Abstract
Translation of small-diameter tissue-engineered vascular grafts (TEVGs) for the treatment of coronary artery disease (CAD) remains an unfulfilled promise. This is largely due to the limited integration of TEVGs into the native vascular wall—a process hampered by the insufficient smooth muscle cell (SMC) infiltration and extracellular matrix deposition, and low vasoactivity. These processes can be promoted through the judicious modulation of the SMC toward a synthetic phenotype to promote remodeling and vascular integration; however, the expression of synthetic markers is often accompanied by a decrease in the expression of contractile proteins. Therefore, techniques that can precisely modulate the SMC phenotypical behavior could have the potential to advance the translation of TEVGs. In this review, we describe the phenotypic diversity of SMCs and the different environmental cues that allow the modulation of SMC gene expression. Furthermore, we describe the emerging biomaterial approaches to modulate the SMC phenotype in TEVG design and discuss the limitations of current techniques. In addition, we found that current studies in tissue engineering limit the analysis of the SMC phenotype to a few markers, which are often the characteristic of early differentiation only. This limited scope has reduced the potential of tissue engineering to modulate the SMC toward specific behaviors and applications. Therefore, we recommend using the techniques presented in this review, in addition to modern single-cell proteomics analysis techniques to comprehensively characterize the phenotypic modulation of SMCs. Expanding the holistic potential of SMC modulation presents a great opportunity to advance the translation of living conduits for CAD therapeutics.
Impact statement
Tissue-engineered vascular grafts (TEVGs) are a promising approach to improve coronary artery bypass graft procedures. However, current approaches to vascular regeneration lack seamless tissue integration and are prone to stenosis and graft failure. In this review, we aim to present the potential of smooth muscle cell (SMC) modulation for TEVGs, targeting long-term patency and full integration. In particular, we describe the diversity of SMCs, state-of-the art techniques for phenotype modulation, and novel methods for translating TEVGs. Overall, this review can serve as a guide for the development of bioactive materials for vascular regeneration.
Keywords: smooth muscle cell phenotype, scaffolds, growth factors, vascular grafts, coronary artery disease, atherosclerosis
Introduction
Coronary artery disease (CAD) is the leading cause of death in adults worldwide.1–3 CAD arises from the accumulation of low-density lipoproteins in the coronary endothelium, which triggers an inflammatory response that recruits leukocytes, macrophages and smooth muscle cells (SMCs) to scavenge lipids.4–6 This subsequently leads to the formation of fibrous atherosclerotic plaques,5 which reduce the vessel diameter and limit myocardial blood circulation. Coronary artery bypass grafting (CABG) is the preferred therapy for restoring blood flow in severe artery occlusion,7,8 with several vessel types as autologous grafts, such as left internal thoracic artery or greater saphenous vein.8–11 Currently, ∼200,000 CABG procedures are performed each year;12,13 however, autologous tissue grafts face critical challenges, including post-implant stenosis, anastomotic compliance mismatch, and a low long-term patency.14,15 Therefore, there is a growing need for effective nonautologous grafts in CABG surgery.16,17
A promising alternative to autologous grafting is vascular tissue-engineered (VTE) conduits. Weinberg and Bell18 created the first TEVG by assembling layers of endothelial cells (ECs) and SMCs, but with insufficient resistance to pulsatile flow. Since then, the field has developed significantly.19–25 Nonetheless, TEVG translation to the clinic still faces limitations, such as the lack of an easily implantable TEVG with integrative potential and mechanical properties resembling those of the native vessel and low thrombogenesis.17,26 These limitations are even accentuated in TEVGs with a smaller vessel diameter (<6 mm) because of intimal hyperplasia or loss of patency.27
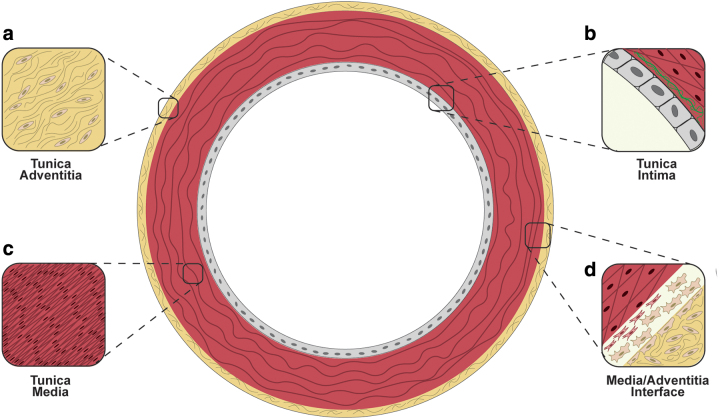
The vascular wall is a dynamic structure composed of three layers: the tunica intima—involved in antithrombogenesis,28 inflammatory processes,29 and mechanotransduction; the tunica media—the main effector of vascular tone due to the high contractility of SMCs and it contains a series of fenestrated elastin sheets (i.e., lamellae); and the tunica adventitia—composed of collagen fibers, myofibroblasts, and fibroblasts (Fig. 1a–d).30–33 This type of layered structure is particularly difficult to mimic due to the limited infiltration of SMCs into the polymeric and decellularized scaffolds.34–37 Such a technical limitation is mainly the failure to promote SMC migration and ECM deposition onto TEVGs.38
FIG. 1.
Layered structure of the coronary arterial wall: (a) the tunica adventitia and its fibrous microstructure, (b) monolayer of endothelial cells and their contact with the elastic lamina (in green) separating the intima and media, (c) highly aligned organization of smooth muscle cells in their contractile phenotype in vivo, and (d) the contact between the tunica media and the adventitia where myofibroblasts can migrate to repopulate the media. Color images are available online.
SMCs are involved in the vascular tone, and play a key role in tissue mechanics and remodeling. In particular, their contractile behavior is a response to sympathetic and parasympathetic cues.39,40 In addition, SMCs also exhibit important plasticity and can undergo phenotype modulation processes (e.g., switching to a noncontractile synthetic phenotype). In this state, contractile markers are downregulated, while proliferation, migration, and extracellular matrix (ECM) deposition are increased.41 This unique SMC phenotype switch occurs in pathological conditions such as vessel injury and atherosclerosis.42,43
Despite the potential of SMC phenotypical switching to promote cell migration and ECM deposition, this process is often overlooked in TEVG design and fabrication. Thus, this review aims to cover the diversity of SMC origins, various cues and modulators that regulate SMC phenotypes, different VTE strategies for SMC modulation, and their potential implications for translation of TEVGs to the clinic.
Embryonic origin of the SMC diversity
SMCs in different regions of the systemic vasculature do not share a unique embryological origin. For example, coronary SMCs arise from proepicardial cells, which are developed at the proepicardium and are precursors of the complete coronary wall.44,45 SMC differentiation involves the transformation of proepicardial cells into mesenchymal cells by FOG-2 protein signaling.45,46 Then, the stimulation of proepicardium-derived mesenchymal cells with the platelet-derived growth factor (PDGF) and the epidermal growth factor results in the differentiated coronary SMCs.47–49 This embryological origin of coronary SMCs is described as an indicator of the characteristic behavior of coronary artery tissues in response to mechanical stimuli, environmental cues, and pathologies.50,51 Besides this contractile/proliferative duality, SMCs also possess in a wide spectrum of genetic expressions.38,52 Therefore, a systematic understanding of the behaviors of SMC phenotype and diversity is crucial for TEVG design.
SMC phenotype modulation
The SMC lineage is diverse and difficult to identify under pathological conditions. Efforts have been made to establish the markers for SMC identification in atherosclerotic lesions and inflammatory processes.41,53 For example, lineage tracking has been developed to characterize the fate of this cell line,43 thereby allowing the detection of various SMC phenotypes (Fig. 2). The key SMC phenotypes and phenotype switching will be described below.
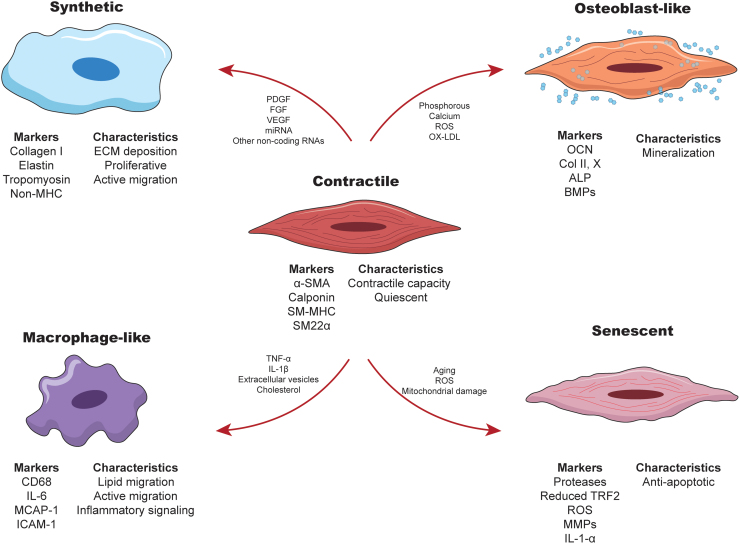
FIG. 2.
Schematics representing the various SMC phenotypes, their respective markers, and the main characteristics. Arrows indicate the modulators described to induce modulation of the contractile SMC toward each phenotype. For abbreviations, please see Table 1. Color images are available online.
Contractile phenotype
The SMC contractile phenotype is the differentiated version of the cell line characterized by the expression of alpha-smooth muscle actin (α-SMA), smooth muscle myosin heavy chain (SM-MHC), SM22α, and SM-calponin, and alpha-tropo-myosin.54 Contractile markers enable the SMCs to exert mechanical forces to control the vascular tone.55 Maintenance of this contractile phenotype can be regulated by the transforming growth factor (TGF),56–58 insulin-like growth factor,59,60 circumferential stress,61 and shear stress.59,60,62
Synthetic phenotype
Alterations in environmental cues and ECM microstructure can lead to a decreased expression of SMC contractile markers, an increased proliferation, and an upregulation of ECM deposition (e.g., type I collagen, elastin, laminin, fibronectin).63,64 Switching from a contractile phenotype to a synthetic/proliferative one is facilitated by a cross talk of signals.63,64 For example, the PDGF has been shown to play an antagonistic role in SMC differentiation65,66 through phosphorylation of Elk-1 that competes with myocardin for the docking site on the serum response factor (SRF).67 Furthermore, the fibroblast growth factors (FGFs) are another group of potent mitogens in the SMCs that promote activation of the Ras/MAPK signaling pathway associated with SMC proliferation and migration.68–70 Interestingly, FGFs can also inhibit the activity of TGF-β in the upregulation of contractile markers.57 Vascular endothelial growth factor (VEGF), S100A4, micro-RNAs, and other noncoding RNAs are found to also promote synthetic phenotype switching.71–75
Other SMC phenotypes
In addition to the canonical duality, the SMCs can undergo modulation to other phenotypes. For example, a proinflammatory SMC exhibits downregulation of traditional contractile markers along with a large increase in the expression of inflammatory markers (e.g., MCP-1, MMP-3, MMP-9, VCAM-1, CCL5, CCL20, CXCL6, CXCR1,76–79 see Table 1 for the definition of these abbreviations). The inflammatory phenotype promotes SMC migration and proliferation during early stages of atherosclerosis and plays a key role in cytokine release to promote inflammatory conditions.80,81 This inflammatory behavior can be induced by different inflammatory signals, such as the tumor necrosis factor-alpha (TNF-α),77 interleukin-1 beta (IL-1β),79 extracellular vesicles78 and cholesterol.76,79
Table 1.
