This cohort study examines incidents rates, microbiological studies, and patient and surgical factors associated with prosthetic joint infection at 3 time points after total knee arthroplasty among US veterans.
Key Points
Question
What are the incidence rates, microbiological culture results, and factors associated with prosthetic joint infection (PJI) at different periods after primary total knee arthroplasty (TKA)?
Findings
In this cohort study of 79 367 veterans who underwent primary TKA in the US Department of Veterans Affairs health system, the incidence of PJI was highest in the first 3 months and remained elevated through 12 months compared with 12 months or more after surgery. Gram-negative organisms were more prevalent in early vs delayed or late PJIs, and differences in factors of PJI were identified for each postoperative period.
Meaning
Findings of this study suggest that empirical gram-negative antibiotic therapy should be considered for early PJI after primary TKA, and the findings have implications for postoperative antibiotic use, stratification of PJI risk according to postoperative time, and PJI risk factor modification.
Abstract
Importance
Despite the frequency of total knee arthroplasty (TKA) and clinical implications of prosthetic joint infections (PJIs), knowledge gaps remain concerning the incidence, microbiological study results, and factors associated with these infections.
Objectives
To identify the incidence rates, organisms isolated from microbiological studies, and patient and surgical factors of PJI occurring early, delayed, and late after primary TKA.
Design, Setting, and Participants
This cohort study obtained data from the US Department of Veterans Affairs (VA) Corporate Data Warehouse on patients who underwent elective primary TKA in the VA system between October 1, 1999, and September 30, 2019, and had at least 1 year of care in the VA prior to TKA. Patients who met these criteria were included in the overall cohort, and patients with linked Veterans Affairs Surgical Quality Improvement Program (VASQIP) data composed the VASQIP cohort. Data were analyzed between December 9, 2021, and September 18, 2023.
Exposures
Primary TKA as well as demographic, clinical, and perioperative factors.
Main Outcomes and Measures
Incident hospitalization with early, delayed, or late PJI. Incidence rate (events per 10 000 person-months) was measured in 3 postoperative periods: early (≤3 months), delayed (between >3 and ≤12 months), and late (>12 months). Unadjusted Poisson regression was used to estimate incidence rate ratios (IRRs) with 95% CIs of early and delayed PJI compared with late PJI. The frequency of organisms isolated from synovial or operative tissue culture results of PJIs during each postoperative period was identified. A piecewise exponential parametric survival model was used to estimate IRRs with 95% CIs associated with demographic and clinical factors in each postoperative period.
Results
The 79 367 patients (median (IQR) age of 65 (60-71) years) in the overall cohort who underwent primary TKA included 75 274 males (94.8%). A total of 1599 PJIs (2.0%) were identified. The incidence rate of PJI was higher in the early (26.8 [95% CI, 24.8-29.0] events per 10 000 person-months; IRR, 20.7 [95% CI, 18.5-23.1]) and delayed periods (5.4 [95% CI, 4.9-6.0] events per 10 000 person-months; IRR, 4.2 [95% CI, 3.7-4.8]) vs the late postoperative period (1.3 events per 10 000 person-months). Staphylococcus aureus was the most common organism isolated overall (489 [33.2%]); however, gram-negative infections were isolated in 15.4% (86) of early PJIs. In multivariable analyses, hepatitis C virus infection, peripheral artery disease, and autoimmune inflammatory arthritis were associated with PJI across all postoperative periods. Diabetes, chronic kidney disease, and obesity (body mass index of ≥30) were not associated factors. Other period-specific factors were identified.
Conclusions and Relevance
This cohort study found that incidence rates of PJIs were higher in the early and delayed vs late post-TKA period; there were differences in microbiological cultures and factors associated with each postoperative period. These findings have implications for postoperative antibiotic use, stratification of PJI risk according to postoperative time, and PJI risk factor modification.
Introduction
Total knee arthroplasty (TKA) is one of the most common elective surgeries in the US due to increasing rates of obesity and an aging population.1,2 Prosthetic joint infection (PJI), one of the most serious complications of TKA, can result in substantial morbidity, mortality, and health care costs.3 Despite the clinical implications of PJIs, there are major knowledge gaps regarding the epidemiological features, microbiological studies, and factors of these infections in the US.
Previous studies of PJI after primary (ie, initial) TKA conducted predominantly with claims data were limited by unvalidated or surrogate end points,4,5,6,7,8 had short follow-up duration,9,10 focused on adults 65 years or older,7,11,12,13 involved single centers,14,15,16,17 and evaluated few risk factors in mainly unadjusted analyses.18,19,20,21 To our knowledge, no US study has compared the incidence, microbiological studies, and factors of PJI occurring in key periods after TKA: early PJI (≤3 months), delayed PJI (between >3 and ≤12 months), and late PJI (>12 months). Prior literature has suggested that the pathogenesis of PJI differs by time points after TKA and that these specified periods may classify the different mechanisms and organisms associated with this infection.22,23 These data could identify subgroups at highest risk for PJI after primary TKA and could inform empirical antibiotic therapy for suspected PJI after this surgery.
To address these knowledge gaps, we evaluated a national cohort of veterans who underwent primary TKA within the US Department of Veterans Affairs (VA) Healthcare System. We aimed to identify the incidence rates, organisms isolated from microbiological cultures, and patient and surgical factors of PJI occurring early, delayed, and late after primary TKA.
Methods
Study Design and Data Source
We conducted a retrospective cohort study of patients who underwent primary TKA in the national VA system. The Human Investigations Committee at the VA Connecticut Health System, Yale University, and University of Pennsylvania approved the study and waived the informed consent requirement because this research could not practicably be conducted without this waiver. We followed the Strengthening the Reporting of Observational Studies in Epidemiology (STROBE) reporting guideline.24
We collected electronic health record (EHR) data from the VA Corporate Data Warehouse. The data set included demographic characteristics, hospital and ambulatory diagnoses (recorded using International Classification of Diseases, Ninth Revision [ICD-9] and International Statistical Classification of Diseases and Related Health Problems, Tenth Revision [ICD-10] diagnosis codes), procedures (recorded using Current Procedural Terminology codes), laboratory results, microbiological culture results, and dispensed medications. Death date was ascertained from the VA Vital Status File.
To obtain operative variables, we linked patients’ records to the Veterans Affairs Surgical Quality Improvement Program (VASQIP), which included surgical data entered by nurses who reviewed records using standardized definitions.25 In general, surgical data at each VA center are identified on an 8-day cycle to ensure that data collection periods begin on different days of the week and are collected for up to 36 surgeries per cycle; thus, not all TKAs are entered into VASQIP. However, exclusion is random and is based on case volume at each center.26
Study Patients
Patients were eligible for inclusion in the study if they (1) underwent elective primary TKA (eTable 1 in Supplement 1) between October 1, 1999, and September 30, 2019, and (2) had at least 1 year of care in the VA prior to TKA. Primary TKAs can be accurately identified from the VA data set with a positive predictive value (PPV) of 95.2% (95% CI, 92%-99%).27 Patients were excluded if they underwent partial or revision TKA, underwent TKA for nonelective reasons (malignant neoplasm or fracture), or had a history of prior PJI or native septic joint arthritis of the knee (eTable 1 in Supplement 1).
We defined the index date as the date of hospital admission for primary TKA. The 12 months prior to admission represented the baseline period. Follow-up continued until the occurrence of one of the following: hospitalization with PJI, death, aseptic revision arthroplasty of the knee or contralateral TKA (since these procedures could affect PJI risk), or last VA visit before October 1, 2020 (to ensure patients had the opportunity for at least 1 year of follow-up after TKA).
