for preventing and controlling infectious diseases (McEwen and Fedorka‐Cray, 2002; Wallinga et al., 2022). Currently, the FDA approves the use of antimicrobials for disease prevention and control only under veterinary supervision (US Food and Drug Administration, 2021), causing such practices to decrease significantly. Furthermore, in 2019 nearly 60% of US broiler chickens were raised without antimicrobials (Wallinga et al., 2022). Even though the use of antibiotics to control Salmonella is restricted, there have been reports of S. Heidelberg, S. Kentucky, S. Typhimurium, and S. Minnesota with high levels of antibiotic resistance and multidrug resistance to many clinically relevant drugs (Venkitanarayanan et al., 2019; Castro-Vargas et al., 2020). In this scenario, the poultry industry has been looking for innovative feeding and nonfeeding-based solutions to manage Salmonella's prevalence in the chicken production chain. In recent years, high interest has arisen in research and potential applications of bacteriophages in both the poultry veterinary medicine and industry for their ability to control enteropathogens, their safety, and high host specificity. Bacteriophage cocktails have been shown to inhibit the growth of Salmonella in vitro (Nale et al., 2021; Kosznik-Kwaśnicka et al., 2022) and in vivo (Toro et al., 2005; Borie et al., 2008; Grant et al., 2017; Clavijo et al., 2019; Wójcik et al., 2020). However, development of optimal bacteriophage cocktails may be challenged by the high diversity of Salmonella serotypes present in the farms.
Here, we aimed to develop a bacteriophage cocktail with high lytic activity against a large and diverse collection of Salmonella isolates. The in vitro activity of the cocktail was tested in different conditions that resemble more closely the chicken gut, such as low oxygen, 42°C and Salmonella mono-strain biofilms. We provide a workflow for development of bacteriophage cocktails that addresses many relevant factors that may be encountered in vivo.
MATERIALS AND METHODS
Isolation of Salmonella
Salmonella samples were obtained from several farms of 11 municipalities of Paraná, Brazil, between January and March of 2021. The samples belong to the historic collection of Salmonella from the company. The details of how many farms, barns and flocks were sampled is confidential information of the company and therefore not available. The isolation of Salmonella from shoe covers was performed on d 28 of the chicken cycle. Shoe covers were transported using a triple packaging system at RT from the farms to the laboratory. Salmonella was isolated from surgical shoe covers following the ISO 6579-1:2017 protocol for the isolation and identification of Salmonella.
Isolation of Bacteriophages
Salmonella strains grouped in bacterial pools were incubated with waters from sewages or water treatment plants mainly located in Santiago, Chile. Incubation was performed in Tryptic Soy Broth (TSB) at 37 °C in agitation overnight to obtain an enrichment of viral particles (EVP). EVPs were then purified using 10% chloroform and passing the supernatant through 0.45 µm syringe filters. Then, EVPs were individually used to evaluate the bactericidal activity against bacterial strains using double layer agar assays, as described (Carlson, 2005; Kropinski et al., 2009). Each EVP with antimicrobial activity was used as inocula to isolate lytic phages, using 10-fold serial dilutions in SM buffer. The plaque forming units (PFU) obtained were isolated 3 consecutive times in their respective hosts on double layer agar plates at 37°C. Once this process was finished, a solid propagation was carried out on Tryptic Soy Agar (TSA) plates to obtain a pure bacteriophage stock.
Double Layer Agar Assays
Bacterial cultures were grown overnight at 37°C in TSB in agitation. 600 µL of overnight culture was added to 6 mL of soft TSA (0.45% agar), poured over plates with TSA (1.5% agar), and solidified at room temperature. PFU (titration) were quantified by spotting 10-fold serial dilutions of bacteriophage on the solidified plates. Plates were incubated overnight at 37°C. For spot assays, bacteriophages were adjusted to 1E+08 PFU/mL, and 5 µL were spotted on the solidified top-agar, and the plates were incubated overnight at 37°C. A qualitative lytic score was defined from the observed lysis zones from 0 (no plaque) to 4 (clear lysis).
Sequencing and Bioinformatic Analysis
DNA was extracted from concentrated bacteriophage stocks (>1E+08 PFU/mL) using the Phage DNA isolation kit (Norgen) following manufacturer's instructions. Libraries were prepared using Nextera XT DNA library preparation kit (Illumina, San Diego, California), and sequencing was performed in a NextSeq 550 (Illumina, San Diego, California). Genomic analyses were performed using a custom bacteriophage workflow pipeline developed at PhageLab SpA. The pipeline includes an initial step of quality control analysis of the raw FASTQ reads, where the sequence quality was assessed, adapters were trimmed, and low-quality reads were discarded to ensure data integrity and reliability. High-quality reads were utilized for de novo genome assembly, enabling the reconstruction of complete or near-complete genomes. Assembled genomes were evaluated to check for completeness and contamination levels. The resulting high-quality genomes were then subjected to gene annotation and the identification of potential virulence factors and antibiotic resistance genes.
Physicochemical Characterization of Bacteriophages
Bacteriophage aliquots were adjusted to 1E+08 PFU/mL. To evaluate bacteriophage tolerance to pH, each bacteriophage was mixed with NaCl 0.9% adjusted to several pHs, between 2 and 10. Each mix was incubated for 4 h at 37°C before being neutralized by the addition of NaHCO3 0.07 M (pH 7). For assessment of bacteriophage temperature tolerance, each bacteriophage was mixed with NaCl 0.9% pH 7 and exposed for 4 h to a range of temperatures from -80 to 70°C. Exposition was ended by the addition of NaCl 0.9% pH 7 at 4°C. After neutralization, antimicrobial activity was evaluated by double layer agar titration.
Transmission Electron Microscopy
A 20 μL sample of each purified bacteriophage (with titers ≥ 5E+09 PFU/mL) was applied to a carbon coated copper grid for 1 min, then negatively stained with uranyl acetate 2% (m/v) and dried at 60°C for 20 min. Images were acquired at a magnification of 92,000× in a Thermo Scientific Talos F200C G2 transmission electron microscope (TEM) with Ceta 16M CMOS camera and C-twin lens. Viruses were analyzed and measured from the images with Fiji (Schindelin et al., 2012) and ImageJ softwares (Schneider et al., 2012). Each bacteriophage's mean and standard deviation were calculated with n = 10 individual particles. Parameters calculated were full length of the bacteriophage (from the top of the head to base plate), head length (from the top of the head to bottom of the head), and tail length (from the bottom of the head to base plate).
Quantitative Assays
Quantitative host range assay was performed in a 96-well plate as described by Xie et al., (2018), with some modifications. Briefly, overnight cultures of bacteria were refreshed and grown until ∼1E+08 CFU/mL (OD600∼0.1, as determined previously) in TSB at 37°C and agitation in a microplate reader EPOCH2 (Agilent Technologies, Santa Clara, California). Then, the culture was diluted to 1E+06 CFU/mL in TSB and mixed with bacteriophage at 2E+08 PFU/mL for a final MOI input of 20. Likewise, for the cocktail, each bacteriophage was adjusted to a final MOI input of 20. The microtiter plate was incubated in EPOCH2 at 37°C or 42°C at maximum agitation. OD600 was measured every 10 min for a total of 18 h. Growth inhibition was calculated as the percentage of reduction of the treatment compared to the area under the curve of the untreated bacteria. The parameter Breadth of a cocktail was considered to select the best combination of bacteriophages (Abedon et al., 2021). Breadth was defined as the fraction of bacteria hit by at least 2 (breadth2) or 3 (breadth3) phages from the cocktail.
Anaerobic Assays
Anaerobic quantitative host range assays were performed in 96 well microtiter plates using an anaerobic system, as described by Eini et al. (2013) with some modifications. Briefly, 16 wells of the 96 well plate were filled with the anaerobic generating substance from a GENbox anaer (bioMerieux, Marcy-l'Étoile, France), and then the plate was sealed with solid petroleum jelly injected from a syringe between the lid and the microplate to ensure gas-tightness. Correct oxygen elimination and maintenance of anaerobic conditions were monitored by visual confirmation of the colorimetric shift of Anaer indicators (bioMerieux, Marcy-l'Étoile, France) at the end of the experiment. Bacterial growth inhibition was calculated as described above.
Cross-Resistance Assays
A single susceptible bacteria to the 3 bacteriophages of the INSPEKTOR cocktail was used as a model wild type (WT) bacteria. A process of isolation of individual phage-resistant mutants was performed. Overnight WT bacteria culture was refreshed by a 1/25 dilution and grown until ∼1E+08 CFU/mL (OD600∼0.1) in TSB at 37°C and agitation. Then, the refreshed culture was diluted 1/20 to 1E+06 CFU/mL. Each bacteriophage was mixed individually with the bacterial culture for a final MOI of 20 and incubated overnight at 37°C and agitation. The surviving bacteria were plated on TSB agar plates (1.5 % w/v agar) and incubated overnight at 37°C. Five different colonies were selected and plated on new TSB agar plates for each bacteriophage and incubated overnight at 37°C. Plating these selected colonies in fresh TSB agar plates was repeated twice. Efficiency of plating (EOP) between individual phage-resistant mutants and WT bacteria was compared by double layer agar titration (Kutter, 2009).