List of Abbreviations Used in This Article and Their Definitions
| Abbreviation | Definition |
|---|---|
| α-SMA | Alpha-smooth muscle actin |
| ABCA1 | ATP-binding cassette transporter A1 |
| Apoe | Apolipoprotein E |
| CABG | Coronary artery bypass grafting |
| CAD | Coronary artery disease |
| CCL20 | Chemokine ligand-20 (CC motif) |
| CCL5 | Chemokine ligand-5 (CC motif) |
| CD31 | Cluster of differentiation 31 |
| CD44 | Cluster of differentiation 44 |
| CD68 | Cluster of differentiation 68 |
| CXCL6 | Chemokind ligand-6 (CXC motif) |
| CXCR1 | Chemokind ligand-1 receptor (CXC motif) |
| EC | Endothelial cell |
| ECM | Extracellular matrix |
| Elk-1 | E26 transformation-specific-like 1 |
| EPL4 | Elastin-like polypeptide-4 |
| FGF | Fibroblast growth factor |
| FOG-2 | Friend of GATA-2 |
| ICAM-1 | Intercellular adhesion molecule-1 |
| IL-1-α | Interleukin-1 alpha |
| IL-1β | Interleukin-1 beta |
| IL-6 | Interleukin-6 |
| MCAP-1 | Mitotic chromosome-associated protein-1 |
| MCP-1 | Monocyte chemoattractant protein-1 |
| MMP-3 | Matrix metalloproteinase-3 |
| MMP-9 | Matrix metalloproteinase-9 |
| MPAK | Mitogen-activated protein kinases |
| PAH-PAA | Poly(allylamine hydrochloride)/poly(acrylic acid) |
| PCL | Polycaprolactone |
| PDGF | Platelet-derived growth factor |
| PELA | poly(DL-lactide)–poly(ethylene glycol) |
| PLLA-PCL | Poly(L-lactide-co-caprolactone)/poly(L-lactic acid) |
| RADA | RADARADARADARADA (Aminoacid sequence) |
| Ras | Rat sarcoma protein |
| RNA | Ribonucleic acid |
| RT-PCR | Reverse-transcription polymerase chain reaction |
| SM-MHC | Smooth muscle myosin heavy chain |
| SM22α | Smooth muscle protein 22-alpha |
| SMC | Smooth muscle cell |
| SRF | Serum response factor |
| TCF21 | Transcription factor 21 |
| TEVG | Tissue-engineered vascular graft |
| TGF | Transforming growth factor |
| TGF-β | Transforming growth factor-beta |
| TNF-α | Tumor necrosis factor-alpha |
| TRF2 | Telomeric repeat-binding factor-2 |
| VCAM-1 | Vascular cell adhesion molecule-1 |
| VEGF | Vascular endothelial growth factor |
| VTE | Vascular tissue engineering |
The SMC may also exhibit a macrophage-like phenotype that shows upregulation of scavenger markers and CD68, without expression of α-SMA.6,76,82–85 In this scavenging process, lipid phagocytosis by SMCs is driven by the ATP-binding cassette transporter A1(ABCA1)—a protein that enables cholesterol influx. However, ABCA1 expression in SMCs is lower than in myeloid macrophages,6,86,87 leading to lipid accumulation and the formation of SMC foam cells.6,86 Interestingly, this macrophage-like transformation is transient and reversible, allowing SMCs to revert to a contractile phenotype, suggesting an atheroprotective role for SMCs in the early stages of CAD.88
Another dynamic fibroblast-like phenotype has also been described for SMCs. As mentioned previously, proepicardial cells can become cardiac fibroblasts or SMCs. These precursor cells express the transcription factor (TCF21) is maintained in cardiac fibroblasts, but downregulated in the SMCs.89,90 However, in atherosclerosis this marker is upregulated in the SMCs.91,92 For example, Wirka et al.92 found that the SMCs in Apoe-/- atherosclerotic mice exhibited a decrease in contractile markers and an upregulation in fibroblast markers. They also observed that these SMCs did not transition into a macrophage-like phenotype, but became more distant to the monocyte-derived macrophages.93
Furthermore, SMCs have been described as a key driver of vascular wall calcification.94,95 This occurs when the SMC is modulated into an osteoblast-like phenotype associated with the downregulation of contractile markers.96,97 This osteogenic phenotype is characterized by the formation and deposition of calcifying vesicles that are mineralization components made of phosphatidylserine and are involved in atherosclerosis.98–100 Modulation to an osteoblast-like phenotype, and hence calcification, is mediated by RUNX2, a transcription factor that drives the differentiation of mineralizing cells.101–103
Finally, the SMC can also undergo senescence due to aging or atherogenic factors (e.g., oxidative stress, inflammation, etc.). Senescence is associated with telomere dysfunction due to reduced expression of telomeric repeat-binding factor-2 (TRF2).104 During atherosclerosis, senescent vascular SMCs may only be present in the intima,105 where they may contribute to plaque instability due to protease release and decreased collagen production.106 The senescent SMCs may also induce proinflammatory behaviors in the neighboring SMCs and ECs,107,108 resulting in the atherogenic behavior of the vascular wall.
Overall, the SMC exhibits a broad spectrum of phenotypical expressions highly dependent on environmental cues. Due to this environment-sensitive nature, the use of SMCs in the production of TEVGs requires a thorough understanding of their behavior when cultured on biomaterials to prevent unwanted marker expressions and adverse effects on the neighboring cells. This advanced knowledge can ultimately promote their integration into the native coronary tissue. In the next section, we will describe the state-of-the-art research on SMC phenotype modulation in the context of TEVG development.
SMC phenotype modulation for vascular tissue engineering
Over the past few decades, VTE has aimed to produce TEVGs, beginning with the work of Weinberg and Bell18 to the recent success of acellular vessels for the access of hemodialysis.109 However, these developments still suffer from the limitations with SMC infiltration into polymeric substrates and the incomplete integration of TEVGs into the native tissue.17 Ideally, modulation of the SMC phenotype through careful biomaterial design to achieve specific SMC behaviors offers a promising approach to advance the development of TEVGs. In the following section, we will summarize different approaches to modulate SMC phenotype with biomaterials (Fig. 3).
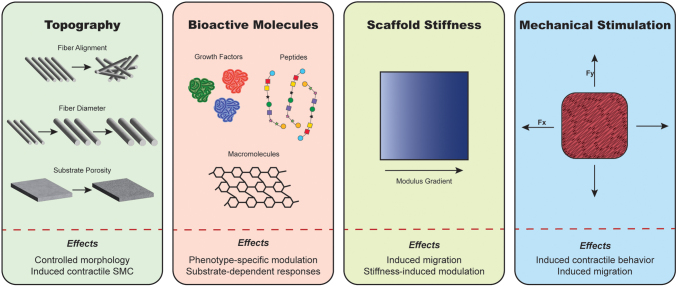
FIG. 3.
Graphical representation of the most prominent methods for modulating SMC phenotype using biomaterial properties and mechanical stimulation, and their most representative effects. Color images are available online.
Tuning of scaffold topography
Modifying scaffold topography is an effective method to modulate SMC phenotypes. The topography of the substrate on which the SMCs are seeded has a strong influence on factors such as cell morphology. Zhu et al.110 characterized the behavior of SMCs when cultured in fibrin-coated polycaprolactone (PCL) electrospun scaffolds and showed that the SMCs were distributed along the fibers and exhibited a similar morphology to the native contractile phenotype. Conversely, random fibers resulted in heterogenous SMC shapes that resembled the synthetic phenotype morphology. Their findings were confirmed by various studies using electrospun and meltspun PCL fibers in conjunction with other polymers,111,112 where it was reported that aligned fibers sustained the expression of α-SMA, calponin, and smoothelin.110–114
Tijore et al.115 used micropatterned gelatin scaffolds for topographic modulation and found that highly aligned cell organization could mimic the native state of the contractile SMCs (Fig. 4a), as evidenced by the upregulation of α-SMA and SM-MHC expressions in the patterned constructs (Fig. 4b). However, the studies on topographical cues in the literature seem to be conflicting: Zeng et al.113 observed increased proliferation on scaffolds with aligned topographies, while Tijore et al.115 found higher proliferation in flat, nonstructured gels.115 In general, aligned fibers and patterned structures promote higher expressions of contractile markers. However, the expression of synthetic and inflammatory markers in different types of topographies has not been systematically characterized.
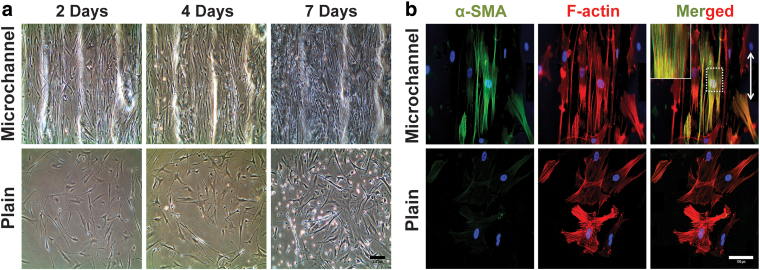
FIG. 4.
(a) Microscopic images of vascular smooth muscle cells grown on microchanneled and plain gelatin substrates: controlled topography induces elongated and aligned SMCs on microchannels and plain substrate allows random elongation of actin fibrils. (Scale bar = 200 μm). (b) Alpha smooth muscle actin (α-SMA, green), F-actin (red), DAPI (nuclei, blue), and merged actin expression in SMCs cultured in the microchanneled and plain substrate after 7 days (white arrow indicates fiber orientation; scale bar = 100 μm). Images by Tijore et al.115 with permission from Springer. Color images are available online.
