Abstract
Vascular plant reproductive structures have undoubtedly become more complex through time, evolving highly differentiated parts that interact in specialized ways. But quantifying these patterns at broad scales is challenging because lineages produce disparate reproductive structures that are often difficult to compare and homologize. We develop a novel approach for analysing interactions within reproductive structures using networks, treating component parts as nodes and a suite of physical and functional interactions among parts as edges. We apply this approach to the plant fossil record, showing that interactions have generally increased through time and that the concentration of these interactions has shifted towards differentiated surrounding organs, resulting in more compact, functionally integrated structures. These processes are widespread across plant lineages, but their extent and timing vary with reproductive biology; in particular, seed-producing structures show them more strongly than spore or pollen-producing structures. Our results demonstrate that major reproductive innovations like the origin of seeds and angiospermy were associated with increased integration through greater interactions among parts. But they also reveal that for certain groups, particularly Mesozoic gymnosperms, millions of years elapsed between the origin of reproductive innovations and increased interactions among parts within their reproductive structures.
Keywords: macroevolution, networks, gymnosperms, angiosperms
1. Introduction
Vascular plants produce an extraordinary variety of reproductive structures, from the simple clusters of sporangia found in many ferns to the elaborate flowers of orchids [1–3]. The complexity of structures such as flowers reflects not only their diverse and highly differentiated parts [3–5], but also the intricacy of interactions among these parts. Botanists have long noted, for example, how specialized floral organs are often borne in specific geometric arrangements that bring anthers, stigmas and animals together to facilitate pollination [6–10]. Floral complexity also reflects extensive fusion and coordinated growth among component parts [2,11–15]. Such functional and developmental integration among reproductive traits has been most widely studied in angiosperms [16–20], but these phenomena are not restricted to the group; reproductive structures with some degree of functional integration are common in extant plant lineages from lycopsids with compact spore-producing strobili [21] to conifers with tightly interlocking seed cone scales [22,23]. The evolution of diverse and specialized functional interactions among reproductive parts then appears to be an important aspect of plant evolutionary history.
Such interactions have undoubtedly increased over vascular plant evolutionary history, given that the earliest known reproductive structures consisted simply of solitary or loose aggregations of sporangia [24–26]. Many later groups appear to have evolved more complex morphologies by co-opting organs to perform new functional roles in spore release, pollination, seed protection and seed dispersal [3,21,27,28], and novel interactions among reproductive parts are an important aspect of this process. For example, major reproductive innovations like the origins of seeds and angiospermy reflect close physical, developmental and functional associations between reproductive organs and enclosing structures, involving either the integument or seed coat alone [29,30] or the integument plus the carpel in angiosperms [31]. Within seed plants, Mesozoic lineages evolved a wide diversity of cupules, scales and compact strobili that cover seeds [25] and which are often assumed to protect them in some capacity [32–34].
That interactions among plant reproductive parts have increased over time and are associated with morphological diversification is intuitive, but the extent and tempo of these trends are difficult to evaluate. Although seed plants are widely regarded as having evolved more integrated and complex reproductive structures (e.g. [2–4,13]), other diverse clades such as leptosporangiate ferns (Polypodiidae sensu [35]) have maintained simple reproductive structures over their evolutionary history [3]. Quantitatively assessing temporal patterns in reproductive part interactions across clades is challenging because they are difficult to consistently define and characterize across lineages with disparate reproductive structures, especially those in which homologies among parts are nonexistent or uncertain (see discussions in [36–38]). In this study, we use a network approach [39] to overcome some of these difficulties. Networks have been used in previous studies of morphological integration [40], morphological complexity [41] and ecological complexity [42,43]. Here they allow us to create a flexible but consistent framework in which to characterize temporal patterns in part interactions and functional integration, regardless of the age, affinities or specific homologies of the reproductive structures.