Biofilm Assays
Biofilm reduction assays were performed as described by Korzeniowski et al. (2022) with some modifications. Overnight cultures of Salmonella strains were incubated at 37°C without agitation. Cultures were then diluted to match optical density (OD600 nm) 0.2, and 150 µL of the suspension was transferred to each well of a 96-well flat-bottomed polystyrene microplate (Falcon, TC-treated). Plates were incubated for 48 h at 37°C without agitation. After incubation, to remove planktonic cells, wells were washed twice with sterile phosphate saline buffer (PBS) 1X. To evaluate biofilm degradation, a volume of 150 µL of the cocktail was added to each well at final titers of 2E+07 PFU/mL. Plates were then incubated at 37°C for 4 h, rinsed with PBS twice, and air-dried (15-20 min). The remaining biofilms were stained with 0.5% crystal violet (CV) for 20 min, followed by 2 washes with PBS. Finally, CV was solubilized using 100% ethanol, and 100 µL of each well was transferred to a new plate for quantification. The absorbance of the soluble CV was measured using an automated EPOCH plate reader at 570 nm. Biofilms not incubated with the cocktail and biofilms incubated with Trypsin-EDTA solution (0.25% Trypsin, 2.21 mM EDTA, 1X [-] sodium bicarbonate) were used as negative treatment and positive control, respectively. The biofilm production ability among different Salmonella strains was classified according to Stepanovic et al., (2004). Briefly, a cut-off O.D. (O.D.c) was defined as 3 standard deviations above the mean O.D. of the negative control. Strains were classified as follows: O.D. ≤ O.D.c = no biofilm producer, O.D.c < O.D. ≤ (2 × O.D.c) = weak biofilm producer, (2 × O.D.c) < O.D. ≤ (4 × O.D.c) = moderate biofilm producer and (4 × O.D.c) < O.D. = strong biofilm producer. All tests were carried out in quintuplicate, and the results were averaged.
Statistical Analysis
Data was analyzed using R. Physicochemical experiments were performed in triplicate and were analyzed with ANOVA and Dunnett post-test (reference group pH 7.0 or 5°C). Bacteriophages and cocktails efficiency were performed in triplicate. Experiments to evaluate the effect of temperature, respiration, and biofilm were performed in quintuplicate and analyzed with mixed-models (lme4 package) using variable intercept and slope for each bacteria.
RESULTS
Isolation of Lytic Bacteriophages
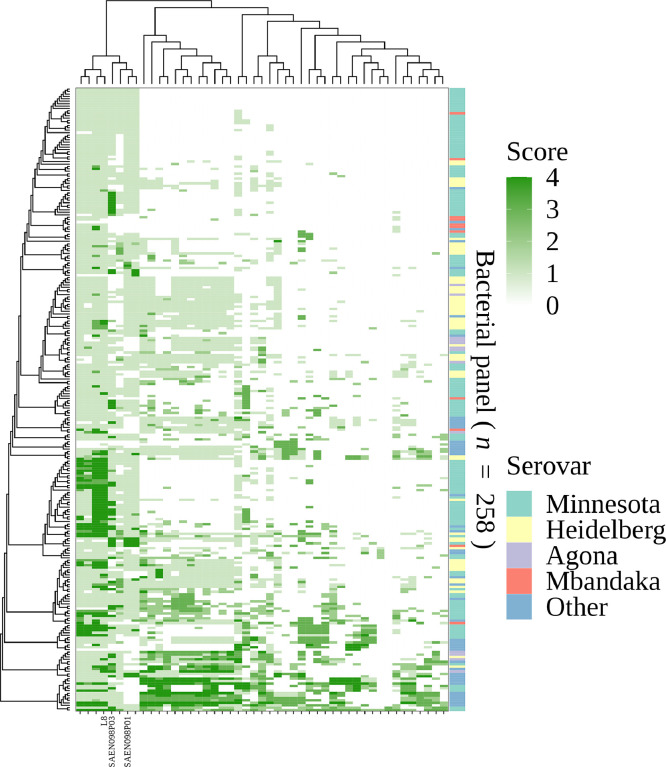
A group of 47 bacteriophages was isolated from water samples of diverse sources. The bacteriophages were sequenced, their genomes characterized using bioinformatic algorithms, and determined to present lytic lifestyles. To determine the lytic potential of these bacteriophages over field-isolated Salmonella, a collection of 258 isolates from a commercial broiler company in Brazil was obtained. Bacteriophages were individually tested using a double layer spot agar qualitative assay, resulting in a matrix of 12,079 interactions (Figure 1). In general, some bacteriophages showed wide host ranges (left half of the matrix), while others displayed lytic activity only against a few isolates (right half of the matrix). Of interest, several bacteriophages had activity against most isolates from the most prevalent serovars of the collection: Minnesota (n = 133), Heidelberg (n = 56), Agona (n = 13), and Mbandaka (n = 11) (Figure 1). From these results, 3 bacteriophages, named L8, SAEN098P01, and SAEN098P03, were selected based on their wide host ranges and complementary lytic activities.
Figure 1.
Heatmap of activity of 47 bacteriophages (horizontal axis) against 258 Salmonella spp. strains (vertical axis) based on double-layer agar test. Lytic score values are shown as a gradient of green. Bacteria and bacteriophages were clustered based on the distance of the score matrix. The top and the left trees show the relationship based on lytic score between phages and bacteria, respectively. The determined serovar of each strain is represented in the right bar. Serovars with less than 7 strains were condensed as “Other.”
Bacteriophages L8 and SAEN098P03 have circular double stranded DNA genomes, while SAEN098P01 has a linear double stranded DNA genome (Table 1). The bacteriophages differed in their genome sizes ranging from 52 Kb (SAEN098P03) to 87 Kb (L8) and up to 158 Kb (SAEN098P01). A taxonomic analysis assigned the 3 bacteriophages to different viral families. Bacteriophages L8, SAEN098P01, and SAEN098P03 were predicted not to carry integrases or antimicrobial resistance genes, and therefore to be suitable for phage therapy (Table 1).
Table 1.
Genomic characteristics of bacteriophages.
| Name | L8 | SAEN098P01 | SAEN098P03 |
|---|---|---|---|
| Genome size (bp) | 87,467 | 158,407 | 52,474 |
| GC content | 38.92% | 44.54% | 45.98% |
| Number of ORFs | 149 | 234 | 86 |
| Number of tRNAs | 20 | 6 | 0 |
| Taxonomy | Autographiviridae | Ackermannviridae | Mesyanzhinovviridae |
| Closest NCBI ID | MZ327261.1 | MW355478.1 | MT074436.1 |
| Antibiotic resistance | Absent | Absent | Absent |
| Integrases | Absent | Absent | Absent |
| Lifestyle | Lytic | Lytic | Lytic |
Physical Properties of Bacteriophages
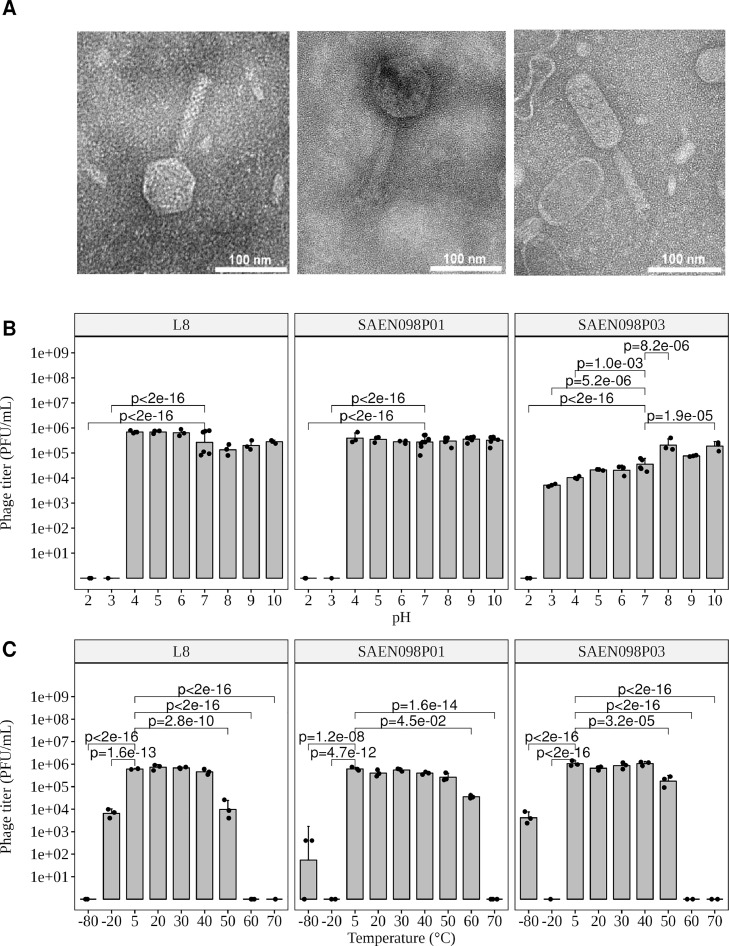
Transmission electron microscopy (TEM) was performed to characterize the selected bacteriophages further. L8, SAEN098P01, and SAEN098P03 bacteriophages are shown in Figure 2A left, middle and right, respectively. The full-length size of L8 is 186.77 ± 5.82 nm, phage head, and tail have a length of 73.67 ± 3.73 nm and 113.10 ± 5.27 nm, respectively; SAEN098P01 had a full size of 198.37 ± 9.99 nm, phage head length of 81.61 ± 4.00 nm and a tail length of 116.76 ± 9.30 nm; finally, the full size of SAEN098P03 was 186.60 ± 5.72 nm, phage head length of 99.13 ± 6.11 nm and tail length of 87.46 ± 1.94 nm.