Main Study Outcome
The primary end point was incident hospitalization with PJI. We classified patients as being hospitalized with PJI after primary TKA if they had (1) principal or contributory PJI ICD-9 or ICD-10 diagnosis codes at hospital discharge; (2) knee radiography within 90 days of PJI diagnosis; and (3) microbiological culture, arthrocentesis, or arthrotomy of the knee within 90 days of PJI diagnosis (eFigure 1 in Supplement 1). This algorithm accurately identified PJI with a PPV of 75.0% (95% CI, 64.1%-84.0%) in the ICD-9 era and 85.0% (95% CI, 75.3%-92.0%) in the ICD-10 era.28
The PJI date was defined as the date of hospital discharge with PJI diagnosis. We defined early PJI as occurring 3 months or less after primary TKA, delayed PJI as occurring between more than 3 months and 12 months or less after TKA, and late PJI as occurring more than 12 months after TKA. Patients stopped contributing to follow-up at their initial PJI.29
Data Collection
At the time of TKA, we collected data on age, sex, self-reported race and ethnicity, year of surgery, urban vs rural VA center,30 body mass index (BMI; calculated as weight in kilograms divided by height in meters squared), and tobacco use (current, former, or never smokers).31 Race and ethnicity data were collected as variables to contribute data on the representativeness of the study sample.
Validated algorithms based on 1 or more inpatient or 2 or more outpatient ICD-9 or ICD-10 diagnosis codes were used to identify baseline comorbidities, including alcohol use disorder (AUD),32 diabetes,33 heart failure,34,35 hypertension,36 hepatitis B virus infection, hepatitis C virus infection,37 HIV infection,38 and peripheral artery disease (PAD)39,40 (eTable 2 in Supplement 1). Autoimmune inflammatory arthritis was defined by 1 inpatient or 2 outpatient ICD-9 or ICD-10 diagnosis codes and required at least 1 rheumatologic clinic visit.41 Baseline hemoglobin and serum creatinine levels were collected from dates closest to, but within 365 days prior to TKA. Surgical factors extracted from VASQIP were recorded at the time of surgery and included anesthesia technique (general vs regional), American Society of Anesthesiology (ASA) Physical Status Classification System score for preanesthesia comorbidity (range: 1 [indicating a healthy patient] to 4 [indicating a patient with incapacitating severe disease that is a constant threat to life]),42 corticosteroid use within 30 days prior to TKA, intraoperative transfusion of packed red blood cells, and operative time (hours).
Results of microbiological cultures performed in the VA were documented in patients’ medical records as free text. All synovial fluid and tissue culture results from the knee within 90 days prior to PJI diagnosis date were obtained. Results were classified as follows: (1) culture-positive, defined as growth of 1 or more bacteria or fungal species from a culture; (2) culture-negative, defined as no growth of organisms; or (3) not collected or missing fluid or tissue culture. Culture-positive specimens were classified according to a hierarchy (eFigure 2 in Supplement 1).
Statistical Analysis
Among the overall cohort, we estimated incidence rates (events per 10 000 person-months) with 95% CIs of early, delayed, and late PJI. We used unadjusted Poisson regression to estimate the incidence rate ratio (IRR) with 95% CI of early and delayed PJI compared with late PJI. The standard Kaplan-Meier method with noninformative censoring was used to demonstrate the cumulative incidence of PJI at 3, 12, and 24 months. We measured the frequency of gram-positive, gram-negative, fungal, polymicrobial, and culture-negative PJI within each postoperative period and compared the frequencies of organisms using χ2 tests.
For the subgroup of patients with linked VASQIP data (referred to as the VASQIP cohort), we explored demographic, baseline clinical, and perioperative factors of early, delayed, and late PJI. The factors we evaluated included age, sex, urban vs rural center, BMI, AUD, hepatitis B virus infection, hepatitis C virus infection, heart failure, HIV infection, hypertension, PAD, autoimmune inflammatory arthritis, tobacco use, anemia (defined as hemoglobin <12.0 g/dL; to convert to milligrams per deciliter, multiply by 10.0), diabetes, chronic kidney disease (CKD; defined by estimated glomerular filtration rate <60 mL/min/1.73 m2, calculated using the Modified Diet in Renal Disease equation43), anesthesia technique, ASA score, corticosteroid use within 30 days prior to surgery, intraoperative transfusion of packed red blood cells, and prolonged operative time (≥2 hours). The degree of missing data for variables in the VASQIP cohort was low, with the highest being 2.9% for BMI data. As a result, we implemented a complete case analysis to identify factors for PJI in the VASQIP cohort.
We used a piecewise exponential parametric survival model fit through Poisson regression to estimate adjusted IRRs (with 95% CIs) of early, delayed, and late PJI associated with risk factors. Variables were retained in multivariable models if they were associated with PJI (statistical significance: P < .10) in univariable analysis and had statistical significance of P < .05 in multivariable analysis. We retained BMI in all models given its clinical importance44 and conflicting association with PJI in prior studies,12 regardless of statistical significance. We used the svy module in Stata to account for clustering by VA center and also adjusted for year of TKA (categorized in 5-year periods from 1999 to 2019).45 Data were analyzed between December 9, 2021, and September 18, 2023, using Stata, version 16.1 (StataCorp LLC).
Results
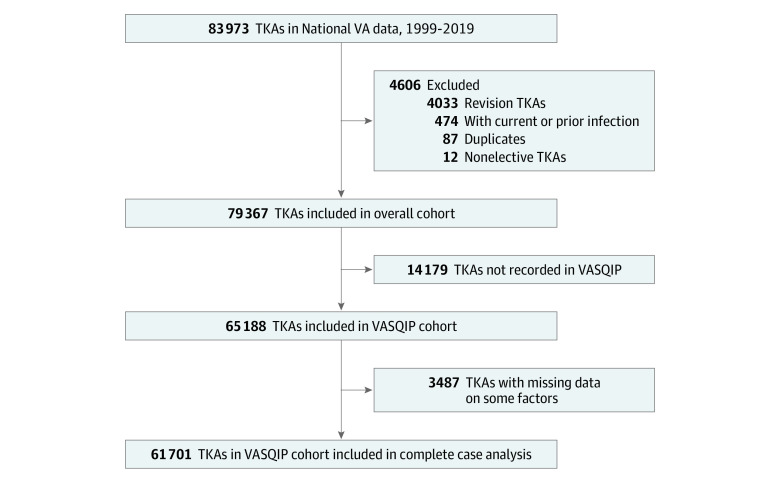
A total of 83 973 patients underwent elective primary TKA during the study period, of whom 4606 were excluded, leaving 79 367 patients in the overall cohort (Figure 1). This cohort comprised 4093 females (5.2%) and 75 274 males (94.8%) with a median (IQR) age of 65 (60-71) years, and most patients identified as non-Hispanic White individuals (73.5%) (Table 1). The median (IQR) duration of follow-up was 58.0 (22.4-108.2) months. Hypertension (86.0%), obesity (BMI of 30.0-39.9 and ≥40; 61.4%), current tobacco use (27.0%), and CKD (18.4%) were common. Among the overall cohort, 82.1% had linked VASQIP data; thus, the VASQIP cohort consisted of 65 188 patients. The characteristics of the VASQIP cohort were similar to those of the overall cohort (eTable 3 in Supplement 1).
Figure 1. Patients Selected for Inclusion.
Analyses of incidence rates of early, delayed, and late prosthetic joint infection and microbiological studies were conducted in the overall cohort. Analyses of factors associated with prosthetic joint infection were evaluated in the Veterans Affairs Surgical Quality Improvement Program (VASQIP) complete case cohort. TKA indicates total knee arthroplasty; VA, US Department of Veterans Affairs.