Another important parameter in the topography of fibrous structures is fiber diameter. Han et al.116 found that thinner fibers (0.5–1 μm) resulted in higher proliferation than thicker ones (5–10 μm). In contrast, cell infiltration was higher (nearly complete) for scaffolds with thicker fibers. To better understand these phenomena, they further characterized the expression of contractile and synthetic markers. Interestingly, they found that α-SMA was similar across all scaffolds, while non-MHC increased over time in scaffolds with thicker fibers, suggesting potential SMC modulation toward a synthetic phenotype.
Bioactive molecules as stimuli
Biochemical cues are fundamental for the modulation of SMC phenotype in vivo and in vitro. Thus, bioactive molecules can be used to modulate the SMC phenotype in cultured scaffolds. For example, Liu et al.117 stimulated SMCs using VEGF-encoding plasmids distributed in a poly(DL-lactide)–poly(ethylene glycol) (PELA) solution to fabricate electrospun scaffolds. They found that when cells were cultured in plasmid-containing scaffolds, SMCs were elongated accompanied by a greater proliferative capacity and increased expressions of α-SMA and type I collagen, suggesting a switch to a synthetic phenotype and an early contractile behavior. This approach takes the advantage of nanotechnology to produce the growth factors essential for SMC proliferation; however, it was unclear whether the SMC phenotype switching could target a specific phenotype, as the advanced contractile markers were not characterized in their work.
In addition, Ardila et al.118 used electrospun scaffolds with different gelatin/fibrinogen mass ratios and stimulated seeded SMCs with TGF-β2. First, they found that all scaffolds exhibited a similar level of cell migration through the entire scaffold depth. Second, the SMC proliferation was found to be higher at a mass ratio of 80:20 (gelatin:fibrinogen), but lower on a pure gelatin scaffold, indicating an important role played by the ECM structures. When the cultured scaffolds were exposed to lower concentrations of TGF-β2 (≤1 ng/mL), it was found that both cell proliferation and migration were promoted, while higher concentrations of TGF-β2 stopped cell replication and reduced migration. These findings correlated with the behavior of SMCs at different TGF-β2 concentrations,53,57,119–121 demonstrating a straightforward method for switching the SMC phenotype in cultured scaffolds.
An alternative approach to biochemically stimulate SMCs is the use of peptides. Peptides are short amino acid chains that can mimic the functionality of whole proteins and can be conjugated to polymeric matrices for tissue engineering applications.122 For example, elastin-like polypeptide-4 (EPL4), with a tropoelastin-like structure and function, has been used to functionalize electrospun polyurethane scaffolds to promote the proliferation and expression of contractile markers (α-SMA and SM-MHC).123 Another example is the use of the RADA peptide to promote angiogenesis and myocardial infarction lesions in vivo.124 Other angiogenic peptides also exhibit the efficacy in EC recruitment to angiogenesis: for example, QK and PAB2–1c can mimic the activities of VEGF and PDGF, respectively.125–127 However, their potential as conjugates to polymeric scaffolds to modulate SMC phenotype has not been fully explored.
Finally, the incorporation of ECM macromolecules into scaffolds was also studied. For example, heparin was covalently linked to electrospun fibers,34 and SMCs were found to be able to infiltrate scaffolds and exhibit higher expression of α-SMA, calponin, and SM-MHC. Later, Geng et al.128 used a heparin/PCL scaffold with a hydrogel precursor for SMC modulation. The hydrogel-enriched scaffold exhibited an increased tensile strength/burst pressure and a higher suture strength than the pure PCL scaffold, but resulted in decreased SMC proliferation. Moreover, these scaffolds exhibited an excellent patency 6 months after implantation in Wistar rats. Additionally, histology confirmed complete SMC infiltration, collagen and elastin deposition, and mild calcification, while immunohistochemistry showed a potential SMC contractile phenotype. The co-localization of von Willebrand factor (EC specific) and α-SMA, as shown in fluorescence microscopy, further suggested that the cells populating the scaffold were of different origins or from distinct differentiation pathways.
Tuning of scaffold stiffness
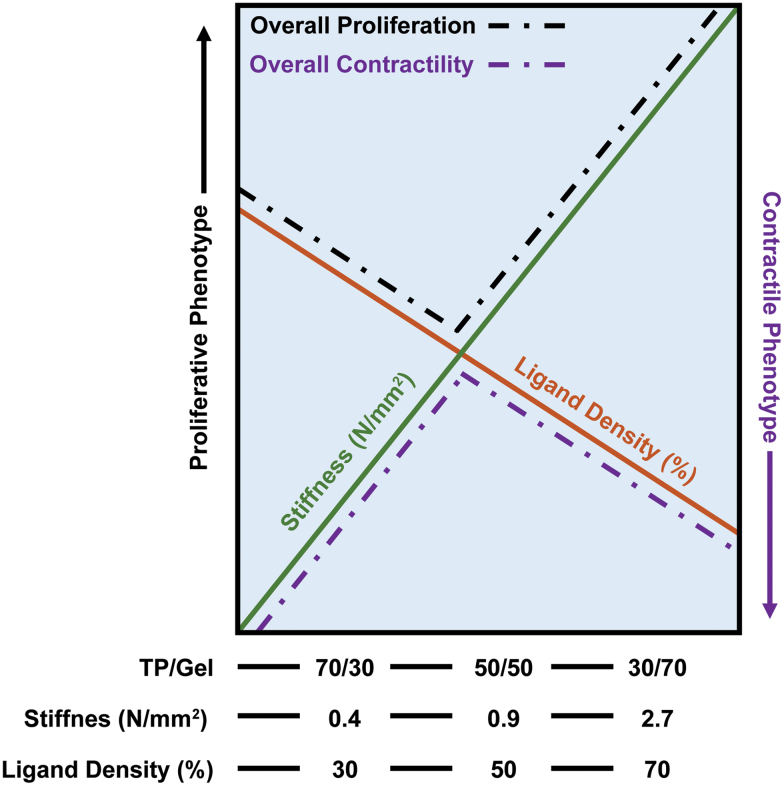
SMCs are inherently mechanosensitive to the expression of several molecules, such as membrane receptors, integrins and ion channels.129 The mechanical properties of biomaterials are fundamental to the modulation of SMC phenotypes. For example, Vatankhah et al.130 studied how the stiffness of electrospun tecophilic/gelatin fibers influenced the phenotypical behavior of human aortic SMCs. This study showed that SMC phenotypic behavior is highly dependent on scaffold stiffness: lower stiffness led to a significant reduction in SMC proliferation than the control (i.e., tissue culture plate, no scaffold), while the stiffest scaffold (30/70 tecophilic/gelatin) achieved the same proliferation rate as the control.
Furthermore, contractile phenotype markers (α-SMA and SM-MHC) were more highly expressed in the stiffest scaffold, while the expression of synthetic markers (e.g., type I collagen deposition) was highest in the most compliant scaffold (70/30 tecophilic/gelatin; Fig. 5). They also coated the compliant scaffold with a gelatin layer, resulting in a more contractile phenotype, together with a reduction in cell proliferation.
FIG. 5.
Diagram of the interaction between ligand density and stiffness of the culture substrate observed in the tecophilic/gelatin electrospun scaffolds. Reprinted (adapted) from Vatankhah et al.130 with permission from the American Chemical Society. Color images are available online.
On the other hand, Moussallem et al.131 used poly(allylamine hydrochloride)/poly(acrylic acid) (PAH-PAA) multilayers to modulate the SMC phenotype. Their RT-PCR results showed that the expression of contractile markers was enhanced in the cross-linked scaffold. Consequently, the noncross-linked material had a higher expression of synthetic markers than its cross-linked counterpart, indicating a contractile phenotype with a stiffer substrate.
In addition, Yi et al.132 examined the effects of electrospun fiber stiffness on SMC modulation using a poly(L-lactide-co-caprolactone)/poly(L-lactic acid) (PLLA-PCL) scaffold. They found that scaffold stiffness during electrospinning was directly proportional to the flow rate of the polymeric solution: that is, stiffer scaffolds promoted a higher rate of cell migration, which was confirmed by an increased expression of CD44.133 Moreover, they showed that α-SMA expression was homogenous among all scaffold groups, but SM-MHC expression was reduced with increasing stiffness, indicating that the transition to a fully contractile phenotype was halted.
They also observed that osteopontin was upregulated in the stiffer scaffolds, indicating a switch of SMCs to a proinflammatory phenotype.134,135 Other markers were also affected by the increased scaffold stiffness: (i) the reduction of desmin—a key structural component in the SMC microstructure136 and (ii) the upregulation of interleukin 6 (IL-6), monocyte chemoattractant protein-1 (MCAP-1), and intercellular adhesion molecule 1 (ICAM-1)—markers observed in the macrophage-like SMC phenotype.136–139
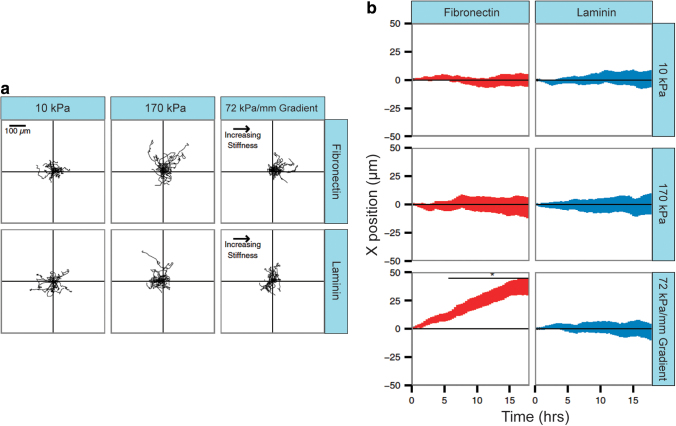
As demonstrated in these studies, SMCs can undergo a migration process depending on stiffness gradients (a process known as durotaxis).140 This process was described by Hartman et al.141 as a substrate-dependent process. They cultured SMCs on polyacrylamide with stiffness gradients coated with different ECM components: fibronectin and laminin (Fig. 6a, b). First, they observed that stiffer gels without specific directionality or preferential ECM coating provoked increased migration velocities. However, when gradient-stiffness gels were prepared, the fibronectin-coated gels exhibited a marked directionality in cell migration.
FIG. 6.