Figure 2.
Physicochemical characterization of bacteriophages. (Left) bacteriophage L8, (Middle) SAEN098P01 and (Right) SAEN098P03. (A) Transmission electron microscopy (TEM) of bacteriophages. (B) pH stability. Reference group was pH 7. (C) Temperature stability. Reference group was 5°C. ANOVA with the Dunnett test was performed to obtain P-values. Bars represent mean and standard deviation.
To characterize the stability of bacteriophages under different pH and temperature conditions, bacteriophages were exposed individually for 4 h to each condition. In general, bacteriophages L8, SAEN098P01 did not lose titer between pHs 4 and 10 (Figure 2B). Bacteriophage SAEN098P03 lost activity below pH 4 but an increase in PFU/mL was detected at pHs 8 and 10. When exposed to different temperatures, bacteriophages L8, SAEN098P01, and SAEN098P03 retained infectivity in the range between 5 and 40°C (Figure 2C). Temperatures above 50°C or below -20°C reduced the titers for all 3 bacteriophages.
Quantitative Assays Against a Collection of Field Isolates
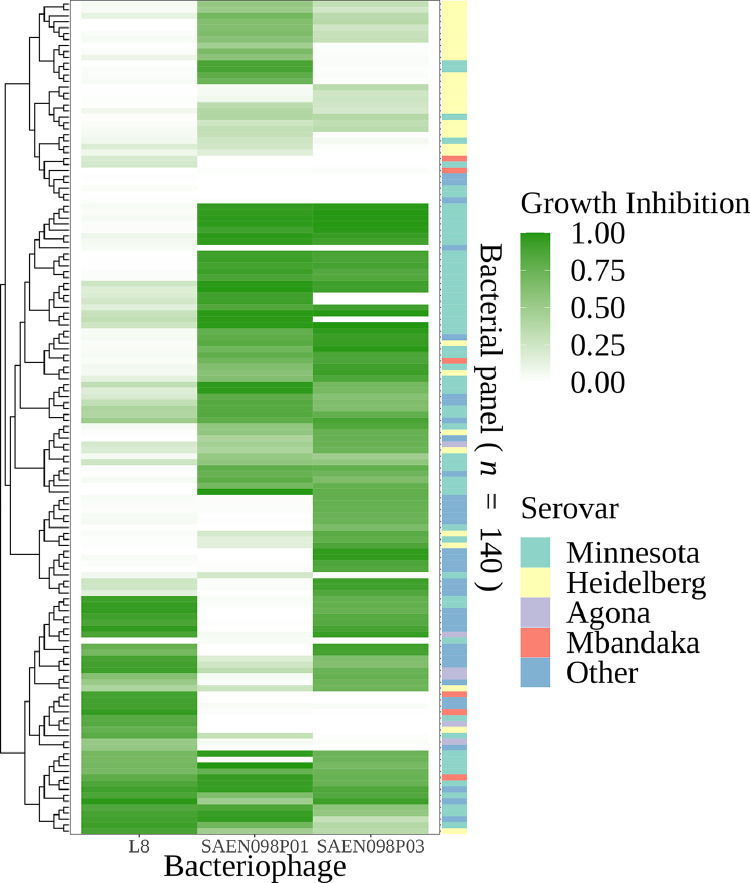
Since the screening of bacteriophage lytic activity by double layer spot agar does not permit controlling MOI, a second quantitative method was additionally used. In this method, a subset of 140 bacteria was chosen based on a qualitative score of hierarchical clustering. Then, the dendrogram was cut into 140 clusters to match the quantitative capability. The 3 bacteriophages were individually co-cultured with each isolate in 96 well plates at an MOI input of 20, and bacterial growth was followed for 6 h at OD600 nm. The initial load of bacteria for the experiments was set up at 1E+06 CFU/mL, which we previously determined to be an average load of Salmonella Infantis in the chicken cecum (data not shown). A pattern of bacterial growth inhibition that corroborated the results of lytic activity by double-layer agar was observed (Figure 3). In most cases, the growth of isolates from the most relevant serovars was inhibited by at least 2 of the 3 bacteriophages. The result highlights the lytic potential of individual bacteriophages L8, SAEN098P01, and SAEN098P03 and their combined use as a cocktail against Salmonella isolates (Figure 3).
Figure 3.
Heatmap of activity of 3 bacteriophages (horizontal axis) against 140 Salmonella spp. strains (vertical axis) based on co-culture assay. Growth inhibition values are shown as a gradient of green. Bacteria and bacteriophages were clustered based on the distance of the growth inhibition. The left tree shows the relationship based on growth inhibition between bacteria. Most abundant serovar of each strain is represented by the right bar. Serovars with less than 7 strains were condensed as “Other.”
Cocktail Assembly
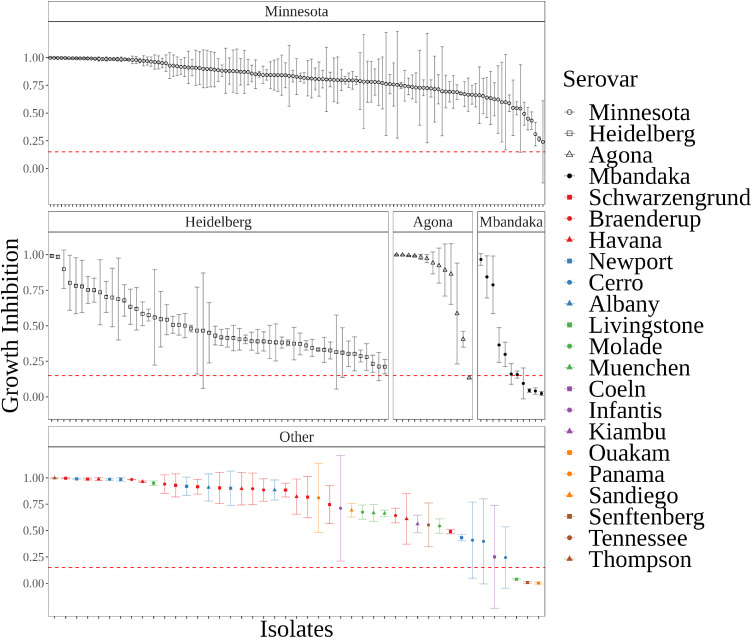
Bacteriophages L8, SAEN098P01, and SAEN098P03 were combined to develop a cocktail named INSPEKTOR. 96 well plates liquid co-culture assays were used to quantitatively analyze the cocktail efficacy. Each bacterial isolate from the commercial broiler farm (n = 258) was grown in the presence of the cocktail at an MOI input of 60 (20 PFU per bacteriophage). Bacterial growth was recorded every 10 min until 18 h post infection. In general, the cocktail inhibited partially (≥15%) or totally (≥85%) the growth of 96.9% of the Salmonella isolates (249 out of 258), with an overall median of 78.1% inhibition. When analyzed by relevant serovars, INSPEKTOR demonstrated median inhibition of 82.8% over Minnesota (n = 133), 42.4% over Heidelberg (n = 56), 94.2% over Agona (n = 13), 16.0% over Mbandaka (n = 11) and 82.1% over other less abundant serovars (n = 45) (Figure 4). Only for 8 isolates of the collection, the growth inhibition remained below 15% (Figure 4, red dotted lines). Remarkably, these isolates corresponded mainly to serovars Mbandaka (n = 4), Agona (n = 1), Livingstone (n = 1), Ouakam (n = 1), and Senftenberg (n = 1). The cocktail demonstrated promising parameters, including a Breadth2 of 71.4% and a Breadth3 of 26.4% (Hyman and Abedon, 2010; Abedon et al., 2021). Overall, the results indicated that the INSPEKTOR cocktail had a high efficacy against a large collection of Salmonella isolates.
Figure 4.
INSPEKTOR performance by serovar based on co-culture assay. Growth inhibition values are shown in the y-axis. Salmonella strains were ordered by growth inhibition mean per serovar. Panels represent each of the most abundant serovar. Serovars with less than 7 strains are detailed in the box “Other.” Each point represents the mean with standard deviation (n = 3). Red dashed line represents 15% of growth inhibition, defined as minimum activity to exert effect.