Table 1. Demographic, Baseline Clinical, and Perioperative Characteristics of the Overall Cohort (n = 79 367).
| Characteristic | Patients, No. (%) | Person-months of follow-up |
|---|---|---|
| Follow-up, median (IQR), mo | 58.0 (22.4-108.2) | 5 647 008 |
| Age, y | ||
| Median (IQR) | 65 (60-71) | NA |
| <60 | 19 682 (24.8) | 1 606 579 |
| 60-69 | 34 632 (43.6) | 2 394 754 |
| ≥70 | 25 053 (31.6) | 1 645 674 |
| Sex | ||
| Female | 4093 (5.2) | 274 224 |
| Male | 75 274 (94.8) | 5 372 783 |
| Race and ethnicitya | ||
| Hispanic | 3484 (4.4) | 243 900 |
| Non-Hispanic Black | 10 642 (13.4) | 734 945 |
| Non-Hispanic White | 58 307 (73.5) | 4 160 585 |
| Otherb | 4181 (5.3) | 328 922 |
| Unknown | 2753 (3.5) | 178 655 |
| Year of TKA | ||
| 1999-2004 | 12 599 (15.9) | 1 503 698 |
| 2005-2009 | 18 767 (23.6) | 1 742 641 |
| 2010-2014 | 23 475 (29.6) | 1 598 900 |
| 2015-2019 | 24 526 (30.9) | 801 767 |
| Urban vs rural center | ||
| Rural | 2551 (3.2) | 205 065 |
| Urban | 76 808 (96.8) | 5 441 347 |
| Missing data | 8 (<0.1) | NA |
| BMI | ||
| <25.0 | 5595 (7.0) | 396 666 |
| 25.0-29.9 | 22 114 (27.9) | 1 591 410 |
| 30.0-39.9 | 43 315 (54.6) | 2 984 255 |
| ≥40 | 5387 (6.8) | 412 417 |
| Missing data | 2956 (3.7) | NA |
| Tobacco use | ||
| Never or former | 56 851 (71.6) | 4 066 693 |
| Current | 21 434 (27.0) | 1 516 583 |
| Missing data | 1082 (1.4) | NA |
| Baseline comorbiditiesc | ||
| AUD | 6436 (8.1) | 409 380 |
| Heart failure | 3839 (4.9) | 220 875 |
| Hypertension | 68 054 (86.0) | 4 767 230 |
| PAD | 3815 (4.8) | 232 108 |
| HIV infection | 133 (0.2) | 9246 |
| HCV infection | 2509 (3.2) | 188 743 |
| HBV infection | 120 (0.2) | 9655 |
| Autoimmune inflammatory arthritis | 2226 (2.8) | 149 622 |
| Anemia | 4009 (5.2) | 247 181 |
| Missing data | 1546 (1.9) | NA |
| Diabetes | 5602 (7.1) | 433 175 |
| CKD | 14 403 (18.4) | 1 018 159 |
| Missing data | 1186 (1.5) | NA |
| Perioperative characteristics obtained from VASQIP cohortd | ||
| Corticosteroids use in prior 30 d | 1114 (1.7) | 72 682 |
| Missing data | 1 (<0.1) | NA |
| ASA score | ||
| 1 | 100 (0.2) | 10 098 |
| 2 | 15 314 (23.5) | 1 244 416 |
| 3 | 48 000 (73.6) | 3 206 147 |
| 4 | 1773 (2.7) | 105 895 |
| Missing data | 1 (<0.1) | NA |
| Anesthesia type | ||
| General | 41 383 (63.5) | 2 980 363 |
| Regionale | 23 805 (36.5) | 1 586 289 |
| Operative time, h | ||
| Median (IQR) | 2.0 (1.7-2.5) | |
| <2 | 31 030 (47.6) | 2 149 297 |
| ≥2 | 34 156 (52.4) | 2 417 239 |
| Missing data | 2 (<0.1) | NA |
| Intraoperative transfusion of packed RBCs | 359 (0.6) | 32 226 |
Abbreviations: ASA, American Society of Anesthesiologists Physical Status Classification System (score range: 1 [indicating a healthy patient] to 4 [indicating a patient with incapacitating severe disease that is a constant threat to life]); AUD, alcohol use disorder; BMI, body mass index (calculated as weight in kilograms divided by height in meters squared); CKD, chronic kidney disease; eGFR, estimated glomerular filtration rate; HBV, hepatitis B virus; HCV, hepatitis C virus; ICD-9, International Classification of Diseases, Ninth Revision; ICD-10, International Statistical Classification of Diseases and Related Health Problems, Tenth Revision; NA, not applicable; PAD, peripheral artery disease; RBC, red blood cell; TKA, total knee arthroplasty; VASQIP, Veterans Affairs Surgical Quality Improvement Project.
Race and ethnicity were self-reported.
Included Asian, American Indian or Alaska native, Native Hawaiian or Other Pacific Islander, and multiracial.
Comorbidities of AUD, heart failure, hypertension, diabetes, PAD, HIV infection, HBV infection, and HCV infection were defined by 1 inpatient or 2 outpatient ICD-9 or ICD-10 diagnosis codes. Autoimmune inflammatory arthritis was defined as 1 inpatient or 2 outpatient ICD-9 or ICD-10 diagnosis codes among those with at least 1 outpatient rheumatologic clinic visit during the baseline period. The ICD-9 or ICD-10 diagnosis codes used to identify comorbidities are provided in eTable 2 in Supplement 1. Anemia was defined as hemoglobin level less than 12 g/dL (to convert to grams per liter, multiply by 10.0), and CKD was defined as an eGFR less than 60 mL/min/1.73 m2; eGFR was calculated using the Modified Diet in Renal Disease equation: 175 × (serum creatinine)–1.154 × (age)–0.203 × (0.742, if female) × (1.212, if Black patient).
Perioperative characteristics were available for only a subset of patients in the VASQIP cohort (n = 65 188). Percentages reflect this subset of patients.
Included epidural, spinal, or monitored anesthesia.
Incidence Rates and Microbiological Studies of Early, Delayed, and Late PJIs
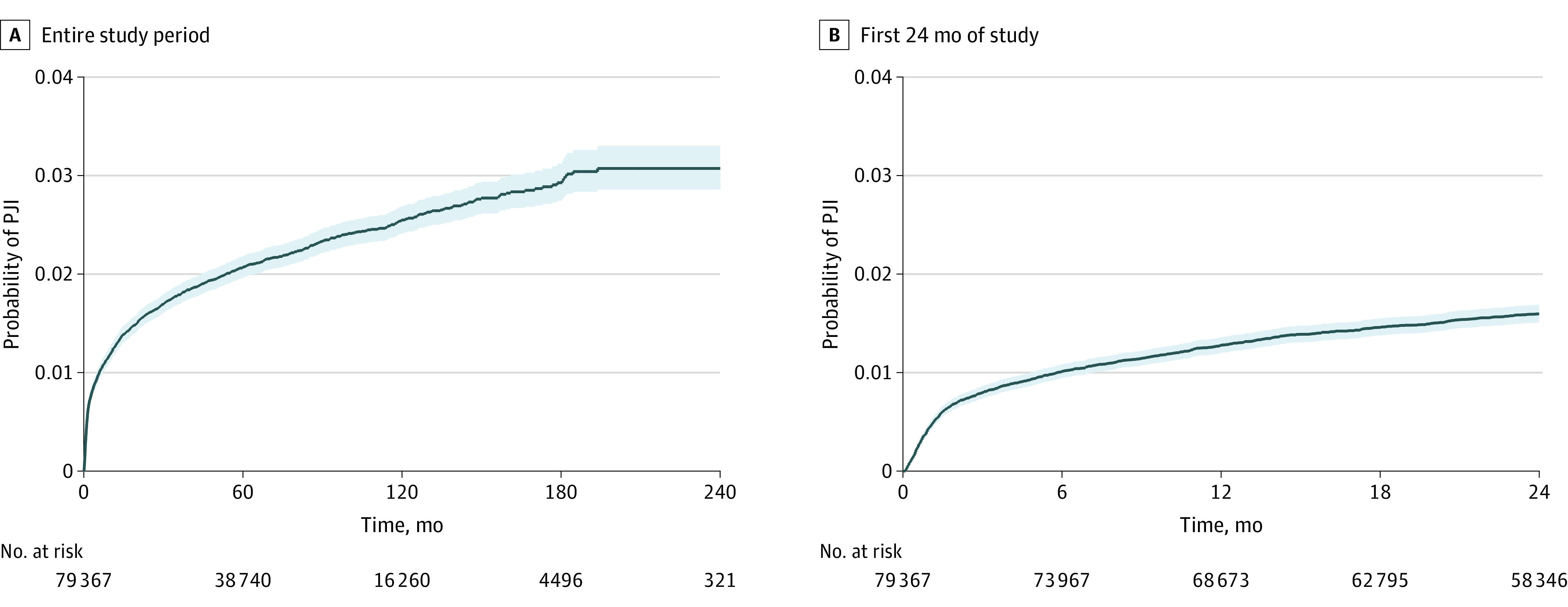
In the overall cohort, we identified 1599 incident PJIs (2.0%) across all 3 postoperative periods. We observed 627 early PJIs (39.2%) during 234 153 person-months of follow-up, yielding an incidence rate of 26.8 (95% CI, 24.8-29.0) events per 10 000 person-months. We identified 356 delayed PJIs (22.2%) during 654 498 person-months of follow-up, yielding an incidence rate of 5.4 (95% CI, 4.9-6.0) events per 10 000 person-months. There were 616 late PJIs (38.5%) during 4 758 356 person-months of follow-up, yielding an incidence rate of 1.3 (95% CI, 1.2-1.4) events per 10 000 person-months. The rate of PJI after primary TKA was significantly higher in the early (IRR, 20.7; 95% CI, 18.5-23.1) and delayed periods (IRR, 4.2; 95% CI, 3.7-4.8) vs the late period. The cumulative incidence of PJI was 0.80% (95% CI, 0.74%-0.86%) at 3 months, 1.28% (95% CI, 1.21%-1.36%) at 12 months, and 1.60% (95% CI, 1.51%-1.70%) at 24 months (Figure 2).