(a) Migration tracks of vSMCs on two different substrates with constant (10 and 170 kPa) and varying (72 kPa/mm) stiffness. VSMCs show a movement toward increased stiffness gradients on fibronectin substrates, but not on laminin. (b) Average position of vascular SMCs for different substrates (fibronectin: red and laminin: blue) and stiffnesses. Reprinted (adapted) from Hartman et al.141 with permission from Proceedings of the National Academy of Sciences. vSMCs, vascular smooth muscle cells. Color images are available online.
These observations indicate not only that SMCs are uniquely sensitive to stiffness gradients but also that their migration processes are selective as a function of the ECM substrate. This group also showed that this ECM-specific durotactic behavior can affect the SMC phenotype: increasing laminin stiffness resulted in a more contractile phenotype (increased myosin light chain expression), while increased stiffness in fibronectin substrates promoted a synthetic phenotype.142 The observed behaviors may explain the increased rate of SMC migration and phenotype modulation during atherosclerosis in vivo.143
Mechanical stimuli through bioreactors
The SMC is subjected to different mechanical stimuli in vivo, including pulsatile pressure and shear stress.55,144 These stimuli are transmitted between cells145,146 and can alter gene expression.147 Therefore, several VTE studies have aimed to modulate the SMC phenotype through mechanical stimuli. An important historical contribution was made by Niklason et al.,23 where they used bioreactors to emulate physiological pulsatile conditions to stimulate cultured scaffolds. Since then, countless studies have emerged to understand the effects of mechanical stimuli on SMC phenotype expression. For example, Sharifpoor et al.148 assessed SMC markers during culture on a polyurethane scaffold and reported that calponin expression increased under cyclic strain, accompanied by proliferation, and increased DNA concentration.
These results were “confounding” because these behaviors could indicate conflicting phenotypes. They later conducted longer-term studies that observed similar behaviors.149 However, it is not uncommon to observe contradictory phenotypical behaviors in vivo: Pan et al.150 described an intermediate SMC phenotype with inflammatory and synthetic behaviors in single-cell genomics and lineage tracing of mice and human atherosclerotic plaques.
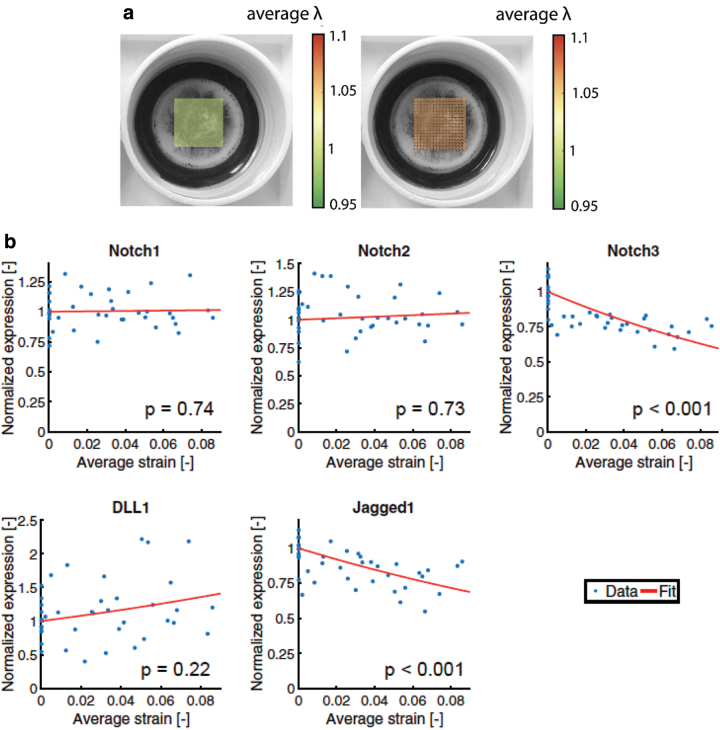
Loerakker et al.151 performed another interesting study of the effects of mechanical strain on SMCs, in which SMCs were cultured on flexible culture plates and subjected to mechanical strain (Fig. 7a). They focused on the Notch signaling pathway, which is crucial for the expression of contractile SMC markers.56 They observed that mechanical strain ranging from 1% to 9% resulted in a decrease in the Notch 3 receptor (which facilitates PDGF-induced proliferation in vivo152) and Jagged1 ligand (promotes signaling to neighboring SMCs152–155), indicating that mechanical strain promotes the quiescent contractile phenotype (Fig. 7b).
FIG. 7.
(a) Digital image correlation to quantify stretch λ applied to elastic culture membranes on which the smooth muscle cells have been cultured. (b) Changes in Notch receptor expression and DLL1 and Jagged1 ligands as a function of average strain applied. Reprinted (adapted) from Loerakker et al.151 with permission from Proceedings of the National Academy of Sciences. Color images are available online.
In addition, Gong and Niklason156 investigated the combined effects of mechanical strain and ECM components on SMC marker expression. They found that fibronectin, elastin, and type I collagen had a significant effect on reducing proliferation under cyclic strain. Furthermore, they observed that α-SMA expression decreased when cells were cultured on fibronectin, but increased when cultured on type I collagen, suggesting that the phenotypic response to mechanical stimuli is substrate dependent, similar to the findings of Hartman et al.141 on the migratory behaviors of SMC. This proliferative behavior after short-term mechanical stimulation could be explained by the observations of Sun et al.,157 where SMCs switched to a pro-inflammatory behavior upon shear stress. Nonetheless, there is currently no consensus on how mechanical stimuli affect the SMC phenotype in cultured scaffolds. See Jensen et al.158 for a more comprehensive review on the mechanical stimulation for SMCs.
Effects of ECM substrates
In the previous sections, we discussed the modulation of SMC phenotype using different techniques (Fig. 3). These studies we reviewed also showed that SMC modulation depended on the presence/absence or concentration of ECM substrates. For example, the study by Geng et al.128 demonstrated the use of heparin-based enrichment in PCL scaffolds; Ardila et al.118 observed the effects of different gelatin:fibrinogen mass ratios on TGF-β2-dependent SMC modulation, and Hartman et al.141 observed substrate- and stiffness-dependent cell migration. Therefore, it is also imperative that SMC modulation is not addressed as a “one-modulator-only” phenomenon, but as a complex adaptive mechanism of SMCs.
Conclusion
The modulation of the SMC phenotype is a complex and controversial topic. The implications of SMC modulation in atherosclerosis and vascular remodeling are still an emerging research topic. In this review, we have discussed various SMC phenotypes described in the literature, the modulators that can induce each phenotype, and the prevailing techniques in VTE for SMC modulation. VTE of small-diameter vessels still faces major challenges that correlate with the phenotypic expression of SMCs in the TEVGs. From this review, we concluded that the development of TEVGs must incorporate a more thorough assessment of the SMC markers and their maintenance/modulation. In particular, studies of the effect of material properties on the SMC phenotype can benefit from the modern lineage tracing and single-cell analysis techniques available today.52,150
These techniques can eventually help inform the design of biomaterial-based modulation methods for the SMC phenotype. Few studies have characterized the full spectrum of the SMC phenotype in response to their proposed stimuli for modulation. Therefore, we recommend that research focus on studying SMC modulation through a holistic combination of the methods presented in this review and benefit from their overall effects on SMC phenotype. For example, the combined use of biomolecules and topographical cues can help obtain dense SMC populations in TEVGs before contractility is induced and, thus, more desirable mechanical properties can be produced.
In particular, the use of peptides or growth factors can promote modulation toward a synthetic phenotype for full cell infiltration and high proliferation across scaffolds. Once these biochemical cues are depleted through degradation or loss of function, topographical cues (e.g., using highly aligned electrospun fibers) can cause the SMC to switch back to a contractile phenotype. With a thorough understanding of the SMC phenotype in response to these stimuli, translation of TEVGs may achieve the long-awaited promise of providing nonautologous grafts for coronary bypass.
Authors' Contributions
S.A.P.-C. contributed to conceptualization, literature search, writing the original draft, and design of in-house figures. H.A. contributed to conceptualization. M.S.D. and G.A.H. contributed to review and editing. C.-H.L. contributed to conceptualization, supervision, and funding acquisition. All authors read and approved the final article.
Disclosure Statement
No competing financial interests exist.
Funding Information
The authors acknowledge support from the National Institutes of Health (NIH) Grant R01 HL159475. S.A.P.-C was supported, in part, by the University of Oklahoma Graduate College Alumni Fellowship.