Activity of the Cocktail in Low Oxygen and High Temperature
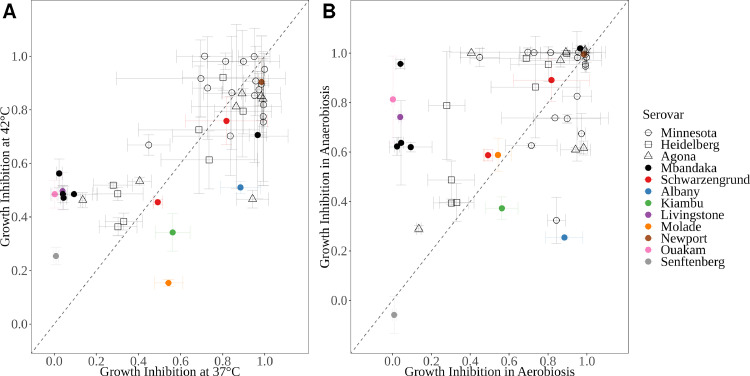
Bacteriophage cocktails against Salmonella spp. must exert their action mainly in the chicken gut. The chicken gut is a hostile environment for bacteriophages, with temperatures reaching 42°C and low-oxygen conditions. To determine the efficacy of INSPEKTOR under these conditions, quantitative assays were established using a selection of 46 isolates that represent the phenotypes of lysis and serovars of the entire collection (Supplementary Table 1). The efficacy of the cocktail at 42°C correlated with what was observed at 37°C, with a 3.7% ± 3.5% increase in bacterial growth inhibition (Figure 5A). No statistically significant differences between both temperatures were observed (χ2(1) = 1.123, P-value = 0.2893). Interestingly, isolates from serovars Senftenberg (n = 2) and Mbandaka (n = 5) were found to be susceptible to INSPEKTOR at 42°C while being resistant at 37°C (Figure 5A, purple and green dots, respectively). Additionally, the efficacy of INSPEKTOR was analyzed in bacteria grown under low-oxygen conditions. In this scenario, shifting from aerobic to anaerobic conditions, both assays at 37°C increased the bacterial growth inhibition by 11.7% ± 4.7% (χ2(1) = 6.0299, P-value = 0.01407) (Figure 5B). A positive shift in susceptibility was found for isolates from serovar Mbandaka (n = 5), but 1 Senftenberg isolate remained resistant to aerobic and anaerobic conditions (Figure 5B). Overall, our results demonstrate the efficacy of INSPEKTOR was not affected by an increase in the temperature from 37°C to 42°C, and it was significantly improved in low oxygen conditions that more closely resemble the chicken gut.
Figure 5.
INSPEKTOR performance in culture modifications. (A) Correlation in growth inhibition between cultures at 37°C vs. 42°C. (B). Correlation in growth inhibition between aerobic vs. anaerobic culture. Serovar of each strain is represented by color. Each point represents the mean with standard deviation (n = 3) in both dimensions. Black dashed line represents the bisector, as a perfect correlation between 2 variables.
Activity of the Cocktail Against Biofilm
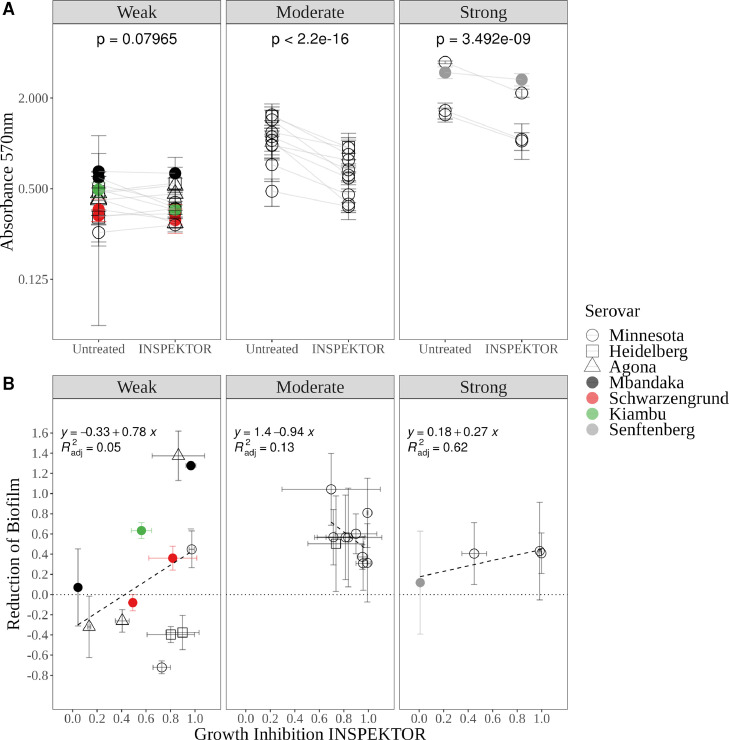
Increased resistance to antimicrobial agents of Salmonella spp. biofilm communities over planktonic cells have been demonstrated in vitro and in vivo (Aleksandrowicz et al., 2023). Here, the ability to form biofilm was determined for the selection of 46 isolates previously analyzed. The isolates were classified as strong, moderate, weak or no biofilm producers (Stepanovic et al., 2004). Of these, 4 were found to be strong biofilm producers, 10 moderate and 12 were weak biofilm producers. Twenty isolates from the selection produced no biofilm (Supplementary Table 1). The activity of the INSPEKTOR cocktail against the biofilm producers (n = 26) was analyzed. Overall, a reduction of 0.30 ± 0.03 units of absorbance was detected in the condition treated with INSPEKTOR compared to nontreated control biofilms (χ2 = 100.89, P-value < 2.2E-16). On relevant serovars, INSPEKTOR reduced the biofilm produced by 13 out of 14 Minnesota strains; 1 out of 3 Heidelberg strains; 1 out of 3 Agona strains; 1 out of 2 Mbandaka strains and 1 out of 1 Kiambu strains. Biofilms produced by Schwarzengrund and Senftenberg strains were not reduced when treated with INSPEKTOR. When analyzing the effect of INSPEKTOR by the classification of biofilm production capabilities, a significant reduction in A570 nm was determined on moderate (0.427 ± 0.03 units, χ2=163.04, P-value < 2.2E-16) and strong (0.655 ± 0.11 units, χ2=34.888, P-value=3.492E-09) biofilm producers (Figure 6A). No reduction was detected over weak biofilm producers’ strains (0.03 ± 0.017 units, χ2=3.072, P-value=0.07965).
Figure 6.
INSPEKTOR effect over biofilms. (A) Effect of INSPEKTOR in biofilms-producer strains, classified as weak, moderate, and strong (see methods). Presence of INSPEKTOR was used as a fixed-factor to model Crystal violet absorbance at 570 nm in a linear mixed-model. For each model, P-value was calculated with the ANOVA function (car package) of the best model (random intercepts and random slopes). Each point represents mean with standard deviation (n = 5), gray lines represent the same strain evaluated in both conditions. Y-axis was transformed to log2 scale. (B) Correlation between INSPEKTOR biofilm reduction and growth inhibition. Linear correlation was calculated, equation and R2adj are shown in each panel. Serovar of each strain is represented by color. Each point represents the mean with standard deviation (n=3 vertically, n=5 horizontally). Black dashed line represents the linear correlation of both variables.
As mentioned above, Salmonella strains with the ability to grow in a biofilm state may be less susceptible to antimicrobial agents. Therefore, the effect of INSPEKTOR on biofilm (sessile) and planktonic cells was compared (Figure 6B). Five strains presented a shift in their phenotype against INSPEKTOR, moving from susceptible in planktonic to resistant in biofilm. Despite the effect of the cocktail on the growth inhibition of planktonic cells and the reduction of biofilm of sessile cells, no statistically significant correlations were detected. This result suggests that an isolate that is highly susceptible to INSPEKTOR in planktonic lifestyle may not have the same susceptibility compared to a sessile lifestyle. Further, the result highlights the importance of evaluating bacteriophage cocktails using different bacterial growth assays to assess maximal efficacy on the different conditions that the target bacteria may encounter.