Figure 2. Kaplan-Meier Failure Curves for Prosthetic Joint Infection (PJI) for the Overall Study Period and the First 24 Months After Primary Total Knee Arthroplasty.
Among the 1599 incident PJIs, 126 (7.9%) did not have a microbiological culture specimen collected or the culture result was missing. Among 1473 patients with culture results, 459 (31.2%) had culture-negative results (Table 2). A culture-negative result was more common in late PJI compared with the combined early and delayed PJIs (200 [35.1%] vs 259 [28.7%]; P = .009). A total of 102 PJIs (6.9%) were polymicrobial, which was more frequently observed in the early postoperative period compared with the combined delayed and late postoperative periods (64 [11.4%] vs 38 [4.2%]; P < .001).
Table 2. Organisms Isolated From Synovial Fluid or Operative Tissue Cultures of Prosthetic Joint Infections (PJIs) After Primary Total Knee Arthroplasty .
| Organism | No. (%) | |||
|---|---|---|---|---|
| Overall (n = 1473)a | Early PJI (n = 560) | Delayed PJI (n = 344) | Late PJI (n = 569) | |
| Culture-negative | 459 (31.2) | 171 (30.5) | 88 (25.6) | 200 (35.1) |
| Polymicrobialb | 102 (6.9) | 64 (11.4) | 18 (5.2) | 20 (3.5) |
| ≥2 Gram-positive | 33 (2.4) | 20 (3.6) | 6 (1.7) | 7 (1.2) |
| ≥2 Gram-negative | 24 (1.6) | 13 (2.3) | 9 (2.6) | 2 (0.4) |
| Gram-positive and gram-negative | 70 (4.8) | 44 (7.9) | 10 (2.9) | 16 (2.8) |
| Gram-positive infections | 710 (48.2) | 286 (51.1) | 177 (51.5) | 247 (43.4) |
| Staphylococcus species | 536 (36.4) | 245 (43.8) | 123 (35.8) | 168 (29.5) |
| Staphylococcus aureus | 489 (33.2) | 223 (39.8) | 108 (31.4) | 158 (27.8) |
| Coagulase-negative staphylococci | 286 (19.4) | 99 (17.7) | 73 (21.2) | 114 (20.0) |
| Streptococcus species | 122 (8.3) | 19 (3.4) | 40 (11.6) | 63 (11.1) |
| Enterococcus species | 65 (4.4) | 35 (6.3) | 16 (4.7) | 14 (2.5) |
| Anaerobes | 10 (0.7) | 4 (0.7) | 4 (1.2) | 2 (0.4) |
| Cutibacterium acnes | 6 (0.4) | 1 (0.2) | 3 (0.9) | 2 (0.4) |
| Otherc | 12 (0.8) | 4 (0.7) | 1 (0.3) | 7 (1.2) |
| Gram-negative infections | 164 (11.1) | 86 (15.4) | 31 (9.0) | 47 (8.3) |
| Pseudomonas aeruginosa | 40 (2.7) | 19 (3.4) | 8 (2.3) | 13 (2.3) |
| Escherichia coli | 34 (2.3) | 19 (3.4) | 8 (2.3) | 7 (1.2) |
| Klebsiella species | 32 (2.2) | 18 (3.2) | 4 (1.2) | 10 (1.8) |
| Enterobacter species | 36 (2.4) | 23 (4.1) | 10 (2.9) | 3 (0.5) |
| Serratia species | 12 (0.8) | 7 (1.3) | 2 (0.6) | 3 (0.5) |
| Anaerobes | 3 (0.2) | 1 (0.2) | 1 (0.3) | 1 (0.2) |
| Otherd | 38 (2.6) | 16 (2.9) | 8 (2.3) | 14 (2.5) |
| Fungal infectionse | 3 (0.2) | 0 (0.0) | 1 (0.3) | 2 (0.4) |
Among the 1599 PJIs in the overall cohort, microbiological data for 126 patients were missing or not collected. Consequently, 1473 PJIs with available microbiological study data were included in these results. Numbers may not sum to group totals or percentages as polymicrobial infections were possible.
Polymicrobial defined by multiple genera of organisms identified. Numbers may not sum to group totals or percentages as polymicrobial infections with more than 1 organism were possible.
Included Micrococcus species and diphtheroids.
Included Salmonella, Pasteurella, Citrobacter, Morganella, Acinetobacter, Pasteurella, Burkholderia species, and gram-negative organisms not otherwise specified.
All fungal infections were identified as Candida species.
Gram-positive infections were the most common across all postoperative periods (710 of 1473 infections [48.2%]), with Staphylococcus aureus being the most frequently identified (489 [33.2%]). Staphylococcus (245 [43.8%]) and Enterococcus (35 [6.3%]) species were more common in early PJIs, whereas Streptococcus species were more common in delayed (40 [11.6%]) and late (63 [11.1%]) PJIs (Table 2). Gram-negative organisms were observed in 164 infections (11.1%) and more commonly occurred in early PJIs than in combined delayed and late PJIs (86 [15.4%] vs 78 [8.5%]; P < .001). Pseudomonas aeruginosa was the most common gram-negative organism overall (40 [2.7%]), but Enterobacter species were the most frequently isolated in early PJI (23 [4.1%]).
Risk Factors for Early, Delayed, and Late PJI
Among the 65 188 patients in the VASQIP cohort, 61 701 (94.6%) had complete data on risk factors and so were included in the complete case analysis. Hepatitis C virus infection, PAD, and autoimmune inflammatory arthritis were associated with PJI across all postoperative periods (Table 3). Early PJI was also associated with current smoking, heart failure, hypertension, urban location, and prolonged operative time (≥2 hours). Autoimmune inflammatory arthritis was a robust factor of early PJI (adjusted IRR, 2.3; 95% CI, 1.5-3.5). Delayed PJI was also associated with BMI of 40 or higher, AUD, general anesthesia, anemia, and prolonged operative time (≥2 hours). Body mass index of 40 or higher was a robust factor of delayed PJI (adjusted IRR, 2.7; 95% CI, 1.5-4.9). Late PJI was also associated with AUD, heart failure, anemia, and younger age (<70 years). Anemia was a factor of late PJI (adjusted IRR, 2.2; 95% CI, 1.5-3.1). Factors asociated with PJI at any time after TKA are reported in eTable 4 in Supplement 1. Diabetes and chronic kidney disease were not associated factors.