References
- 1. Virani SS, Alonso A, Benjamin EJ, et al. Heart disease and stroke statistics-2020 update: A report from the American Heart Association. Circulation 2020;141(9):e139–e596; doi: 10.1161/CIR.0000000000000757 [DOI] [PubMed] [Google Scholar]
- 2. Roth GA, Johnson C, Abajobir A, et al. Global, regional, and national nurden of cardiovascular diseases for 10 causes, 1990 to 2015. J Am Coll Cardiol 2017;70(1):1–25; doi: 10.1016/j.jacc.2017.04.052 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. GBD 2019 Diseases and Injuries Collaborators. Global burden of 369 diseases and injuries in 204 countries and territories, 1990–2019: A systematic analysis for the Global Burden of Disease Study 2019. Lancet 2020;396(10258):1204–1222; doi: 10.1016/S0140-6736(20)30925-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Yu XH, Fu YC, Zhang DW, et al. Foam cells in atherosclerosis. Clin Chim Acta 2013;424: 245–252; doi: 10.1016/j.cca.2013.06.006 [DOI] [PubMed] [Google Scholar]
- 5. Malakar AK, Choudhury D, Halder B., et al. A review on coronary artery disease, its risk factors, and therapeutics. J Cell Physiol 2019;234(10):16812–16823; doi: 10.1002/jcp.28350 [DOI] [PubMed] [Google Scholar]
- 6. Allahverdian S, Chehroudi AC, McManus BM, et al. Contribution of intimal smooth muscle cells to cholesterol accumulation and macrophage-like cells in human atherosclerosis. Circulation 2014;129(15):1551–1559; doi: 10.1161/CIRCULATIONAHA.113.005015 [DOI] [PubMed] [Google Scholar]
- 7. Khan MS. Coronary Artery Bypass Grafting: Surgical Anastomosis: Tips and Tricks. In: The Current Perspectives on Coronary Artery Bypass Grafting. (Murashita T. ed.) IntechOpen: London, United Kingdom; 2020; pp. 79–108. [Google Scholar]
- 8. Alexander JH, Smith PK. Coronary-artery bypass grafting. N Engl J Med 2016;375(10):e22; doi: 10.1056/NEJMc1608042 [DOI] [PubMed] [Google Scholar]
- 9. Al-Sabti HA, Al Kindi A, Al-Rasadi K, et al. Saphenous vein graft vs. radial artery graft searching for the best second coronary artery bypass graft. J Saudi Heart Assoc 2013;25(4):247–254; doi: 10.1016/j.jsha.2013.06.001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Otsuka F, Yahagi K, Sakakura K, et al. Why is the mammary artery so special and what protects it from atherosclerosis? Ann Cardiothorac Surg 2013;2(4):519–526; doi: 10.3978/j.issn.2225-319X.2013.07.06 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Hu X, Zhao Q. Systematic comparison of the effectiveness of radial artery and saphenous vein or right internal thoracic artery coronary bypass grafts in non-left anterior descending coronary arteries. J Zhejiang Univ Sci B 2011;12(4):273–279; doi: 10.1631/jzus.B1000241 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Kim LK, Looser P, Swaminathan RV, et al. Outcomes in patients undergoing coronary artery bypass graft surgery in the United States based on hospital volume, 2007 to 2011. J Thorac Cardiovasc Surg 2016;151(6):1686–1692; doi: 10.1016/j.jtcvs.2016.01.050 [DOI] [PubMed] [Google Scholar]
- 13. Weiss AJ, Elixhauser A.. Trends in operating room procedures in U.S. hospitals, 2001–2011: Statistical Brief #171. In: Healthcare Cost and Utilization Project (HCUP) Statistical Briefs. Rockville, MD; 2006. [Google Scholar]
- 14. Tinica G, Chistol RO, Enache M, et al. Long-term graft patency after coronary artery bypass grafting: Effects of morphological and pathophysiological factors. Anatol J Cardiol 2018;20(5):275–282; doi: 10.14744/AnatolJCardiol.2018.51447 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Tiwari A, Cheng KS, Salacinski H, et al. Improving the patency of vascular bypass grafts: The role of suture materials and surgical techniques on reducing anastomotic compliance mismatch. Eur J Vasc Endovasc Surg 2003;25(4):287–295; doi: 10.1053/ejvs.2002.1810 [DOI] [PubMed] [Google Scholar]
- 16. Lampridis S, George SJ. Non-autologous grafts in coronary artery bypass surgery: A systematic review. Ann Thorac Surg 2020; 112(6): 2094–2103; doi: 10.1016/j.athoracsur.2020.11.028 [DOI] [PubMed] [Google Scholar]
- 17. Dimitrievska S, Niklason LE. Historical perspective and future direction of blood vessel developments. Cold Spring Harb Perspect Med 2018;8(2):a025742; doi: 10.1101/cshperspect.a025742 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Weinberg CB, Bell E. A blood vessel model constructed from collagen and cultured vascular cells. Science 1986;231(4736):397–400; doi: 10.1126/science.2934816 [DOI] [PubMed] [Google Scholar]
- 19. L'Heureux N, Dusserre N, Konig G, et al. Human tissue-engineered blood vessels for adult arterial revascularization. Nat Med 2006;12(3):361–365; doi: 10.1038/nm1364 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. L'Heureux N, Germain L, Labbe R, et al. In vitro construction of a human blood vessel from cultured vascular cells: A morphologic study. J Vasc Surg 1993;17(3):499–509; doi: 10.1067/mva.1993.38251 [DOI] [PubMed] [Google Scholar]
- 21. L'Heureux N, Paquet S, Labbe R, et al. A completely biological tissue-engineered human blood vessel. FASEB J 1998;12(1):47–56; doi: 10.1096/fasebj.12.1.47 [DOI] [PubMed] [Google Scholar]
- 22. L'Heureux N, McAllister T. Cytograft tissue sngineering: A new paradigm in cardiovascular tissue engineering. Regen Med 2008;3: 471–475; doi: 10.2217/17460751.3.4.471 [DOI] [Google Scholar]
- 23. Niklason LE, Gao J, Abbott WM, et al. Functional arteries grown in vitro. Science 1999;284(5413):489–493; doi: 10.1126/science.284.5413.489 [DOI] [PubMed] [Google Scholar]
- 24. Dahl SLM, Koh J, Prabhakar V, et al. Decellularized native and engineered arterial scaffolds for transplantation. Cell Transpl 2003;12(6):659–666; doi: 10.3727/000000003108747136 [DOI] [PubMed] [Google Scholar]
- 25. Higgins SP, Solan AK, Niklason LE. Effects of polyglycolic acid on porcine smooth muscle cell growth and differentiation. J Biomed Mater Res A 2003;67(1):295–302; doi: 10.1002/jbm.a.10599 [DOI] [PubMed] [Google Scholar]
- 26. Song HHG, Rumma RT, Ozaki CK, et al. Vascular tissue engineering: Progress, challenges, and clinical promise. Cell Stem Cell 2018;22(3):340–354; doi: 10.1016/j.stem.2018.02.009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Dahl SL, Kypson AP, Lawson JH, et al. Readily available tissue-engineered vascular grafts. Sci Transl Med 2011;3(68):68ra9; doi: 10.1126/scitranslmed.3001426 [DOI] [PubMed] [Google Scholar]
- 28. Yau JW, Teoh H, Verma S. Endothelial cell control of thrombosis. BMC Cardiovasc Disord 2015;15: 130; doi: 10.1186/s12872-015-0124-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Muller WA. How endothelial cells regulate transmigration of leukocytes in the inflammatory response. Am J Pathol 2014;184(4):886–896; doi: 10.1016/j.ajpath.2013.12.033 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Pugsley MK, Tabrizchi R. The vascular system. An overview of structure and function. J Pharmacol Toxicol Meth 2000;44(2):333–340; doi: 10.1016/s1056-8719(00)00125-8 [DOI] [PubMed] [Google Scholar]
- 31. Zeng Y, Tarbell JM. The adaptive remodeling of endothelial glycocalyx in response to fluid shear stress. PLoS ONE 2014;9(1):e86249; doi: 10.1371/journal.pone.0086249 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Wagenseil JE, Mecham RP. Vascular extracellular matrix and arterial mechanics. Physiol Rev 2009;89(3):957–989; doi: 10.1152/physrev.00041.2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Sherifova S, Holzapfel GA. Biomechanics of aortic wall failure with a focus on dissection and aneurysm: A review. Acta Biomater 2019;99: 1–17; doi: 10.1016/j.actbio.2019.08.017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Cao J, Geng X, Wen J, et al. The penetration and phenotype modulation of smooth muscle cells on surface heparin modified poly(varepsilon-caprolactone) vascular scaffold. J Biomed Mater Res A 2017;105(10):2806–2815; doi: 10.1002/jbm.a.36144 [DOI] [PubMed] [Google Scholar]
- 35. Ju YM, Ahn H, Arenas-Herrera J, et al. Electrospun vascular scaffold for cellularized small diameter blood vessels: A preclinical large animal study. Acta Biomater 2017;59: 58–67; doi: 10.1016/j.actbio.2017.06.027 [DOI] [PubMed] [Google Scholar]
- 36. Ye L, Cao J, Chen L, et al. The fabrication of double layer tubular vascular tissue engineering scaffold via coaxial electrospinning and its 3D cell coculture. J Biomed Mater Res A 2015;103(12):3863–3871; doi: 10.1002/jbm.a.35531 [DOI] [PubMed] [Google Scholar]
- 37. Duan N, Geng X, Ye L, et al. A vascular tissue engineering scaffold with core–shell structured nano-fibers formed by coaxial electrospinning and its biocompatibility evaluation. Biomed Mater 2016;11(3):035007; doi: 10.1088/1748-6041/11/3/035007 [DOI] [PubMed] [Google Scholar]
- 38. Hao H, Ropraz P, Verin V, et al. Heterogeneity of smooth muscle cell populations cultured from pig coronary artery. Arterioscler Thromb Vasc Biol 2002;22(7):1093–1099; doi: 10.1161/01.atv.0000022407.91111.e4 [DOI] [PubMed] [Google Scholar]
- 39. Halper J. Basic components of vascular connective tissue and extracellular matrix. Adv Pharmacol 2018;81: 95–127; doi: 10.1016/bs.apha.2017.08.012 [DOI] [PubMed] [Google Scholar]
- 40. Bowens N, Parmacek MS. Chapter 82 - Development of the Smooth Muscle Cell Lineage. In: Muscle. (Hill JA, Olson EN. eds.) Academic Press: Boston/Waltham, MA; 2012; pp. 1109–1116. [Google Scholar]
- 41. Gomez D, Owens GK. Smooth muscle cell phenotypic switching in atherosclerosis. Cardiovasc Res 2012;95(2):156–164; doi: 10.1093/cvr/cvs115 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Sinha S, Iyer D, Granata A. Embryonic origins of human vascular smooth muscle cells: Implications for in vitro modeling and clinical application. Cell Mol Life Sci 2014;71(12):2271–2288; doi: 10.1007/s00018-013-1554-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Bentzon JF, Majesky MW. Lineage tracking of origin and fate of smooth muscle cells in atherosclerosis. Cardiovasc Res 2018;114(4):492–500; doi: 10.1093/cvr/cvx251 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Majesky MW. Development of coronary vessels. Curr Top Dev Biol 2004;62: 225–259; doi: 10.1016/S0070-2153(04)62008-4 [DOI] [PubMed] [Google Scholar]