Cross Resistance of the Cocktail
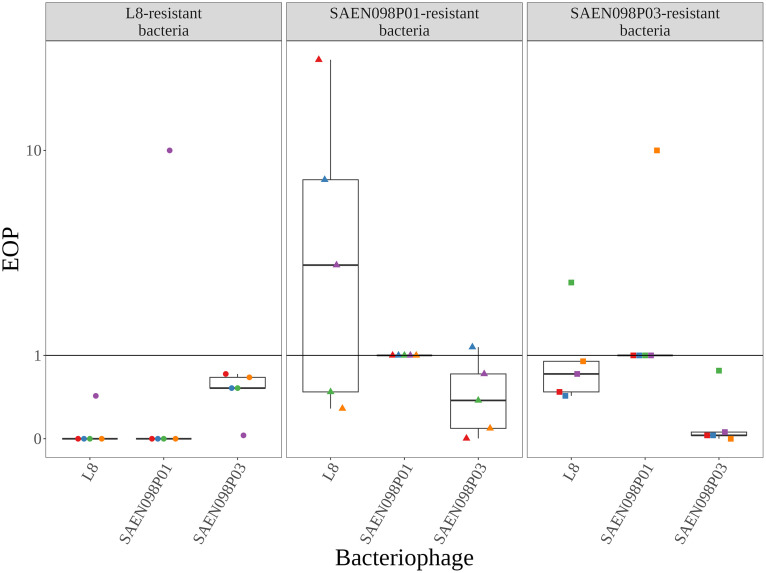
One critical aspect to analyze when designing bacteriophage cocktails for biocontrol is the potential rise of resistant bacteria. In this aspect, it is desirable that bacteriophages within a cocktail share minimal mechanisms of infection, thus minimizing the risk of cross-resistance (Abedon et al., 2021). To determine the potential rise of cross-resistance between bacteriophages L8, SAEN098P01 and SAEN098P03, bacterial isolates susceptible to the 3 bacteriophages were analyzed. Efficiency of plating (EOP) of bacteriophages L8, SAEN098P01 and SAEN098P03 on 8 isolates was analyzed. EOP was lower for bacteriophages L8 and SAEN098P01 on all the isolates tested compared to their original host. For bacteriophage SAEN098P03, EOP was higher in 7 out of 8 isolates (Supplementary Table 2). Based on these results, the isolate 1026 was chosen as a model bacteria for the next steps. To determine potential cross-resistance, the isolate 1026 was infected independently with each individual bacteriophage at an MOI input of 20. Five clones of infection-resistant bacteria were selected after infection with each bacteriophage. EOP was then determined for each resistant-isolated colony (Figure 7, red, blue, green, purple and orange symbols, respectively). Four L8 resistant clones remained resistant to bacteriophage L8 and 1 clone (purple dot) remained mildly resistant (Figure 7, left panel). These mutants were mildly resistant to infection by bacteriophage SAEN098P03 and in 4 out of 5 clones completely resistant to SAEN098P01. Interestingly, the clone that remained mildly resistant to L8 was 10 times more sensitive to infection by bacteriophage SAEN098P01 (purple dot). When analyzing SAEN098P01 resistant clones, none of the resistant isolates to the bacteriophage SAEN098P01 retained this phenotype. Only 1 isolate presented complete resistance to SAEN098P03 (red triangle). Interestingly, in 3 out of the 5 clones, an increased susceptibility to bacteriophage L8 was observed (Figure 7, middle panel). This suggests that cross-resistance between bacteriophages L8 and SAEN098P01 is not reciprocal. Finally, in the case of the resistant clones to the bacteriophage SAEN098P03, partial cross resistance of 4 clones was found for L8, and no cross-resistance was determined for SAEN098P01 (Figure 7, right panel). Again, increased susceptibility to infection was found for L8 (green square) and SAEN098P01 (orange square) in 2 clones. Only 1 clone retained a completely resistant phenotype to infection by SAEN098P03 (orange square), while the remaining isolates were less susceptible to infection by SAEN098P03 than the original isolate. Thus, our results indicate a limited emergence of cross-resistance between the 3 bacteriophages of the cocktail INSPEKTOR.
Figure 7.
INSPEKTOR cross resistance assays. Wild type strain 1026 was challenged with L8, SAEN098P01 or SAEN098P03. With the resulting resistant strains, EOP of each bacteriophage was determined by comparing the titers on these bacteria and wild type strain (baseline). Values below 1 represent resistance against the respective bacteriophage and values above 1 represent susceptibility to the bacteriophage. Finally, values equal or close to 1 represent no changes of the sensibility to the bacteriophages. Each shape represents the strains derived from bacteriophage-resistant bacteria.
DISCUSSION
Bacteriophages have gained relevance over the last years as a potential solution for control of Salmonella in both the poultry veterinary medicine and industry. In this work, a cocktail named INSPEKTOR was developed using bacteriophages from the collection of PhageLab. In order to develop a cocktail with high lytic efficacy that can be suitable for use in farms, several considerations were taken. First, a large and diverse collection of Salmonella isolates that was recently obtained from commercial broiler farms was used as our cocktail target. Bacteriophages with activity against the most prevalent serovars of the collection were readily identified using spot assays, and 3 of these were selected to assemble the cocktail. To ensure the selected bacteriophages will not be quickly inactivated in chicken gut-like conditions, a step of in vitro analysis of tolerance of individual bacteriophages to pH and temperature was made. Then, the lytic activity of these bacteriophages individually, or together as a cocktail, was analyzed in quantitative assays. Further, all subsequent steps of characterization demonstrated that conditions in which the cocktail could be exposed in vivo such as anaerobiosis, 42°C and Salmonella mono-strain biofilms did not affect, or even improved the lytic activity of INSPEKTOR. Remarkably, analysis of bacterial cross-resistance between the 3 bacteriophages composing the cocktail revealed limited or no generation of cross-resistance. Altogether, our in vitro data demonstrates a high lytic activity of INSPEKTOR against Salmonella and argues for its potential use in vivo.
The success of bacteriophage cocktails with high efficacies against Salmonella in vivo largely depends on the use of an updated and diverse group of bacteria during in vitro characterization. For this purpose, a collection of 258 Salmonella isolates was obtained from a commercial Brazilian broiler company in 2021. The collection was dominated by isolates from serovars Minnesota (52.5%) and Heidelberg (21.8%), followed by Agona (4.7%), Mbandaka (3.5%) and 20 other, less abundant serovars (17.5%). Recent reports from the EFSA determined the most prevalent serovars in broiler chickens in Europe were Infantis, Enteritidis, Livingstone and Mbandaka (EFSA and ECDC, 2022). However, in Brazilian farms the recent rising of Minnesota and Heidelberg serovars is of high concern for the poultry industry (Kipper et al., 2021; Alikhan et al., 2022; Saidenberg et al., 2023). Thus, the collection of strains used in this study is likely an updated representation of what is circulating in Brazilian farms. An initial load of 1E+06 CFU/mL of Salmonella was defined by us for our quantitative assays. This number was determined to be the average load of Salmonella Infantis on previous analysis of chicken cecum obtained from farm animals (n = 20). Additional reports support our estimations, as loads of Salmonella in the range of 2.8 to 6.4 log10 cells/g of cecal content (mean 4.3 ± 1.9) have been found (Souza et al., 2022). Our results indicate that the cocktail INSPEKTOR had a high lytic efficacy against strains from the 2 most prevalent serovars, Minnesota and Heidelberg (Figure 4). The growth inhibition of all isolates from both serovars (n = 189) was above 15%, which we interpreted as at least partial inhibition. A high lytic efficacy was also shown against isolates from the third most prevalent serovar found in this collection, Agona (n = 13). Further, INSPEKTOR demonstrated high lytic efficacy against twenty other additional serovars from the collection. Even when in some instances inhibition of bacterial growth was not above 85% (interpreted by us as total inhibition), our experimental in vitro setting included only 1 dose of the cocktail. To maximize the effect of bacterial growth inhibition, we propose that multiple doses of INSPEKTOR should be tested in vivo in experimental farms.
Multiple micro-environments can be found along the poultry gastrointestinal tract, ranging from pH 2.5 in proventriculus to 7.1 at the rectum, and an internal temperature of 42°C. The permanence in each segment of the digestive tract fluctuates from 10 min at duodenum up to 120 min at the cecum (Denbow, 2015). Individual bacteriophage tolerance to pH and temperature within these parameters was evaluated in this work. Regarding pH tolerance, acidity stays over pH 4.7 from gizzard to cloaca (Denbow, 2015), therefore all 3 bacteriophages should retain near complete activity under such conditions. In upper digestive tract segments however, exposition to high acidity conditions (pH 2.5) for long periods might compromise bacteriophage infectivity. However, even when some infectivity may be lost during passage through the proventriculus, several articles have described that infectious bacteriophages can be recovered from chicken feces after treatment through drinking water (Toro et al., 2005; Clavijo et al., 2019). To retain the cocktail efficacy and to ensure minimal loss of antimicrobial activity, in vivo delivery of INSPEKTOR will require formulation strategies such as addition of adequate buffers and excipients, coatings and/or microencapsulation (Rosner and Clark, 2021). Regarding temperature tolerance, the internal temperature in poultry is within the range of tolerance for all 3 bacteriophages of the cocktail (Figure 2). Remarkably, this study demonstrates that our prototype cocktail INSPEKTOR host range can even be expanded at 42°C (Figure 5A). It has been demonstrated that infection by bacteriophages can be affected by host factors including availability of cell surface receptors or active metabolic pathways, which can vary by environmental conditions, such as pH, temperature and amount of nutrients (Taj et al., 2014; Nilsson et al., 2022). Interestingly, isolates from serovars Senftenberg and Mbandaka that were resistant to infection at 37°C were found to be affected by INSPEKTOR at 42°C (Figure 5A). This temperature-dependent susceptibility could be due to a differential expression of surface receptors in these serovars, as described for the expression of OmpF in Yersinia enterocolitica (Leon-Velarde et al., 2016) or rhamnose in Listeria monocytogenes (Tokman et al., 2016).