Table 3. Adjusted Incidence Rate Ratios (IRRs) of Prosthetic Joint Infections (PJIs) After Primary Total Knee Arthroplasty (TKA) in the Veterans Affairs Surgical Quality Improvement Project Complete Case Cohort (n = 61 701).
| Characteristic | Adjusted IRR (95% CI)a | ||
|---|---|---|---|
| Early PJI: ≤3 mo | Delayed PJI: >3 and ≤12 mo | Late PJI: >12 mo | |
| No. of PJIs | 513 | 286 | 500 |
| Age, y | |||
| <60 | 1 [Reference] | 1 [Reference] | 1 [Reference] |
| 60-69 | 0.9 (0.7-1.1) | 0.8 (0.6-1.1) | 0.9 (0.7-1.2) |
| ≥70 | 0.8 (0.6-1.0) | 0.8 (0.6-1.2) | 0.6 (0.5-0.9) |
| Urban vs rural center | |||
| Rural | 1 [Reference] | 1 [Reference] | 1 [Reference] |
| Urban | 2.3 (1.1-4.9) | 1.3 (0.7-2.6) | 1.6 (0.8-3.3) |
| BMI | |||
| <25.0 | 1 [Reference] | 1 [Reference] | 1 [Reference] |
| 25.0-29.9 | 0.8 (0.6-1.2) | 1.7 (0.9-2.9) | 0.8 (0.6-1.2) |
| 30.0-39.9 | 0.8 (0.6-1.2) | 1.7 (1.0-2.7) | 0.9 (0.7-1.3) |
| ≥40 | 0.9 (0.6-1.4) | 2.7 (1.5-4.9) | 1.3 (0.8-1.9) |
| AUD | |||
| No | 1 [Reference] | 1 [Reference] | 1 [Reference] |
| Yes | 1.1 (0.8-1.6) | 2.0 (1.4-2.9) | 1.5 (1.1-2.0) |
| Tobacco use | |||
| Former or never | 1 [Reference] | 1 [Reference] | 1 [Reference] |
| Current | 1.3 (1.0-1.6) | 0.9 (0.7-1.2) | 1.0 (0.8-1.3) |
| Heart failure | |||
| No | 1 [Reference] | 1 [Reference] | 1 [Reference] |
| Yes | 1.8 (1.3-2.4) | 0.8 (0.5-1.3) | 1.9 (1.3-2.8) |
| Hypertension | |||
| No | 1 [Reference] | 1 [Reference] | 1 [Reference] |
| Yes | 1.5 (1.2-2.0) | 1.2 (0.8-1.7) | 1.1 (0.8-1.4) |
| PAD | |||
| No | 1 [Reference] | 1 [Reference] | 1 [Reference] |
| Yes | 1.6 (1.1-2.2) | 1.6 (1.0-2.4) | 1.9 (1.3-2.7) |
| HCV infection | |||
| No | 1 [Reference] | 1 [Reference] | 1 [Reference] |
| Yes | 1.9 (1.3-2.8) | 1.7 (1.0-2.9) | 2.1 (1.5-3.0) |
| Autoimmune inflammatory arthritis | |||
| No | 1 [Reference] | 1 [Reference] | 1 [Reference] |
| Yes | 2.3 (1.5-3.5) | 1.7 (1.0-2.9) | 1.9 (1.2-3.0) |
| Anemia | |||
| No | 1 [Reference] | 1 [Reference] | 1 [Reference] |
| Yes | 1.4 (0.9-1.9) | 1.8 (1.3-2.7) | 2.2 (1.5-3.1) |
| Anesthesia type | |||
| Regional | 1 [Reference] | 1 [Reference] | 1 [Reference] |
| General | 1.1 (0.9-1.4) | 1.3 (1.0-1.6) | 1.1 (0.8-1.3) |
| Operative time, h | |||
| <2 | 1 [Reference] | 1 [Reference] | 1 [Reference] |
| ≥2 | 1.3 (1.0-1.6) | 1.3 (1.0-1.7) | 1.1 (0.9-1.4) |
Abbreviations: AUD, alcohol use disorder; BMI, body mass index (calculated as weight in kilograms divided by height in meters squared); HCV, hepatitis C virus; PAD, peripheral artery disease; VA, US Department of Veterans Affairs.
The IRR for each variable was adjusted for all other variables as well as by VA center and year of TKA (categorized in 5-year periods from 1999 to 2019).
Discussion
Using EHR data from the national VA system, we found that PJI was uncommon after TKA (2.0%), and the incidence rate was highest in the early and delayed periods (20.7 and 4.2 times higher, respectively) compared with the late period. Staphylococcus and Enterococcus species were more commonly isolated from early PJIs, whereas Streptococcus species were more commonly isolated from delayed or late PJIs. Gram-negative infections were more common in the early postoperative period. Hepatitis C infection, PAD, and autoimmune inflammatory arthritis were associated with PJI across all postoperative periods.
This study demonstrated that the rate of PJI was highest within the initial 3 months after primary TKA, and the cumulative incidence at 24 months was 1.60%. These results are consistent with the findings of a cohort study of 69 663 US Medicare beneficiaries in which the cumulative incidence of PJI diagnoses was 1.55% within the first 24 months after elective TKA.7 Since the incidence of PJI was highest soon after primary TKA, development and testing of interventions to modify risk factors for early PJI should be prioritized.
Few studies have examined the microbiological characteristics of PJI after TKA. In a cross-sectional study of 1651 patients who had a primary or revision TKA or total hip arthroplasty at Mayo Clinic (2010-2019) and who were observed for PJI, coagulase-negative staphylococci were the most common organisms isolated from cultures.46 In a separate cohort study of 231 patients with hip or knee PJI from a single hospital in China (2006-2015), coagulase-negative staphylococci were also the most common organisms isolated.47 The finding of the present study that Staphylococcus aureus was the most frequently identified causative organism may be due to inclusion of only primary TKAs, whereas previous studies included PJIs of the knee and hip as well as primary and revision arthroplasties.
Gram-negative organisms were isolated from 11.1% of all PJIs and 15.4% of early PJIs. The gram-negative organisms identified from the PJIs in this study, including the early infections, are typically resistant to first-generation cephalosporins. Given these findings, empirical gram-negative antibiotic therapy, in addition to the usual gram-positive coverage, should be considered during suspected early PJI. Cefazolin, the recommended antibiotic for perioperative TKA prophylaxis, would not provide adequate protection against most of the gram-negative organisms identified in this study.48 To date, the only intervention associated with a decreased rate of PJI is perioperative antibiotic prophylaxis.49,50 Future studies should examine the antimicrobial susceptibility profiles of gram-negative infections that were isolated during early PJI to determine the extent to which broader perioperative gram-negative antibiotic prophylaxis is warranted.
We explored factors of early, delayed, and late PJI. Factors associated with inflammation and tissue hypoxia were associated with PJI in each postoperative period. Hepatitis C virus infection and autoimmune inflammatory arthritis were associated with PJI across all postoperative periods and contributed to systemic inflammation that compromised wound healing.51,52,53 Peripheral artery disease was also a significant factor in tissue hypoxia and compromised skin or soft tissue integrity54 across the 3 postoperative periods. Early PJI was associated with factors that promoted tissue hypoxia (smoking, heart failure, prolonged operative time, and hypertension).20,55,56,57,58 Delayed PJI was associated with factors contributing to tissue hypoxia (prolonged operative time, general anesthesia, and anemia) and impaired wound healing and falls (morbid obesity and AUD).58,59,60,61,62,63 Additional factors of late PJI were associated with impaired wound healing and falls (AUD, heart failure, and anemia).56,59,60,61,62 Prognostic models should be developed to classify patients according to risk of PJI, which could help guide informed consent, surgical planning, and postoperative monitoring.
We observed a reduced rate of late infections among patients 70 years or older compared with those younger than 60 years, which is consistent with findings of large European and Canadian studies and may reflect a tendency to avoid invasive diagnostic testing and workup for painful joints in patients 70 years or older.17,64 Chronic kidney disease and diabetes were not associated with PJI during any period after TKA. The lack of association between CKD and PJI has been reported in a meta-analysis.65 Diabetes has been a factor of PJI in most prior studies.15,16,17,66 The lack of a similar association in the present study might be due to the definition of diabetes used. No pattern was observed between BMI and PJI risk, making the clinical significance of the association between BMI of 40 or higher and risk of PJI in the delayed postoperative period unclear. The role that obesity plays in postoperative infection risk has been inconsistent, with some studies reporting no association2,14,21,67 and other studies observing an increased risk.12,17,68 Results of the present study suggest that patients with obesity or CKD should not be restricted from undergoing TKA because of concerns about increased PJI risk.