- 45. Tomanek RJ. Formation of the coronary vasculature during development. Angiogenesis 2005;8(3):273–284; doi: 10.1007/s10456-005-9014-9 [DOI] [PubMed] [Google Scholar]
- 46. Reese DE, Mikawa T, Bader DM. Development of the coronary vessel system. Circ Res 2002;91(9):761–768; doi: 10.1161/01.res.0000038961.53759.3c [DOI] [PubMed] [Google Scholar]
- 47. Waldo KL, Willner W, Kirby ML. Origin of the proximal coronary artery stems and a review of ventricular vascularization in the chick embryo. Am J Anat 1990;188(2):109–120; doi: 10.1002/aja.1001880202 [DOI] [PubMed] [Google Scholar]
- 48. Bogers AJ, Gittenberger-de Groot AC, Poelmann RE, et al. Development of the origin of the coronary arteries, a matter of ingrowth or outgrowth? Anat Embryol 1989;180(5):437–441; doi: 10.1007/BF00305118 [DOI] [PubMed] [Google Scholar]
- 49. Majesky MW. Developmental basis of vascular smooth muscle diversity. Arterioscler Thromb Vasc Biol 2007;27(6):1248–1258; doi: 10.1161/ATVBAHA.107.141069 [DOI] [PubMed] [Google Scholar]
- 50. Badimon JJ, Ortiz AF, Meyer B, et al. Different response to balloon angioplasty of carotid and coronary arteries: Effects on acute platelet deposition and intimal thickening. Atherosclerosis 1998;140(2):307–314; doi: 10.1016/s0021-9150(98)00134-8 [DOI] [PubMed] [Google Scholar]
- 51. Nabzdyk CS, Chun M, Pradhan Nabzdyk L, et al. Differential susceptibility of human primary aortic and coronary artery vascular cells to RNA interference. Biochem Biophys Res Commun 2012;425(2):261–265; doi: 10.1016/j.bbrc.2012.07.078 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Liu M, Gomez D. Smooth muscle cell phenotypic diversity. Arterioscler Thromb Vasc Biol 2019;39(9):1715–1723; doi: 10.1161/ATVBAHA.119.312131 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Beamish JA, He P, Kottke-Marchant K, et al. Molecular regulation of contractile smooth muscle cell phenotype: implications for vascular tissue engineering. Tissue Eng Part B Rev 2010;16(5):467–491; doi: 10.1089/ten.TEB.2009.0630 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Alexander MR, Owens GK. Epigenetic control of smooth muscle cell differentiation and phenotypic switching in vascular development and disease. Annu Rev Physiol 2012;74: 13–40; doi: 10.1146/annurev-physiol-012110-142315 [DOI] [PubMed] [Google Scholar]
- 55. Ye GJ, Nesmith AP, Parker KK. The role of mechanotransduction on vascular smooth muscle myocytes' cytoskeleton and contractile function. Anat Rec (Hoboken) 2014;297(9):1758–1769; doi: 10.1002/ar.22983 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Tang Y, Urs S, Boucher J, et al. Notch and transforming growth factor-beta signaling pathways cooperatively regulate vascular smooth muscle cell differentiation. J Biol Chem 2010;285(23):17556–17563; doi: 10.1074/jbc.M109.076414 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57. Chen P-Y, Qin L, Li G, et al. Fibroblast growth factor signaling regulates transforming growth factor beta-dependent smooth muscle cell phenotype modulation. Sci Rep 2016;6(1):33407; doi: 10.1038/srep33407 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58. Kurpinski K, Lam H, Chu J, et al. Transforming growth factor-beta and notch signaling mediate stem cell differentiation into smooth muscle cells. Stem Cells 2010;28(4):734–742; doi: 10.1002/stem.319 [DOI] [PubMed] [Google Scholar]
- 59. Hayashi K, Saga H, Chimori Y, et al. Differentiated phenotype of smooth muscle cells depends on signaling pathways through insulin-like growth factors and phosphatidylinositol 3-kinase. J Biol Chem 1998;273(44):28860–28867; doi: 10.1074/jbc.273.44.28860 [DOI] [PubMed] [Google Scholar]
- 60. Shai SY, Sukhanov S, Higashi Y, et al. Smooth muscle cell-specific insulin-like growth factor-1 overexpression in Apoe-/- mice does not alter atherosclerotic plaque burden but increases features of plaque stability. Arterioscler Thromb Vasc Biol 2010;30(10):1916–1924; doi: 10.1161/ATVBAHA.110.210831 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61. Lacolley P, Regnault V, Segers P, et al. Vascular smooth muscle cells and arterial stiffening: Relevance in development, aging, and disease. Physiol Rev 2017;97(4):1555–1617; doi: 10.1152/physrev.00003.2017 [DOI] [PubMed] [Google Scholar]
- 62. Tsai MC, Chen L, Zhou J, et al. Shear stress induces synthetic-to-contractile phenotypic modulation in smooth muscle cells via peroxisome proliferator-activated receptor alpha/delta activations by prostacyclin released by sheared endothelial cells. Circ Res 2009;105(5):471–480; doi: 10.1161/CIRCRESAHA.109.193656 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63. Yang L, Gao L, Nickel T, et al. Lactate promotes synthetic phenotype in vascular smooth muscle cells. Circ Res 2017;121(11):1251–1262; doi: 10.1161/CIRCRESAHA.117.311819 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64. Maryam H, Craig AM, Stephanie L. Vascular smooth muscle cell phenotypic modulation and the extracellular matrix. Artery Res 2015;9(C):14–18; doi: 10.1016/j.artres.2014.12.002 [DOI] [Google Scholar]
- 65. Holycross BJ, Blank RS, Thompson MM, et al. Platelet-derived growth factor-BB-induced suppression of smooth muscle cell differentiation. Circ Res 1992;71(6):1525–1532; doi: 10.1161/01.res.71.6.1525 [DOI] [PubMed] [Google Scholar]
- 66. Thomas JA, Deaton RA, Hastings NE, et al. PDGF-DD, a novel mediator of smooth muscle cell phenotypic modulation, is upregulated in endothelial cells exposed to atherosclerosis-prone flow patterns. Am J Physiol Heart Circ Physiol 2009;296(2):H442–H452; doi: 10.1152/ajpheart.00165.2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67. Wang Z, Wang DZ, Hockemeyer D, et al. Myocardin and ternary complex factors compete for SRF to control smooth muscle gene expression. Nature 2004;428(6979):185–189; doi: 10.1038/nature02382 [DOI] [PubMed] [Google Scholar]
- 68. Kouhara H, Hadari YR, Spivak-Kroizman T, et al. A lipid-anchored Grb2-binding protein that links FGF-receptor activation to the Ras/MAPK signaling pathway. Cell 1997;89(5):693–702; doi: 10.1016/S0092-8674(00)80252-4 [DOI] [PubMed] [Google Scholar]
- 69. Jackson CL, Reidy MA. Basic fibroblast growth factor: Its role in the control of smooth muscle cell migration. American J Pathol 1993;143(4):1024–1031. [PMC free article] [PubMed] [Google Scholar]
- 70. Lindner V, Reidy MA. Proliferation of smooth muscle cells after vascular injury is inhibited by an antibody against basic fibroblast growth factor. Proc Natl Acad Sci U S A 1991;88(9):3739–3743; doi: 10.1073/pnas.88.9.3739 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71. Liao XH, Xiang Y, Li H, et al. VEGF-α stimulates STAT3 activity via nitrosylation of myocardin to regulate the expression of vascular smooth muscle cell differentiation markers. Sci Rep 2017;7(1):2660; doi: 10.1038/s41598-017-02907-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72. Chaabane C, Heizmann CW, Bochaton-Piallat ML. Extracellular S100A4 induces smooth muscle cell phenotypic transition mediated by RAGE. Biochim Biophys Acta 2015;1853(9):2144–2157; doi: 10.1016/j.bbamcr.2014.07.022 [DOI] [PubMed] [Google Scholar]
- 73. Alshanwani AR, Riches-Suman K, O'Regan DJ, et al. MicroRNA-21 drives the switch to a synthetic phenotype in human saphenous vein smooth muscle cells. IUBMB Life 2018;70(7):649–657; doi: 10.1002/iub.1751 [DOI] [PubMed] [Google Scholar]
- 74. Kim S, Kang H. miR-15b induced by platelet-derived growth factor signaling is required for vascular smooth muscle cell proliferation. BMB Rep 2013;46(11):550–554; doi: 10.5483/bmbrep.2013.46.11.057 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75. Coll-Bonfill N, de la Cruz-Thea B, Pisano MV, et al. Noncoding RNAs in smooth muscle cell homeostasis: Implications in phenotypic switch and vascular disorders. Pflugers Arch 2016;468(6):1071–1087; doi: 10.1007/s00424-016-1821-x [DOI] [PubMed] [Google Scholar]
- 76. Shankman LS, Gomez D, Cherepanova OA, et al. KLF4-dependent phenotypic modulation of smooth muscle cells has a key role in atherosclerotic plaque pathogenesis. Nature Med 2015;21(6):628–637; doi: 10.1038/nm.3866 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77. Ali MS, Starke RM, Jabbour PM, et al. TNF-α induces phenotypic modulation in cerebral vascular smooth muscle cells: Implications for cerebral aneurysm pathology. J Cereb Blood Flow Metab 2013;33(10):1564–1573; doi: 10.1038/jcbfm.2013.109 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78. Vajen T, Benedikter BJ, Heinzmann ACA, et al. Platelet extracellular vesicles induce a pro-inflammatory smooth muscle cell phenotype. J Extracell Vesicles 2017;6(1):1322454; doi: 10.1080/20013078.2017.1322454 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79. Alexander MR, Murgai M, Moehle CW, et al. Interleukin-1β modulates smooth muscle cell phenotype to a distinct inflammatory state relative to PDGF-DD via NF-κB-dependent mechanisms. Physiol Genomics 2012;44(7):417–429; doi: 10.1152/physiolgenomics.00160.2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80. Orr AW, Hastings NE, Blackman BR, et al. Complex regulation and function of the inflammatory smooth muscle cell phenotype in atherosclerosis. J Vasc Res 2010;47(2):168–180; doi: 10.1159/000250095 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81. Yang X, Coriolan D, Murthy V, et al. Proinflammatory phenotype of vascular smooth muscle cells: role of efficient Toll-like receptor 4 signaling. Am J Physiol Heart Circ Physiol 2005;289(3):H1069–H1076; doi: 10.1152/ajpheart.00143.2005 [DOI] [PubMed] [Google Scholar]
- 82. Andreeva ER, Pugach IM, Orekhov AN. Subendothelial smooth muscle cells of human aorta express macrophage antigen in situ and in vitro. Atherosclerosis 1997;135(1):19–27; doi: 10.1016/s0021-9150(97)00136-6 [DOI] [PubMed] [Google Scholar]