The effect of oxygen on host-phage interaction was evaluated in this work. The effect of environmental oxygen availability over activity of bacteriophages is of high interest for Salmonella, a facultative anaerobic bacteria. Interestingly, a significant improvement of INSPEKTOR efficacy was found in anaerobic conditions compared to aerobic conditions (Figure 5B). In the absence of oxygen, modifications occur in metabolic pathways that favor anaerobic respiration and carbon metabolism (Encheva et al., 2009; Unden et al., 2014). In Salmonella Typhimurium, ArcA and FNR repress pathways associated with aerobic metabolism, and activate gene expression involved with anaerobic metabolism, flagellar biosynthesis, mobility and sugar transportation (Fink et al., 2007; Evans et al., 2011). However, although these changes allow Salmonella to grow in anaerobiosis, the expression of these pathways can simultaneously be affected by bacteriophages (Hernández and Vives, 2020). We speculate that specific host factors not present in aerobic conditions favored infection by the bacteriophages of the cocktail INSPEKTOR. The exact mechanism of how low oxygen improvement of lytic activity by the cocktail occurs warrants future research.
Biofilms play an important role in colonization and persistence of bacteria on inert surfaces and organ tissues, such as the respiratory tract and the gut, among others (Satpathy et al., 2016). Interestingly, biofilm communities can be more resistant to antimicrobial agents compared to planktonic bacteria (Høiby et al., 2010; Hall and Mah, 2017). Therefore, it was relevant for us to determine the effect of INSPEKTOR against biofilm formed by Salmonella isolates. In vitro conditions of biofilm formation may not be equivalent to biofilm persistence on organ tissues, mainly due to the nutrient, physiologic and environmental differences between both conditions. Of the selected panel of 46 Salmonella isolates, 26 were able to produce biofilm on a polystyrene 96-well plate. The cocktail INSPEKTOR demonstrated a significant activity against biofilm on strong and moderate biofilm-producers bacteria (Figure 6A). Under our experimental conditions, 20 strains were unable to form biofilm on a 96-well plate, despite being isolated from farms, which are sites where the persistence of bacteria greatly depends on biofilm formation (Steenackers et al., 2012). We speculate the standardization of a biofilm formation matrix that resembles as much as possible the environment where the target bacteria is found is of great importance in the development of a bacteriophage cocktail. This becomes even more relevant when comparing the effect of INSPEKTOR on quantitative assays where bacteria have a planktonic lifestyle, and biofilm disruption assays where bacteria is embedded on the extracellular polymeric substance of biofilm, as our results showed no correlation between both experimental conditions. Several reasons could explain the shift of phenotype of 5 isolates from susceptible to resistant against INSPEKTOR (Figure 6B). First, 90% of biofilm mass consists of extracellular polymeric substance, which could cover bacteria, preventing phage diffusion and limiting its antibacterial activity (Flemming and Wingender, 2010). Additional reports have described a rapid growth of a phage resistant sub-population after the primary reduction of biofilm cells treated with phages (Lacqua et al., 2006; Donlan, 2009; Fu et al., 2010; Pires et al., 2011). Furthermore, bacteria present in biofilms are under nutrient-limited conditions and they grow slowly compared to planktonic cells. Since phage infection depends on the growth condition of the host, a reduction of the metabolism in the bacteria could significantly decrease phage activity (Sillankorva et al., 2004; Łoś et al., 2007; Yonezawa et al., 2015). Overall, our results emphasize the importance of studying the effect of bacteriophage cocktails over biofilms.
Microbiological assays were made in this work to assess potential rise of cross-resistance between the 3 bacteriophages of the INSPEKTOR cocktail. In this experiment, mainly 2 phenotypes of resistance were observed. Colonies that were isolated as resistant for an specific bacteriophage, and remained resistant to the bacteriophage could be an irreversible resistant phenotype. On the other hand, colonies that were isolated as resistant and turned out to be susceptible to the same virus after passages represent a reversible type of resistance. Irreversible resistance phenotypes suggest the presence of a stable genetic mechanism of resistance (Hyman and Abedon, 2010; Labrie et al., 2010). Meanwhile, reversible resistance phenotypes may indicate a transient mechanism of resistance such as epigenetic changes (Labrie et al., 2010; Gurney et al., 2019). A detailed analysis of the EOP assays revealed a possible cross-resistance event between bacteriophages L8 and SAEN098P01. Resistance to SAEN098P01 occurred in isolates that were also irreversible resistant to L8, which suggests similarities in the infection mechanisms between these 2 bacteriophages (Adler et al., 2021). Although the reciprocal effect was not observed since no isolates with irreversible resistance to SAEN098P01 were obtained, we did observe isolates with reversible phenotypes for SAEN098P01 and increased sensitivity to L8. Finally, the presence of SAEN098P03 in INSPEKTOR could compensate for the possible cross-resistance of the other 2 bacteriophages because it was capable of infecting the majority of resistant bacteria, while the others could not.
Overall, the present report represents a detailed workflow of bacteriophage cocktail development in vitro that addresses many relevant factors that may be encountered in vivo. Our workflow has allowed us to rapidly assemble bacteriophage cocktails that fill the specific needs of commercial poultry companies.
ACKNOWLEDGMENTS
This work was supported by the Chilean Economic Development Agency (CORFO) [grant number 22CYE-201546, 2022]; and by private funding. Special thanks to Loreto Hidalgo, Nicolás Méndez, Juan Sacre, Johanna Saldías, Héctor Garcias, Braulio Paillavil, Kristen Oviedo and Gastón Higuera for their help with experiments of this manuscript.
DISCLOSURES
Pablo Cifuentes, Nicolás Ferreira and Hans Pieringer are co-founders of PhageLab Chile SpA. Matías Aguilera, Rodrigo Norambuena, Eduardo Tobar, Andrea Sabag, Onix Cifuentes, Nicolás Cifuentes, Victoria Rojas, María Jesus Serrano, Daniel Castillo, Pablo Cifuentes and Hans Pieringer are inventors of a patent application directed to protect INSPEKTOR. All authors are employees of PhageLab Chile SpA or its affiliates.
Footnotes
Supplementary material associated with this article can be found in the online version at doi:10.1016/j.psj.2023.103125.
Appendix. Supplementary materials
REFERENCES
- Abedon S.T., Danis-Wlodarczyk K.M., Wozniak D.J. Phage cocktail development for bacteriophage therapy: toward improving spectrum of activity breadth and depth. Pharmaceuticals. 2021;14:1019. doi: 10.3390/ph14101019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Adler B.A., Kazakov A.E., Zhong C., Liu H., Kutter E., Lui L.M., Nielsen T.N., Carion H., Deutschbauer A.M., Mutalik V.K., Arkin A.P. The genetic basis of phage susceptibility, cross-resistance and host-range in Salmonella. Microbiology. 2021;167 doi: 10.1099/mic.0.001126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aleksandrowicz A., Carolak E., Dutkiewicz A., Błachut A., Waszczuk W., Grzymajlo K. Better together–Salmonella biofilm-associated antibiotic resistance. Gut Microbes. 2023;15 doi: 10.1080/19490976.2023.2229937. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alikhan N.-F., Moreno L.Z., Castellanos L.R., Chattaway M.A., McLauchlin J., Lodge M., O'Grady J., Zamudio R., Doughty E., Petrovska L., Cunha M.P.V., Knöbl T., Moreno A.M., Mather A.E. Dynamics of Salmonella enterica and antimicrobial resistance in the Brazilian poultry industry and global impacts on public health. PLoS Genet. 2022;18 doi: 10.1371/journal.pgen.1010174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Borie C., Albala I., Sánchez P., Sánchez M.L., Ramírez S., Navarro C., Morales M.A., Retamales A.J., Robeson J. Bacteriophage treatment reduces Salmonella colonization of infected chickens. Avian Dis. 2008;52:64–67. doi: 10.1637/8091-082007-Reg. [DOI] [PubMed] [Google Scholar]
- Carlson K. CRC press; Boca Raton, FL: 2005. Working With Bacteriophages: Common Techniques and Methodological Approaches. [Google Scholar]
- Castello A., Piraino C., Butera G., Alio V., Cardamone C., Oliveri G., Cascone G., Ciravolo C., Costa A. Prevalence and antimicrobial resistance profiles of Salmonella spp. in poultry meat. Ital. J. Food Saf. 2023;12:11135. doi: 10.4081/ijfs.2023.11135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Castro-Vargas R.E., Herrera-Sánchez M.P., Rodríguez-Hernández R., Rondón-Barragán I.S. Antibiotic resistance in Salmonella spp. isolated from poultry: a global overview. Vet. World. 2020;13:2070–2084. doi: 10.14202/vetworld.2020.2070-2084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clavijo V., Baquero D., Hernandez S., Farfan J.C., Arias J., Arévalo A., Donado-Godoy P., Vives-Flores M. Phage cocktail SalmoFREE® reduces Salmonella on a commercial broiler farm. Poult. Sci. 2019;98:5054–5063. doi: 10.3382/ps/pez251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Denbow D.M. In: Pages 337–366 in Sturkie's Avian Physiology. 6th ed. Scanes C.G., editor. Academic Press; San Diego, CA: 2015. Chapter 14 - gastrointestinal anatomy and physiology. [Google Scholar]