Strengths and Limitations
Strengths of this study included the large sample of veterans undergoing primary TKAs, access to national EHR data that included microbiological culture results, and linkage to surgical-specific data. This study also had several potential limitations. First, misclassification of outcomes was possible, but use of a validated PJI end point minimized this risk. It is possible that some PJI outcomes were missed in patients who presented to a non-VA hospital. Second, some culture results could have included organisms that were contaminants. Third, residual confounding by unmeasured factors, such as perioperative antibiotic prophylaxis and chronic skin conditions, could have affected risk factor analyses. Moreover, we evaluated comorbidities as binary (present or absent), without accounting for illness severity, and accounted only for baseline comorbidities or surgical characteristics without evaluating time-varying factors. Fourth, the sample consisted of mostly males with a high prevalence of comorbid conditions. Results may have less generalizability to females and the overall population receiving TKAs.
Conclusions
In this cohort study of patients who underwent primary TKA in the VA system, incidence rates of PJI occurring in the early and delayed post-TKA periods were higher than incidence of PJI occurring later. Staphylococcus and Enterococcus species were more commonly isolated from PJIs occurring in the early postoperative period, whereas Streptococcus species were more commonly isolated from PJIs occurring in the delayed or late postoperative periods. Gram-negative infections were more commonly observed in the early postoperative period, which suggests that empirical gram-negative antibiotic therapy should be considered along with the usual gram-positive coverage for early PJIs. Additionally, differences in demographic, clinical, and perioperative factors associated with early, delayed, and late PJIs were identified. These findings have implications for postoperative antibiotic use, stratification of PJI risk according to post-TKA periods, and PJI risk factor modification.
eTable 1. Diagnosis and Procedures Codes Used to Define Inclusion and Exclusion Criteria, Prosthetic Joint Infection Outcomes, and Censoring Events
eFigure 1. Validated Case-Finding Algorithms Used to Identify Prosthetic Joint Infection After Total Knee Arthroplasty Within United States Veterans Health Administration Data
eTable 2. Definitions of Medical Comorbidities and Surgical Characteristics as well as ICD-9 and ICD-10 Diagnosis Codes Used to Identify Comorbidities of Interest
eFigure 2. Hierarchy Used for Organization of Microbiological Culture Data
eTable 3. Baseline Demographics, Medical Comorbidities, and Surgical Characteristics at Time of Total Knee Arthroplasty Among Those Not in the Veterans Affairs Surgical Quality Improvement Program Compared to Patients Included in This Cohort
eTable 4. Adjusted Incidence Rate Ratios of Prosthetic Joint Infection at Any Time After Primary Total Knee Arthroplasty Associated With Demographic, Baseline Clinical, and Peri-Operative Factors Among Patients Included in the Veterans Affairs Surgical Quality Improvement Project (n = 61 701)
Data Sharing Statement
References
- 1.Cram P, Lu X, Kates SL, Singh JA, Li Y, Wolf BR. Total knee arthroplasty volume, utilization, and outcomes among Medicare beneficiaries, 1991-2010. JAMA. 2012;308(12):1227-1236. doi: 10.1001/2012.jama.11153 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Singh JA, Yu S, Chen L, Cleveland JD. Rates of total joint replacement in the United States: future projections to 2020-2040 using the National Inpatient Sample. J Rheumatol. 2019;46(9):1134-1140. doi: 10.3899/jrheum.170990 [DOI] [PubMed] [Google Scholar]
- 3.Beam E, Osmon D. Prosthetic joint infection update. Infect Dis Clin North Am. 2018;32(4):843-859. doi: 10.1016/j.idc.2018.06.005 [DOI] [PubMed] [Google Scholar]
- 4.Badawy M, Espehaug B, Fenstad AM, et al. Patient and surgical factors affecting procedure duration and revision risk due to deep infection in primary total knee arthroplasty. BMC Musculoskelet Disord. 2017;18(1):544. doi: 10.1186/s12891-017-1915-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Brochin R, Poeran J, Vig KS, et al. Trends in periprosthetic knee infection and associated costs: a population-based study using national data. J Knee Surg. 2021;34(10):1110-1119. doi: 10.1055/s-0040-1701516 [DOI] [PubMed] [Google Scholar]
- 6.Kurtz SM, Lau EC, Son MS, Chang ET, Zimmerli W, Parvizi J. Are we winning or losing the battle with periprosthetic joint infection: trends in periprosthetic joint infection and mortality risk for the Medicare population. J Arthroplasty. 2018;33(10):3238-3245. doi: 10.1016/j.arth.2018.05.042 [DOI] [PubMed] [Google Scholar]
- 7.Kurtz SM, Ong KL, Lau E, Bozic KJ, Berry D, Parvizi J. Prosthetic joint infection risk after TKA in the Medicare population. Clin Orthop Relat Res. 2010;468(1):52-56. doi: 10.1007/s11999-009-1013-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Kamath AF, Ong KL, Lau E, et al. Quantifying the burden of revision total joint arthroplasty for periprosthetic infection. J Arthroplasty. 2015;30(9):1492-1497. doi: 10.1016/j.arth.2015.03.035 [DOI] [PubMed] [Google Scholar]
- 9.Baier C, Adelmund S, Schwab F, et al. Incidence and risk factors of surgical site infection after total knee arthroplasty: results of a retrospective cohort study. Am J Infect Control. 2019;47(10):1270-1272. doi: 10.1016/j.ajic.2019.04.010 [DOI] [PubMed] [Google Scholar]
- 10.Grammatico-Guillon L, Baron S, Rosset P, et al. Surgical site infection after primary hip and knee arthroplasty: a cohort study using a hospital database. Infect Control Hosp Epidemiol. 2015;36(10):1198-1207. doi: 10.1017/ice.2015.148 [DOI] [PubMed] [Google Scholar]
- 11.Kurtz SM, Lau E, Schmier J, Ong KL, Zhao K, Parvizi J. Infection burden for hip and knee arthroplasty in the United States. J Arthroplasty. 2008;23(7):984-991. doi: 10.1016/j.arth.2007.10.017 [DOI] [PubMed] [Google Scholar]
- 12.Namba RS, Inacio MC, Paxton EW. Risk factors associated with deep surgical site infections after primary total knee arthroplasty: an analysis of 56,216 knees. J Bone Joint Surg Am. 2013;95(9):775-782. doi: 10.2106/JBJS.L.00211 [DOI] [PubMed] [Google Scholar]
- 13.Rasouli MR, Restrepo C, Maltenfort MG, Purtill JJ, Parvizi J. Risk factors for surgical site infection following total joint arthroplasty. J Bone Joint Surg Am. 2014;96(18):e158. doi: 10.2106/JBJS.M.01363 [DOI] [PubMed] [Google Scholar]
- 14.Berbari EF, Hanssen AD, Duffy MC, et al. Risk factors for prosthetic joint infection: case-control study. Clin Infect Dis. 1998;27(5):1247-1254. doi: 10.1086/514991 [DOI] [PubMed] [Google Scholar]
- 15.Blanco JF, Díaz A, Melchor FR, da Casa C, Pescador D. Risk factors for periprosthetic joint infection after total knee arthroplasty. Arch Orthop Trauma Surg. 2020;140(2):239-245. doi: 10.1007/s00402-019-03304-6 [DOI] [PubMed] [Google Scholar]