- 83. Rong JX, Shapiro M, Trogan E, et al. Transdifferentiation of mouse aortic smooth muscle cells to a macrophage-like state after cholesterol loading. Proc Natl Acad Sci 2003;100(23):13531–13536; doi: 10.1073/pnas.1735526100 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84. Vengrenyuk Y, Nishi H, Long X, et al. Cholesterol loading reprograms the microRNA-143/145-myocardin axis to convert aortic smooth muscle cells to a dysfunctional macrophage-like phenotype. Arterioscler Thromb Vasc Biol 2015;35(3):535–546; doi: 10.1161/ATVBAHA.114.304029 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85. Chattopadhyay A, Kwartler CS, Kaw K, et al. Cholesterol-induced phenotypic modulation of smooth muscle cells to macrophage/fibroblast-like cells is driven by an unfolded protein response. Arterioscler Thromb Vasc Biol 2021;41(1):302–316; doi: 10.1161/ATVBAHA.120.315164 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86. Miller CL, Zhang H. Clarifying the distinct roles of smooth muscle cell-derived versus macrophage foam cells and the implications in atherosclerosis. Arterioscler Thromb Vasc Biol 2021;41(6):2035–2037; doi: 10.1161/ATVBAHA.121.316287 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87. Dubland JA, Allahverdian S, Besler KJ, et al. Low LAL (lysosomal acid lipase) expression by smooth muscle cells relative to macrophages as a mechanism for arterial foam cell formation. Arterioscler Thromb Vasc Biol 2021;41(6):e354–e368; doi: 10.1161/ATVBAHA.120.316063 [DOI] [PubMed] [Google Scholar]
- 88. Li Y, Zhu H, Zhang Q, et al. Smooth muscle-derived macrophage-like cells contribute to multiple cell lineages in the atherosclerotic plaque. Cell Discov 2021;7(1):111; doi: 10.1038/s41421-021-00328-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89. Dettman RW, Denetclaw W Jr, Ordahl CP, et al. Common epicardial origin of coronary vascular smooth muscle, perivascular fibroblasts, and intermyocardial fibroblasts in the avian heart. Dev Biol 1998;193(2):169–181; doi: 10.1006/dbio.1997.8801 [DOI] [PubMed] [Google Scholar]
- 90. Acharya A, Baek ST, Huang G, et al. The bHLH transcription factor TCF21 is required for lineage-specific EMT of cardiac fibroblast progenitors. Development 2012;139(12):2139–2149; doi: 10.1242/dev.079970 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91. Nurnberg ST, Cheng K, Raiesdana A, et al. Coronary artery disease associated transcription factor TCF21 regulates smooth muscle precursor cells that contribute to the fibrous cap. PLoS Genet 2015;11(5):e1005155; doi: 10.1371/journal.pgen.1005155 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92. Wirka RC, Wagh D, Paik DT, et al. Atheroprotective roles of smooth muscle cell phenotypic modulation and the TCF21 disease gene as revealed by single-cell analysis. Nature Med 2019;25(8):1280–1289; doi: 10.1038/s41591-019-0512-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93. Zhang P, Guan Y, Chen J, et al. Contribution of p62/SQSTM1 to PDGF-BB-induced myofibroblast-like phenotypic transition in vascular smooth muscle cells lacking SMPD1 gene. Cell Death Dis 2018;9(12):1145; doi: 10.1038/s41419-018-1197-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94. Alves RD, Eijken M, van de Peppel J, et al. Calcifying vascular smooth muscle cells and osteoblasts: Independent cell types exhibiting extracellular matrix and biomineralization-related mimicries. BMC Genomics 2014;15(1):965; doi: 10.1186/1471-2164-15-965 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95. Hortells L, Sur S, St Hilaire C. Cell phenotype transitions in cardiovascular calcification. Front Cardiovasc Med 2018;5: 27; doi: 10.3389/fcvm.2018.00027 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96. Leopold JA. Vascular calcification: Mechanisms of vascular smooth muscle cell calcification. Trends Cardiovasc Med 2015;25(4):267–274; doi: 10.1016/j.tcm.2014.10.021 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97. Cao G, Xuan X, Hu J, et al. How vascular smooth muscle cell phenotype switching contributes to vascular disease. Cell Comm Signal 2022;20(1):180; doi: 10.1186/s12964-022-00993-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98. Steitz SA, Speer MY, Curinga G, et al. Smooth muscle cell phenotypic transition associated with calcification: Upregulation of Cbfa1 and downregulation of smooth muscle lineage markers. Circ Res 2001;89(12):1147–1154; doi: 10.1161/hh2401.101070 [DOI] [PubMed] [Google Scholar]
- 99. Kapustin AN, Shanahan CM. Calcium regulation of vascular smooth muscle cell–derived matrix vesicles. Trends Cardiovasc Med 2012;22(5):133–137; doi: 10.1016/j.tcm.2012.07.009 [DOI] [PubMed] [Google Scholar]
- 100. Canet-Soulas E, Bessueille L, Mechtouff L, et al. The elusive origin of atherosclerotic plaque calcification. Front Cell Develop Biol 2021;9: 622736; doi: 10.3389/fcell.2021.622736 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101. Lin ME, Chen TM, Wallingford MC, et al. Runx2 deletion in smooth muscle cells inhibits vascular osteochondrogenesis and calcification but not atherosclerotic lesion formation. Cardiovasc Res 2016;112(2):606–616; doi: 10.1093/cvr/cvw205 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102. Sun Y, Byon CH, Yuan K, et al. Smooth muscle cell-specific Runx2 deficiency inhibits vascular calcification. Circ Res 2012;111(5):543–552; doi: 10.1161/circresaha.112.267237 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103. Komori T, Yagi H, Nomura S, et al. Targeted disruption of Cbfa1 results in a complete lack of bone formation owing to maturational arrest of osteoblasts. Cell 1997;89(5):755–764; doi: 10.1016/S0092-8674(00)80258-5 [DOI] [PubMed] [Google Scholar]
- 104. Wang J, Uryga AK, Reinhold J, et al. Vascular smooth muscle cell senescence promotes atherosclerosis and features of plaque vulnerability. Circulation 2015;132(20):1909–1919; doi: 10.1161/CIRCULATIONAHA.115.016457 [DOI] [PubMed] [Google Scholar]
- 105. Minamino T. Role of cellular senescence in lifestyle-related disease. Circ J 2010;74(12):2527–2533; doi: 10.1253/circj.cj-10-0916 [DOI] [PubMed] [Google Scholar]
- 106. Zha Y, Zhuang W, Yang Y, et al. Senescence in vascular smooth muscle cells and atherosclerosis. Front Cardiovasc Med 2022;9: 910580; doi: 10.3389/fcvm.2022.910580 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107. Gardner SE, Humphry M, Bennett MR, et al. Senescent vascular smooth muscle cells drive inflammation through an interleukin-1α–dependent senescence-associated secretory phenotype. Arteriosclerosis Thromb Vas Biol 2015;35(9):1963–1974; doi: 10.1161/ATVBAHA.115.305896 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108. Stojanović SD, Fiedler J, Bauersachs J, et al. Senescence-induced inflammation: An important player and key therapeutic target in atherosclerosis. Eur Heart J 2020;41(31):2983–2996; doi: 10.1093/eurheartj/ehz919 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109. Jakimowicz T, Przywara S, Turek J, et al. Five Year Outcomes in patients with end stage renal disease who received a bioengineered human acellular vessel for dialysis access. EJVES Vasc Forum 2022;54: 58–63; doi: 10.1016/j.ejvsvf.2022.01.003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110. Zhu Y, Cao Y, Pan J, et al. Macro-alignment of electrospun fibers for vascular tissue engineering. J Biomed Mater Res Part B Appl Biomat 2010;92B(2):508–516; doi: 10.1002/jbm.b.31544 [DOI] [PubMed] [Google Scholar]
- 111. Shalumon KT, Sreerekha PR, Sathish D, et al. Hierarchically designed electrospun tubular scaffolds for cardiovascular applications. J Biomed Nanotechnol 2011;7(5):609–620; doi: 10.1166/jbn.2011.1337 [DOI] [PubMed] [Google Scholar]
- 112. Agrawal A, Lee BH, Irvine SA, et al. Smooth muscle cell alignment and phenotype control by melt spun polycaprolactone fibers for seeding of tissue engineered blood vessels. Int J Biomater 2015;2015:434876; doi: 10.1155/2015/434876 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113. Zeng YN, Kang YL, Rau LR, et al. Construction of cell-containing, anisotropic, three-dimensional collagen fibril scaffolds using external vibration and their influence on smooth muscle cell phenotype modulation. Biomed Mater 2017;12(4):045019; doi: 10.1088/1748-605X/aa766d [DOI] [PubMed] [Google Scholar]
- 114. Liu R, Jin JP. Deletion of calponin 2 in macrophages alters cytoskeleton-based functions and attenuates the development of atherosclerosis. J Mol Cell Cardiol 2016;99: 87–99; doi: 10.1016/j.yjmcc.2016.08.019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115. Tijore A, Behr JM, Irvine SA, et al. Bioprinted gelatin hydrogel platform promotes smooth muscle cell contractile phenotype maintenance. Biomed Microdev 2018;20(2):32; doi: 10.1007/s10544-018-0274-8 [DOI] [PubMed] [Google Scholar]
- 116. Han DG, Ahn CB, Lee JH, et al. Optimization of electrospun poly(caprolactone) fiber diameter for vascular scaffolds to maximize smooth muscle cell infiltration and phenotype modulation. Polymers 2019;11(4): 643; doi: 10.3390/polym11040643 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117. Liu Y, Lu J, Li H, et al. Engineering blood vessels through micropatterned co-culture of vascular endothelial and smooth muscle cells on bilayered electrospun fibrous mats with pDNA inoculation. Acta Biomater 2015;11: 114–125; doi: 10.1016/j.actbio.2014.10.004 [DOI] [PubMed] [Google Scholar]
- 118. Ardila DC, Tamimi E, Danford FL, et al. TGFβ2 differentially modulates smooth muscle cell proliferation and migration in electrospun gelatin-fibrinogen constructs. Biomaterials 2015;37: 164–173; doi: 10.1016/j.biomaterials.2014.10.021 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119. Moses MA, Klagsbrun M, Shing Y. The role of growth factors in vascular cell development and differentiation. Int Rev Cytol 1995;161:1–48; doi: 10.1016/s0074-7696(08)62495-x [DOI] [PubMed] [Google Scholar]