- Donlan R.M. Preventing biofilms of clinically relevant organisms using bacteriophage. Trends Microbiol. 2009;17:66–72. doi: 10.1016/j.tim.2008.11.002. [DOI] [PubMed] [Google Scholar]
- Eini A., Sol A., Coppenhagen-Glazer S., Skvirsky Y., Zini A., Bachrach G. Oxygen deprivation affects the antimicrobial action of LL-37 as determined by microplate real-time kinetic measurements under anaerobic conditions. Anaerobe. 2013;22:20–24. doi: 10.1016/j.anaerobe.2013.04.014. [DOI] [PubMed] [Google Scholar]
- Encheva V., Shah H.N., Gharbia S.E. Proteomic analysis of the adaptive response of Salmonella enterica serovar Typhimurium to growth under anaerobic conditions. Microbiology. 2009;155:2429–2441. doi: 10.1099/mic.0.026138-0. [DOI] [PubMed] [Google Scholar]
- European Food Safety Authority and European Centre for Disease Prevention and Control (EFSA and ECDC) The European Union One Health 2018 Zoonoses Report. EFSA J. 2019;17:e05926. doi: 10.2903/j.efsa.2019.5926. [DOI] [PMC free article] [PubMed] [Google Scholar]
- European Food Safety Authority and European Centre for Disease Prevention and Control (EFSA and ECDC) The European Union One Health 2021 Zoonoses Report. EFSA J. 2022;20:e07666. doi: 10.2903/j.efsa.2022.7666. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Evans M.R., Fink R.C., Vazquez-Torres A., Porwollik S., Jones-Carson J., McClelland M., Hassan H.M. Analysis of the ArcA regulon in anaerobically grown Salmonella enterica sv. Typhimurium. BMC Microbiol. 2011;11:58. doi: 10.1186/1471-2180-11-58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ferrari R.G., Rosario D.K.A., Cunha-Neto A., Mano S.B., Figueiredo E.E.S., Conte-Junior C.A. Worldwide epidemiology of Salmonella serovars in animal-based foods: a meta-analysis. Appl. Environ. Microbiol. 2019;85 doi: 10.1128/AEM.00591-19. e00591-19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fink R.C., Evans M.R., Porwollik S., Vazquez-Torres A., Jones-Carson J., Troxell B., Libby S.J., McClelland M., Hassan H.M. FNR is a global regulator of virulence and anaerobic metabolism in Salmonella enterica serovar typhimurium (ATCC 14028s) J. Bacteriol. 2007;189:2262–2273. doi: 10.1128/JB.00726-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Flemming H.-C., Wingender J. The biofilm matrix. Nat. Rev. Microbiol. 2010;8:623–633. doi: 10.1038/nrmicro2415. [DOI] [PubMed] [Google Scholar]
- Fu W., Forster T., Mayer O., Curtin J.J., Lehman S.M., Donlan R.M. Bacteriophage cocktail for the prevention of biofilm formation by Pseudomonas aeruginosa on catheters in an In Vitro model system. Antimicrob. Agents Chemother. 2010;54:397–404. doi: 10.1128/AAC.00669-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grant A., Parveen S., Schwarz J., Hashem F., Vimini B. Reduction of Salmonella in ground chicken using a bacteriophage. Poult. Sci. 2017;96:2845–2852. doi: 10.3382/ps/pex062. [DOI] [PubMed] [Google Scholar]
- Gurney J., Pleška M., Levin B.R. Why put up with immunity when there is resistance: an excursion into the population and evolutionary dynamics of restriction–modification and CRISPR-Cas. Phil. Trans. R. Soc. B. 2019;374 doi: 10.1098/rstb.2018.0096. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gut A.M., Vasiljevic T., Yeager T., Donkor O.N. Salmonella infection – prevention and treatment by antibiotics and probiotic yeasts: a review. Microbiology. 2018;164:1327–1344. doi: 10.1099/mic.0.000709. [DOI] [PubMed] [Google Scholar]
- Hall C.W., Mah T.-F. Molecular mechanisms of biofilm-based antibiotic resistance and tolerance in pathogenic bacteria. FEMS Microbiol. Rev. 2017;41:276–301. doi: 10.1093/femsre/fux010. [DOI] [PubMed] [Google Scholar]
- Hernández S., Vives M.J. Phages in anaerobic systems. Viruses. 2020;12:1091. doi: 10.3390/v12101091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Høiby N., Bjarnsholt T., Givskov M., Molin S., Ciofu O. Antibiotic resistance of bacterial biofilms. Int. J. Antimicrob. Agents. 2010;35:322–332. doi: 10.1016/j.ijantimicag.2009.12.011. [DOI] [PubMed] [Google Scholar]
- Hyman P., Abedon S.T. Pages 217–248 in Advances in Applied Microbiology. Elsevier; Amsterdam, Netherlands: 2010. Chapter 7 - bacteriophage host range and bacterial resistance. [DOI] [PubMed] [Google Scholar]
- Ikuta K.S., Swetschinski L.R., Robles Aguilar G., et al. Global mortality associated with 33 bacterial pathogens in 2019: a systematic analysis for the Global Burden of Disease Study 2019. Lancet. 2022;400:2221–2248. doi: 10.1016/S0140-6736(22)02185-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kabir S.M.L. Avian Colibacillosis and Salmonellosis: a closer look at epidemiology, pathogenesis, diagnosis, control and public health concerns. Int. J. Environ. Res. Public Health. 2010;7:89–114. doi: 10.3390/ijerph7010089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kipper D., Orsi R.H., Carroll L.M., Mascitti A.K., Streck A.F., Fonseca A.S.K., Ikuta N., Tondo E.C., Wiedmann M., Lunge V.R. Recent evolution and genomic profile of Salmonella enterica serovar Heidelberg isolates from poultry flocks in Brazil. Appl. Environ. Microbiol. 2021;87 doi: 10.1128/AEM.01036-21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kirk M.D., Pires S.M., Black R.E., Caipo M., Crump J.A., Devleesschauwer B., Döpfer D., Fazil A., Fischer-Walker C.L., Hald T., Hall A.J., Keddy K.H., Lake R.J., Lanata C.F., Torgerson P.R., Havelaar A.H., Angulo F.J. Correction: World Health Organization estimates of the global and regional disease burden of 22 foodborne bacterial, protozoal, and viral diseases, 2010: a data synthesis. PLOS Med. 2015;12 doi: 10.1371/journal.pmed.1001921. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Korzeniowski P., Śliwka P., Kuczkowski M., Mišić D., Milcarz A., Kuźmińska-Bajor M. Bacteriophage cocktail can effectively control Salmonella biofilm in poultry housing. Front. Microbiol. 2022;13 doi: 10.3389/fmicb.2022.901770. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kosznik-Kwaśnicka K., Stasiłojć M., Grabowski Ł., Zdrojewska K., Węgrzyn G., Węgrzyn A. Efficacy and safety of phage therapy against Salmonella enterica serovars Typhimurium and Enteritidis estimated by using a battery of in vitro tests and the Galleria mellonella animal model. Microbiol. Res. 2022;261 doi: 10.1016/j.micres.2022.127052. [DOI] [PubMed] [Google Scholar]
- Kropinski A.M., Mazzocco A., Waddell T.E., Lingohr E., Johnson R.P. In: Pages 69–76 in Bacteriophages: Methods and Protocols, Volume 1: Isolation, Characterization, and Interactions. Clokie M.R.J., Kropinski A.M., editors. Humana Press; Totowa, NJ: 2009. Enumeration of bacteriophages by double agar overlay plaque assay. [DOI] [PubMed] [Google Scholar]
- Kutter E. In: Pages 141–149 in Bacteriophages: Methods and Protocols, Volume 1: Isolation, Characterization, and Interactions. Clokie M.R.J., Kropinski A.M., editors. Humana Press; Totowa, NJ: 2009. Phage host range and efficiency of plating. [Google Scholar]
- Labrie S.J., Samson J.E., Moineau S. Bacteriophage resistance mechanisms. Nat. Rev. Microbiol. 2010;8:317–327. doi: 10.1038/nrmicro2315. [DOI] [PubMed] [Google Scholar]
- Lacqua A., Wanner O., Colangelo T., Martinotti M.G., Landini P. Emergence of biofilm-forming subpopulations upon exposure of Escherichia coli to environmental bacteriophages. Appl. Environ. Microbiol. 2006;72:956–959. doi: 10.1128/AEM.72.1.956-959.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leon-Velarde C.G., Happonen L., Pajunen M., Leskinen K., Kropinski A.M., Mattinen L., Rajtor M., Zur J., Smith D., Chen S., Nawaz A., Johnson R.P., Odumeru J.A., Griffiths M.W., Skurnik M. Yersinia enterocolitica-specific infection by bacteriophages TG1 and ϕR1-RT is dependent on temperature-regulated expression of the phage host receptor OmpF (HL Drake, Ed.) Appl. Environ. Microbiol. 2016;82:5340–5353. doi: 10.1128/AEM.01594-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Łoś M., Golec P., Łoś J.M., Węglewska-Jurkiewicz A., Czyż A., Węgrzyn A., Węgrzyn G., Neubauer P. Effective inhibition of lytic development of bacteriophages λ, P1 and T4 by starvation of their host, Escherichia coli. BMC Biotechnol. 2007;7:13. doi: 10.1186/1472-6750-7-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McEwen S.A., Fedorka-Cray P.J. Antimicrobial use and resistance in animals. Clin. Infect. Dis. 2002;34:S93–S106. doi: 10.1086/340246. [DOI] [PubMed] [Google Scholar]
- Nale J.Y., Vinner G.K., Lopez V.C., Thanki A.M., Phothaworn P., Thiennimitr P., Garcia A., AbuOun M., Anjum M.F., Korbsrisate S., Galyov E.E., Malik D.J., Clokie M.R.J. An optimized bacteriophage cocktail can effectively control Salmonella in vitro and in Galleria mellonella. Front. Microbiol. 2021;11 doi: 10.3389/fmicb.2020.609955. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nilsson E., Li K., Hoetzinger M., Holmfeldt K. Nutrient driven transcriptional changes during phage infection in an aquatic Gammaproteobacterium. Environ. Microbiol. 2022;24:2270–2281. doi: 10.1111/1462-2920.15904. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Official Journal of the European Union. 2006. Commission Regulation (EC) No 1177/2006 of 1 August 2006 implementing Regulation (EC) No 2160/2003 of the European Parliament and of the Council as regards requirements for the use of specific control methods in the framework of the national programmes for the control of salmonella in poultry (Text with EEA relevance).