- 16.Eka A, Chen AF. Patient-related medical risk factors for periprosthetic joint infection of the hip and knee. Ann Transl Med. 2015;3(16):233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Lenguerrand E, Whitehouse MR, Beswick AD, et al. ; National Joint Registry for England, Wales, Northern Ireland and the Isle of Man . Risk factors associated with revision for prosthetic joint infection following knee replacement: an observational cohort study from England and Wales. Lancet Infect Dis. 2019;19(6):589-600. doi: 10.1016/S1473-3099(18)30755-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Babu JM, Kalagara S, Durand W, Antoci V, Deren ME, Cohen E. Sarcopenia as a risk factor for prosthetic infection after total hip or knee arthroplasty. J Arthroplasty. 2019;34(1):116-122. doi: 10.1016/j.arth.2018.09.037 [DOI] [PubMed] [Google Scholar]
- 19.Chrastil J, Anderson MB, Stevens V, Anand R, Peters CL, Pelt CE. Is hemoglobin A1c or perioperative hyperglycemia predictive of periprosthetic joint infection or death following primary total joint arthroplasty? J Arthroplasty. 2015;30(7):1197-1202. doi: 10.1016/j.arth.2015.01.040 [DOI] [PubMed] [Google Scholar]
- 20.Gonzalez AI, Luime JJ, Uçkay I, Hannouche D, Hoffmeyer P, Lübbeke A. Is there an association between smoking status and prosthetic joint infection after primary total joint arthroplasty? J Arthroplasty. 2018;33(7):2218-2224. doi: 10.1016/j.arth.2018.02.069 [DOI] [PubMed] [Google Scholar]
- 21.Nelson CL, Elkassabany NM, Kamath AF, Liu J. Low albumin levels, more than morbid obesity, are associated with complications after TKA. Clin Orthop Relat Res. 2015;473(10):3163-3172. doi: 10.1007/s11999-015-4333-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Fernández-Sampedro M, Fariñas-Alvarez C, Garces-Zarzalejo C, et al. Accuracy of different diagnostic tests for early, delayed and late prosthetic joint infection. BMC Infect Dis. 2017;17(1):592. doi: 10.1186/s12879-017-2693-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Triffault-Fillit C, Ferry T, Laurent F, et al. ; Lyon BJI Study Group . Microbiologic epidemiology depending on time to occurrence of prosthetic joint infection: a prospective cohort study. Clin Microbiol Infect. 2019;25(3):353-358. doi: 10.1016/j.cmi.2018.04.035 [DOI] [PubMed] [Google Scholar]
- 24.von Elm E, Altman DG, Egger M, Pocock SJ, Gøtzsche PC, Vandenbroucke JP; STROBE Initiative . The Strengthening the Reporting of Observational Studies in Epidemiology (STROBE) statement: guidelines for reporting observational studies. Ann Intern Med. 2007;147(8):573-577. doi: 10.7326/0003-4819-147-8-200710160-00010 [DOI] [PubMed] [Google Scholar]
- 25.Khuri SF, Daley J, Henderson W, et al. ; National VA Surgical Quality Improvement Program . The Department of Veterans Affairs’ NSQIP: the first national, validated, outcome-based, risk-adjusted, and peer-controlled program for the measurement and enhancement of the quality of surgical care. Ann Surg. 1998;228(4):491-507. doi: 10.1097/00000658-199810000-00006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Massarweh NN, Kaji AH, Itani KMF. Practical guide to surgical data sets: Veterans Affairs Surgical Quality Improvement Program (VASQIP). JAMA Surg. 2018;153(8):768-769. doi: 10.1001/jamasurg.2018.0504 [DOI] [PubMed] [Google Scholar]
- 27.Singh JA, Ayub S. Accuracy of VA databases for diagnoses of knee replacement and hip replacement. Osteoarthritis Cartilage. 2010;18(12):1639-1642. doi: 10.1016/j.joca.2010.10.003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Weinstein EJ, Stephens-Shields A, Loabile B, et al. Development and validation of case-finding algorithms to identify prosthetic joint infections after total knee arthroplasty in Veterans Health Administration data. Pharmacoepidemiol Drug Saf. 2021;30(9):1184-1191. doi: 10.1002/pds.5316 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Tande AJ, Patel R. Prosthetic joint infection. Clin Microbiol Rev. 2014;27(2):302-345. doi: 10.1128/CMR.00111-13 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.US Department of Veterans Affairs . Rural veterans. Accessed May 18, 2022. https://www.ruralhealth.va.gov/aboutus/ruralvets.asp#def
- 31.McGinnis KA, Brandt CA, Skanderson M, et al. Validating smoking data from the Veteran’s Affairs Health Factors dataset, an electronic data source. Nicotine Tob Res. 2011;13(12):1233-1239. doi: 10.1093/ntr/ntr206 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Justice AC, McGinnis KA, Atkinson JH, et al. ; Veterans Aging Cohort 5-Site Study Project Team . Psychiatric and neurocognitive disorders among HIV-positive and negative veterans in care: Veterans Aging Cohort Five-Site Study. AIDS. 2004;18(suppl 1):S49-S59. doi: 10.1097/00002030-200401001-00008 [DOI] [PubMed] [Google Scholar]
- 33.Butt AA, McGinnis K, Rodriguez-Barradas MC, et al. ; Veterans Aging Cohort Study . HIV infection and the risk of diabetes mellitus. AIDS. 2009;23(10):1227-1234. doi: 10.1097/QAD.0b013e32832bd7af [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Saczynski JS, Andrade SE, Harrold LR, et al. A systematic review of validated methods for identifying heart failure using administrative data. Pharmacoepidemiol Drug Saf. 2012;21(suppl 1):129-140. doi: 10.1002/pds.2313 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Freiberg MS, Chang CH, Skanderson M, et al. Association between HIV infection and the risk of heart failure with reduced ejection fraction and preserved ejection fraction in the antiretroviral therapy era: results from the Veterans Aging Cohort Study. JAMA Cardiol. 2017;2(5):536-546. doi: 10.1001/jamacardio.2017.0264 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Tamariz L, Palacio A, Denizard J, Schulman Y, Contreras G. The use of claims data algorithms to recruit eligible participants into clinical trials. Am J Manag Care. 2015;21(2):e114-e118. [PubMed] [Google Scholar]
- 37.Kramer JR, Davila JA, Miller ED, Richardson P, Giordano TP, El-Serag HB. The validity of viral hepatitis and chronic liver disease diagnoses in Veterans Affairs administrative databases. Aliment Pharmacol Ther. 2008;27(3):274-282. doi: 10.1111/j.1365-2036.2007.03572.x [DOI] [PubMed] [Google Scholar]
- 38.Fultz SL, Skanderson M, Mole LA, et al. Development and verification of a “virtual” cohort using the National VA Health Information System. Med Care. 2006;44(8 suppl 2):S25-S30. doi: 10.1097/01.mlr.0000223670.00890.74 [DOI] [PubMed] [Google Scholar]
- 39.Beckman JA, Duncan MS, Alcorn CW, et al. Association of human immunodeficiency virus infection and risk of peripheral artery disease. Circulation. 2018;138(3):255-265. doi: 10.1161/CIRCULATIONAHA.117.032647 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Beckman JA, Duncan MS, Damrauer SM, et al. Microvascular disease, peripheral artery disease, and amputation. Circulation. 2019;140(6):449-458. doi: 10.1161/CIRCULATIONAHA.119.040672 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Ng B, Aslam F, Petersen NJ, Yu HJ, Suarez-Almazor ME. Identification of rheumatoid arthritis patients using an administrative database: a Veterans Affairs study. Arthritis Care Res (Hoboken). 2012;64(10):1490-1496. doi: 10.1002/acr.21736 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Mayhew D, Mendonca V, Murthy BVS. A review of ASA physical status–historical perspectives and modern developments. Anaesthesia. 2019;74(3):373-379. doi: 10.1111/anae.14569 [DOI] [PubMed] [Google Scholar]
- 43.Levey AS, Bosch JP, Lewis JB, Greene T, Rogers N, Roth D; Modification of Diet in Renal Disease Study Group . A more accurate method to estimate glomerular filtration rate from serum creatinine: a new prediction equation. Ann Intern Med. 1999;130(6):461-470. doi: 10.7326/0003-4819-130-6-199903160-00002 [DOI] [PubMed] [Google Scholar]