- 120. Stegemann JP, Nerem RM. Phenotype modulation in vascular tissue engineering using biochemical and mechanical stimulation. Ann Biomed Eng 2003;31(4):391–402; doi: 10.1114/1.1558031 [DOI] [PubMed] [Google Scholar]
- 121. Guo X, Chen SY. Transforming growth factor-β and smooth muscle differentiation. World J Biol Chem 2012;3(3):41–52; doi: 10.4331/wjbc.v3.i3.41 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122. Shu JY, Panganiban B, Xu T. Peptide-polymer conjugates: From fundamental science to application. Annu Rev Phys Chem 2013;64: 631–657; doi: 10.1146/annurev-physchem-040412-110108 [DOI] [PubMed] [Google Scholar]
- 123. Blit PH, Battiston KG, Yang M, et al. Electrospun elastin-like polypeptide enriched polyurethanes and their interactions with vascular smooth muscle cells. Acta Biomater 2012;8(7):2493–2503; doi: 10.1016/j.actbio.2012.03.032 [DOI] [PubMed] [Google Scholar]
- 124. Kim JH, Jung Y, Kim S-H, et al. The enhancement of mature vessel formation and cardiac function in infarcted hearts using dual growth factor delivery with self-assembling peptides. Biomaterials 2011;32(26):6080–6088; doi: 10.1016/j.biomaterials.2011.05.003 [DOI] [PubMed] [Google Scholar]
- 125. D'Andrea LD, Iaccarino G, Fattorusso R, et al. Targeting angiogenesis: structural characterization and biological properties of a de novo engineered VEGF mimicking peptide. Proc Natl Acad Sci U S A 2005;102(40):14215–14220; doi: 10.1073/pnas.0505047102 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126. Leslie-Barbick JE, Saik JE, Gould DJ, et al. The promotion of microvasculature formation in poly(ethylene glycol) diacrylate hydrogels by an immobilized VEGF-mimetic peptide. Biomaterials 2011;32(25):5782–5789; doi: 10.1016/j.biomaterials.2011.04.060 [DOI] [PubMed] [Google Scholar]
- 127. Lin X, Takahashi K, Liu Y, et al. A synthetic, bioactive PDGF mimetic with binding to both α-PDGF and β-PDGF receptors. Growth Fact 2007;25(2):87–93; doi: 10.1080/08977190701553449 [DOI] [PubMed] [Google Scholar]
- 128. Geng X, Xu Z-Q, Tu C-Z, et al. Hydrogel complex electrospun scaffolds and their multiple functions in in situ vascular tissue engineering. ACS Appl Bio Mater 2021;4(3):2373–2384; doi: 10.1021/acsabm.0c01225 [DOI] [PubMed] [Google Scholar]
- 129. Liu S, Lin Z. Vascular smooth muscle cells mechanosensitive regulators and vascular remodeling. J Vasc Res 2022;59(2):90–113; doi: 10.1159/000519845 [DOI] [PubMed] [Google Scholar]
- 130. Vatankhah E, Prabhakaran MP, Semnani D, et al. Phenotypic modulation of smooth muscle cells by chemical and mechanical cues of electrospun tecophilic/gelatin nanofibers. ACS Appl Mater Interf 2014;6(6):4089–4101; doi: 10.1021/am405673h [DOI] [PubMed] [Google Scholar]
- 131. Moussallem MD, Olenych SG, Scott SL, et al. Smooth muscle cell phenotype modulation and contraction on native and cross-linked polyelectrolyte multilayers. Biomacromolecules 2009;10(11):3062–3068; doi: 10.1021/bm9007309 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132. Yi B, Shen Y, Tang H, et al. Stiffness of aligned fibers regulates the phenotypic expression of vascular smooth muscle cells. ACS Appl Mater Interf 2019;11(7):6867–6880; doi: 10.1021/acsami.9b00293 [DOI] [PubMed] [Google Scholar]
- 133. Vigetti D, Viola M, Karousou E, et al. Hyaluronan-CD44-ERK1/2 regulate human aortic smooth muscle cell motility during aging. J Biol Chem 2008;283(7):4448–4458; doi: 10.1074/jbc.M709051200 [DOI] [PubMed] [Google Scholar]
- 134. Cho HJ, Cho HJ, Kim HS. Osteopontin: A multifunctional protein at the crossroads of inflammation, atherosclerosis, and vascular calcification. Curr Atheroscler Rep 2009;11(3):206–213; doi: 10.1007/s11883-009-0032-8 [DOI] [PubMed] [Google Scholar]
- 135. Kang N, Ng CS, Hu J, et al. Role of osteopontin in the development of neointimal hyperplasia in vein grafts. Eur J Cardiothorac Surg 2012;41(6):1384–1389; doi: 10.1093/ejcts/ezr200 [DOI] [PubMed] [Google Scholar]
- 136. Tang DD. Intermediate filaments in smooth muscle. Am J Physiol Cell Physiol 2008;294(4):C869–C878; doi: 10.1152/ajpcell.00154.2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137. Zampetaki A, Zhang Z, Hu Y, et al. Biomechanical stress induces IL-6 expression in smooth muscle cells via Ras/Rac1–p38 MAPK-NF-κB signaling pathways. Am J Phys-Heart Circul Phys 2005;288(6):H2946–H2954; doi: 10.1152/ajpheart.00919.2004 [DOI] [PubMed] [Google Scholar]
- 138. Owsiany K, Nguyen A, Owens GK. Abstract 582: Vascular smooth muscle-derived macrophage are a major source of MCP1 in atherosclerosis. Arterioscler Thromb Vasc Biol 2018;38(Suppl_1):A582–A582; doi: 10.1161/atvb.38.suppl_1.582 [DOI] [Google Scholar]
- 139. Zou Y, Hu Y, Mayr M, et al. Reduced neointima hyperplasia of vein bypass grafts in intercellular adhesion molecule-1-deficient mice. Circ Res 2000;86(4):434–440; doi: 10.1161/01.res.86.4.434 [DOI] [PubMed] [Google Scholar]
- 140. Rens EG, Merks RMH. Cell shape and durotaxis explained from cell-extracellular matrix forces and focal adhesion dynamics. iScience 2020;23(9):101488; doi: 10.1016/j.isci.2020.101488 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141. Hartman CD, Isenberg BC, Chua SG, et al. Vascular smooth muscle cell durotaxis depends on extracellular matrix composition. Proc Natl Acad Sci U S A 2016;113(40):11190–11195; doi: 10.1073/pnas.1611324113 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142. Sazonova OV, Isenberg BC, Herrmann J, et al. Extracellular matrix presentation modulates vascular smooth muscle cell mechanotransduction. Matrix Biol 2015;41: 36–43; doi: 10.1016/j.matbio.2014.11.001 [DOI] [PubMed] [Google Scholar]
- 143. Matter CM, Schuler PK, Alessi P, et al. Molecular imaging of atherosclerotic plaques using a human antibody against the extra-domain B of fibronectin. Circ Res 2004;95(12):1225–1233; doi: 10.1161/01.RES.0000150373.15149.ff [DOI] [PubMed] [Google Scholar]
- 144. Opitz F, Schenke-Layland K, Cohnert TU, et al. Phenotypical plasticity of vascular smooth muscle cells-effect of in vitro and in vivo shear stress for tissue engineering of blood vessels. Tissue Eng 2007;13(10):2505–2514; doi: 10.1089/ten.2006.0424 [DOI] [PubMed] [Google Scholar]
- 145. Wiesner S, Legate KR, Fässler R. Integrin-actin interactions. Cell Mol Life Sci 2005;62(10):1081–1099; doi: 10.1007/s00018-005-4522-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146. Philippova MP, Bochkov VN, Stambolsky DV, et al. T-cadherin and signal-transducing molecules co-localize in caveolin-rich membrane domains of vascular smooth muscle cells. FEBS Lett 1998;429(2):207–210; doi: 10.1016/s0014-5793(98)00598-5 [DOI] [PubMed] [Google Scholar]
- 147. Wang N, Tytell JD, Ingber DE. Mechanotransduction at a distance: mechanically coupling the extracellular matrix with the nucleus. Nat Rev Mol Cell Biol 2009;10(1):75–82; doi: 10.1038/nrm2594 [DOI] [PubMed] [Google Scholar]
- 148. Sharifpoor S, Simmons CA, Labow RS, et al. A study of vascular smooth muscle cell function under cyclic mechanical loading in a polyurethane scaffold with optimized porosity. Acta Biomater 2010;6(11):4218–4228; doi: 10.1016/j.actbio.2010.06.018 [DOI] [PubMed] [Google Scholar]
- 149. Sharifpoor S, Simmons CA, Labow RS, et al. Functional characterization of human coronary artery smooth muscle cells under cyclic mechanical strain in a degradable polyurethane scaffold. Biomaterials 2011;32(21):4816–4829; doi: 10.1016/j.biomaterials.2011.03.034 [DOI] [PubMed] [Google Scholar]
- 150. Pan H, Xue C, Auerbach BJ, et al. Single-cell genomics reveals a novel cell state during smooth muscle cell phenotypic switching and potential therapeutic targets for atherosclerosis in mouse and human. Circulation 2020;142(21):2060–2075; doi: 10.1161/CIRCULATIONAHA.120.048378 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 151. Loerakker S, Stassen OMJA, Huurne FMt, et al. Mechanosensitivity of Jagged-Notch signaling can induce a switch-type behavior in vascular homeostasis. Proc Natl Acad Sci U S A 2018;115(16):E3682–E3691; doi: 10.1073/pnas.1715277115 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 152. Baeten JT, Lilly B. Differential regulation of Notch2 and Notch3 contribute to their unique functions in vascular smooth muscle cells. J Biolog Chem 2015;290(26):16226–16237; doi: 10.1074/jbc.M115.655548 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 153. Liu H, Kennard S, Lilly B. Notch3 expression is induced in mural cells through an autoregulatory loop that requires endothelial-expressed JAGGED1. Circ Res 2009;104(4):466–475; doi: 10.1161/CIRCRESAHA.108.184846 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 154. High FA, Lu MM, Pear WS, et al. Endothelial expression of the Notch ligand JAGGED1 is required for vascular smooth muscle development. Proc Natl Acad Sci U S A 2008;105(6):1955–1959; doi: 10.1073/pnas.0709663105 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 155. Boucher J, Gridley T, Liaw L. Molecular pathways of Notch signaling in vascular smooth muscle cells. Front Phys 2012;3:00081; doi: 10.3389/fphys.2012.00081 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 156. Gong Z, Niklason LE. Small-diameter human vessel wall engineered from bone marrow-derived mesenchymal stem cells (hMSCs). FASEB J 2008;22(6):1635–1648; doi: 10.1096/fj.07-087924 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 157. Sun L, Zhao M, Liu A, et al. Shear stress induces phenotypic modulation of vascular smooth muscle cells via AMPK/mTOR/ULK1-mediated autophagy. Cell Mol Neurobiol 2018;38(2):541–548; doi: 10.1007/s10571-017-0505-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 158. Jensen LF, Bentzon JF, Albarran-Juarez J. The phenotypic responses of vascular smooth muscle cells exposed to mechanical cues. Cells 2021;10(9): 2209; doi: 10.3390/cells10092209 [DOI] [PMC free article] [PubMed] [Google Scholar]