- Pires D., Sillankorva S., Faustino A., Azeredo J. Use of newly isolated phages for control of Pseudomonas aeruginosa PAO1 and ATCC 10145 biofilms. Res. Microbiol. 2011;162:798–806. doi: 10.1016/j.resmic.2011.06.010. [DOI] [PubMed] [Google Scholar]
- Popoff M.Y., Bockemühl J., Gheesling L.L. Supplement 2002 (no. 46) to the Kauffmann–White scheme. Res. Microbiol. 2004;155:568–570. doi: 10.1016/j.resmic.2004.04.005. [DOI] [PubMed] [Google Scholar]
- Pui C.F., Wong W.C., Chai L.C., Tunung R., Jeyaletchumi P., Noor Hidayah M.S., Ubong A., Farinazleen M.G., Cheah Y.K., Son R. Salmonella: a foodborne pathogen. Int. Food Res. J. 2011;18:465–473. [Google Scholar]
- Rosner D., Clark J. Formulations for bacteriophage therapy and the potential uses of immobilization. Pharmaceuticals. 2021;14:359. doi: 10.3390/ph14040359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saidenberg A.B.S., Franco L.S., Reple J.N., Hounmanou Y.M.G., Casas M.R.T., Cardoso B., Esposito F., Lincopan N., Dalsgaard A., Stegger M., Knöbl T. Salmonella Heidelberg and Salmonella Minnesota in Brazilian broilers: genomic characterization of third-generation cephalosporin and fluoroquinolone-resistant strains. Environ. Microbiol. Rep. 2023;15:119–128. doi: 10.1111/1758-2229.13132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Satpathy S., Sen S.K., Pattanaik S., Raut S. Review on bacterial biofilm: an universal cause of contamination. Biocatal. Agric. Biotechnol. 2016;7:56–66. [Google Scholar]
- Schindelin J., Arganda-Carreras I., Frise E., Kaynig V., Longair M., Pietzsch T., Preibisch S., Rueden C., Saalfeld S., Schmid B., Tinevez J.-Y., White D.J., Hartenstein V., Eliceiri K., Tomancak P., Cardona A. Fiji: an open-source platform for biological-image analysis. Nat. Methods. 2012;9:676–682. doi: 10.1038/nmeth.2019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schneider C.A., Rasband W.S., Eliceiri K.W. NIH Image to ImageJ: 25 years of image analysis. Nat. Methods. 2012;9:671–675. doi: 10.1038/nmeth.2089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sillankorva S., Oliveira R., Vieira M.J., Sutherland I., Azeredo J. Pseudomonas fluorescens infection by bacteriophage ΦS1: the influence of temperature, host growth phase and media. FEMS Microbiol. Lett. 2004;241:13–20. doi: 10.1016/j.femsle.2004.06.058. [DOI] [PubMed] [Google Scholar]
- Souza M., Wolf J., Zanetti N., Fonseca A., Ikuta N., Lunge V. Direct detection and quantification of bacterial pathogens from broiler cecal samples in the slaughter line by real-time PCR. Braz. J. Poult. Sci. 2022;24 eRBCA-2021-1505. [Google Scholar]
- Stanaway J.D., Parisi A., Sarkar K., et al. The global burden of non-typhoidal salmonella invasive disease: a systematic analysis for the Global Burden of Disease Study 2017. Lancet Infect. Dis. 2019;19:1312–1324. doi: 10.1016/S1473-3099(19)30418-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Steenackers H., Hermans K., Vanderleyden J., De Keersmaecker S.C.J. Salmonella biofilms: an overview on occurrence, structure, regulation and eradication. Food Res. Int. 2012;45:502–531. [Google Scholar]
- Stepanovic S., Cirkovic I., Ranin L., Svabic-Vlahovic M. Biofilm formation by Salmonella spp. and Listeria monocytogenes on plastic surface. Lett. Appl. Microbiol. 2004;38:428–432. doi: 10.1111/j.1472-765X.2004.01513.x. [DOI] [PubMed] [Google Scholar]
- Tack D.M., Ray L., Griffin P.M., Cieslak P.R., Dunn J., Rissman T., Jervis R., Lathrop S., Muse A., Duwell M., Smith K., Tobin-D'Angelo M., Vugia D.J., Kufel J.Z., Wolpert B.J., Tauxe R., Payne D.C. Preliminary incidence and trends of infections with pathogens transmitted commonly through food — foodborne diseases active surveillance network, 10 U.S. sites, 2016–2019. Mor. Mortal Wkly. Rep. 2020;69:510–514. doi: 10.15585/mmwr.mm6917a1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taj M.K., Ling J.X., Bing L.L., Qi Z., Taj I., Hassani T.M., Samreen Z., Yunlin W. Effect of dilution, temperature and pH on the lysis activity of T4 phage against E. coli BL21. J. Anim. Plant Sci. 2014;24:1252–1255. [Google Scholar]
- Tariq S., Samad A., Hamza M., Ahmer A., Muazzam A., Ahmad S., Amhabj A.M.A. Salmonella in poultry; an overview. Int. J. Mult. Sci. Arts. 2022;1:80–84. [Google Scholar]
- Tokman J.I., Kent D.J., Wiedmann M., Denes T. Temperature significantly affects the plaquing and adsorption efficiencies of Listeria phages. Front. Microbiol. 2016;7:631. doi: 10.3389/fmicb.2016.00631. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Toro H., Price S.B., McKee A.S., Hoerr F.J., Krehling J., Perdue M., Bauermeister L. Use of bacteriophages in combination with competitive exclusion to reduce Salmonella from infected chickens. Avian Dis. 2005;49:118–124. doi: 10.1637/7286-100404R. [DOI] [PubMed] [Google Scholar]
- Unden G., Steinmetz P.A., Degreif-Dünnwald P. The aerobic and anaerobic respiratory chain of Escherichia coli and Salmonella enterica: enzymes and energetics. EcoSal Plus. 2014;6 doi: 10.1128/ecosalplus.ESP-0005-2013. [DOI] [PubMed] [Google Scholar]
- US Food and Drug Administration (FDA). 2021. CVM GFI #263: Recommendations for sponsors of medically important antimicrobial drugs approved for use in animals to voluntarily bring under veterinary oversight all products that continue to be available over-the-counter.
- Venkitanarayanan K., Thakur S., Ricke S.C., editors. Food Safety in Poultry Meat Production. Springer International Publishing; Cham: 2019. [Google Scholar]
- Wallinga D., Smit L.A.M., Davis M.F., Casey J.A., Nachman K.E. A review of the effectiveness of current US policies on antimicrobial use in meat and poultry production. Curr. Envir. Health Rpt. 2022;9:339–354. doi: 10.1007/s40572-022-00351-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wójcik E.A., Stańczyk M., Wojtasik A., Kowalska J.D., Nowakowska M., Łukasiak M., Bartnicka M., Kazimierczak J., Dastych J. Comprehensive evaluation of the safety and efficacy of BAFASAL® bacteriophage preparation for the reduction of Salmonella in the food chain. Viruses. 2020;12:742. doi: 10.3390/v12070742. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xie Y., Wahab L., Gill J. Development and validation of a microtiter plate-based assay for determination of bacteriophage host range and virulence. Viruses. 2018;10:189. doi: 10.3390/v10040189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yonezawa H., Osaki T., Kamiya S. Biofilm formation by Helicobacter pylori and its involvement for antibiotic resistance. Biomed. Res. Int. 2015;2015 doi: 10.1155/2015/914791. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.