- 44.DeMik DE, Muffly SA, Carender CN, Glass NA, Brown TS, Bedard NA. What is the impact of body mass index cutoffs on total knee arthroplasty complications? J Arthroplasty. 2022;37(4):683-687.e1. doi: 10.1016/j.arth.2021.12.024 [DOI] [PubMed] [Google Scholar]
- 45.StataCorp LLC . Stata Survey Data Reference Manual. Release 17. Stata Press Publication; 2021. [Google Scholar]
- 46.Tai DBG, Patel R, Abdel MP, Berbari EF, Tande AJ. Microbiology of hip and knee periprosthetic joint infections: a database study. Clin Microbiol Infect. 2022;28(2):255-259. doi: 10.1016/j.cmi.2021.06.006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Hu L, Fu J, Zhou Y, et al. Trends in microbiological profiles and antibiotic resistance in periprosthetic joint infections. J Int Med Res. 2021;49(3):3000605211002784. doi: 10.1177/03000605211002784 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Bratzler DW, Dellinger EP, Olsen KM, et al. ; American Society of Health-System Pharmacists (ASHP); Infectious Diseases Society of America (IDSA); Surgical Infection Society (SIS); Society for Healthcare Epidemiology of America (SHEA) . Clinical practice guidelines for antimicrobial prophylaxis in surgery. Surg Infect (Larchmt). 2013;14(1):73-156. doi: 10.1089/sur.2013.9999 [DOI] [PubMed] [Google Scholar]
- 49.Gillespie WJ. Prevention and management of infection after total joint replacement. Clin Infect Dis. 1997;25(6):1310-1317. doi: 10.1086/516134 [DOI] [PubMed] [Google Scholar]
- 50.Marculescu CE, Osmon DR. Antibiotic prophylaxis in orthopedic prosthetic surgery. Infect Dis Clin North Am. 2005;19(4):931-946. doi: 10.1016/j.idc.2005.07.002 [DOI] [PubMed] [Google Scholar]
- 51.Schwarzkopf R, Novikov D, Anoushiravani AA, et al. The preoperative management of Hepatitis C may improve the outcome after total knee arthroplasty. Bone Joint J. 2019;101-B(6):667-674. doi: 10.1302/0301-620X.101B6.BJJ-2018-0723.R3 [DOI] [PubMed] [Google Scholar]
- 52.Smolen JS, Aletaha D, McInnes IB. Rheumatoid arthritis. Lancet. 2016;388(10055):2023-2038. doi: 10.1016/S0140-6736(16)30173-8 [DOI] [PubMed] [Google Scholar]
- 53.Yeganeh MH, Kheir MM, Shahi A, Parvizi J. Rheumatoid arthritis, disease modifying agents, and periprosthetic joint infection: what does a joint surgeon need to know? J Arthroplasty. 2018;33(4):1258-1264. doi: 10.1016/j.arth.2017.11.031 [DOI] [PubMed] [Google Scholar]
- 54.Fraval A, Hozack WJ. Managing the patient with peripheral vascular disease before total knee arthroplasty surgery. Orthop Clin North Am. 2023;54(3):259-267. doi: 10.1016/j.ocl.2023.02.011 [DOI] [PubMed] [Google Scholar]
- 55.Ahmed AA, Mooar PA, Kleiner M, Torg JS, Miyamoto CT. Hypertensive patients show delayed wound healing following total hip arthroplasty. PLoS One. 2011;6(8):e23224. doi: 10.1371/journal.pone.0023224 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Curtis GL, Newman JM, George J, Klika AK, Barsoum WK, Higuera CA. Perioperative outcomes and complications in patients with heart failure following total knee arthroplasty. J Arthroplasty. 2018;33(1):36-40. doi: 10.1016/j.arth.2017.07.043 [DOI] [PubMed] [Google Scholar]
- 57.Porter SE, Hanley EN Jr. The musculoskeletal effects of smoking. J Am Acad Orthop Surg. 2001;9(1):9-17. doi: 10.5435/00124635-200101000-00002 [DOI] [PubMed] [Google Scholar]
- 58.Wang Q, Goswami K, Shohat N, Aalirezaie A, Manrique J, Parvizi J. Longer operative time results in a higher rate of subsequent periprosthetic joint infection in patients undergoing primary joint arthroplasty. J Arthroplasty. 2019;34(5):947-953. doi: 10.1016/j.arth.2019.01.027 [DOI] [PubMed] [Google Scholar]
- 59.Greenky M, Gandhi K, Pulido L, Restrepo C, Parvizi J. Preoperative anemia in total joint arthroplasty: is it associated with periprosthetic joint infection? Clin Orthop Relat Res. 2012;470(10):2695-2701. doi: 10.1007/s11999-012-2435-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Rantala A, Lehtonen OP, Niinikoski J. Alcohol abuse: a risk factor for surgical wound infections? Am J Infect Control. 1997;25(5):381-386. doi: 10.1016/S0196-6553(97)90082-1 [DOI] [PubMed] [Google Scholar]
- 61.Sequeira SB, Quinlan ND, Althoff AD, Werner BC. Iron deficiency anemia is associated with increased early postoperative surgical and medical complications following total hip arthroplasty. J Arthroplasty. 2021;36(3):1023-1028. doi: 10.1016/j.arth.2020.09.043 [DOI] [PubMed] [Google Scholar]
- 62.Shabanzadeh DM, Sørensen LT. Alcohol consumption increases post-operative infection but not mortality: a systematic review and meta-analysis. Surg Infect (Larchmt). 2015;16(6):657-668. doi: 10.1089/sur.2015.009 [DOI] [PubMed] [Google Scholar]
- 63.Treschan TA, Taguchi A, Ali SZ, et al. The effects of epidural and general anesthesia on tissue oxygenation. Anesth Analg. 2003;96(6):1553-1557. doi: 10.1213/01.ANE.0000063824.43113.DB [DOI] [PubMed] [Google Scholar]
- 64.McMaster Arthroplasty Collaborative (MAC) . Incidence and predictors of prosthetic joint infection following primary total knee arthroplasty: a 15-year population-based cohort study. J Arthroplasty. 2022;37(2):367-372.e1. doi: 10.1016/j.arth.2021.10.006 [DOI] [PubMed] [Google Scholar]
- 65.Kim CW, Kim HJ, Lee CR, Wang L, Rhee SJ. Effect of chronic kidney disease on outcomes of total joint arthroplasty: a meta-analysis. Knee Surg Relat Res. 2020;32(1):12. doi: 10.1186/s43019-020-0029-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Kunutsor SK, Whitehouse MR, Blom AW, Beswick AD; INFORM Team . Patient-related risk factors for periprosthetic joint infection after total joint arthroplasty: a systematic review and meta-analysis. PLoS One. 2016;11(3):e0150866. doi: 10.1371/journal.pone.0150866 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Turcotte J, Kelly M, Aja J, King P, MacDonald J. Complication rates and resource utilization after total hip and knee arthroplasty stratified by body mass index. J Orthop. 2021;24:111-120. doi: 10.1016/j.jor.2021.02.024 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Onggo JR, Ang JJM, Onggo JD, de Steiger R, Hau R. Greater risk of all-cause revisions and complications for obese patients in 3 106 381 total knee arthroplasties: a meta-analysis and systematic review. ANZ J Surg. 2021;91(11):2308-2321. doi: 10.1111/ans.17138 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
eTable 1. Diagnosis and Procedures Codes Used to Define Inclusion and Exclusion Criteria, Prosthetic Joint Infection Outcomes, and Censoring Events
eFigure 1. Validated Case-Finding Algorithms Used to Identify Prosthetic Joint Infection After Total Knee Arthroplasty Within United States Veterans Health Administration Data
eTable 2. Definitions of Medical Comorbidities and Surgical Characteristics as well as ICD-9 and ICD-10 Diagnosis Codes Used to Identify Comorbidities of Interest
eFigure 2. Hierarchy Used for Organization of Microbiological Culture Data
eTable 3. Baseline Demographics, Medical Comorbidities, and Surgical Characteristics at Time of Total Knee Arthroplasty Among Those Not in the Veterans Affairs Surgical Quality Improvement Program Compared to Patients Included in This Cohort
eTable 4. Adjusted Incidence Rate Ratios of Prosthetic Joint Infection at Any Time After Primary Total Knee Arthroplasty Associated With Demographic, Baseline Clinical, and Peri-Operative Factors Among Patients Included in the Veterans Affairs Surgical Quality Improvement Project (n = 61 701)
Data Sharing Statement