To create reproductive interaction networks, we divide reproductive structures into their basic component part types following a previous study [3], which we treat as nodes in a network. We then score five kinds of interactions among these parts and represent these interactions as edges linking network nodes. Interactions represent basic physical and geometric relationships among parts, including three types describing physical contact (attachment, fusion and conforming, which refers to closely adpressed parts), and two describing nested spatial relationships (envelopment and enclosure of one or more part types by another). The simple networks that we build from these interactions, which can often be clearly recognized in well-preserved fossils, encode a suite of potential functional relationships among the basic units of a plant reproductive structure. By analysing networks from both extant and extinct plants, we ask if interactions among reproductive parts have increased through time generally and within major plant lineages specifically. We also use the topological structure of our networks to ask if the location and concentration of part interactions has changed through time; in particular, we ask if interactions have shifted away from the reproductive organs themselves and onto auxillary structures, as a greater variety of organs have become incorporated into reproductive structures over plant evolutionary history [27,28].
2. Material and methods
We used published literature descriptions supplemented with direct observations from herbarium specimens to score 1461 reproductive structures (905 fossil, 556 extant) from 1306 vascular plant taxa (we treat conspecific micro- and megasporangiate structures as separate units of analysis). A full list of taxa and reproductive structures can be found in the electronic supplementary materials, and sources are listed in files on Dryad [44]. The number of nodes for each reproductive structure is based on a list of part types derived from a previous study (the ‘morphological element types' of Leslie et al. [3]), although modified in some cases to include updated information. We defined part types as distinct geometric regions of reproductive structures summed over ontogeny; in angiosperms, for example, the total number includes parts at pollination and fruit maturity. It is important to emphasize that the number of network nodes represents distinct part types rather than the absolute number of parts; for example, a single part type such as a petal may be expressed many times in a reproductive structure. As in [3], we sampled reproductive structures at the genus level to minimize uneven sampling because many fossil reproductive structures belong to monotypic genera. Species within genera often do not vary in part types present (i.e. node number) or part interactions as we define them, although in cases with known variation we did include multiple representatives. For fossil taxa with a wide stratigraphic range, we generally sampled one representative per geologic epoch. Among extant angiosperms, we sampled one representative taxon per major clade (i.e. monophyletic families following APG IV [45]) due to their extreme species richness (although not all families were sampled). This dramatically undersamples extant angiosperms and our results should not be taken to encompass their full range of diversity or complexity, although our analyses are not specifically focused on angiosperms and exhaustive sampling of their diversity is not required for the basic comparisons with other vascular plant groups that we discuss here. See electronic supplementary materials for a description of part types in each taxon as well as a more in-depth discussion of sampling and scoring concepts).
For each reproductive structure, we scored five kinds of part interactions: (i) attachment, (ii) fusion, (iii) conforming, (iv) envelopment and (v) enclosure. Attachment describes organic or anatomical connection among part types at a small point or thin edge, while fusion describes part types that are attached for more than one-third of their length or surface area. We represent fusion in our networks as two edges, one representing attachment (a necessary condition of being fused) and one representing the fusion interaction itself (figure 1a,b). Conforming or adpression interactions are represented by a single edge and refer to part types with regular, close contact (although not direct anatomical attachment) for much of their ontogeny; this relationship creates a closed surface as the structure grows and develops (figure 1a,b). Envelopment occurs when one part type surrounds another; this interaction most often involves a cup-like structure although it may also include parts that are sunken into subtending organs. An enveloping part need not physically contact internal part type(s) but as defined here must form a continuous wall or tissue groundmass surrounding them for half or more of their length or surface area. Enclosure is an extension of envelopment where internal part type(s) are completely sealed off from the outside environment throughout their ontogeny and only interact with it through mechanical fracture (e.g. seeds released from a dehiscent fruit) or a dedicated opening (e.g. a seed micropyle). Enclosure necessarily includes envelopment, but the distinction between these two is important because enclosure necessitates a functional change in how reproductive propagules (spores, pollen or seeds) are released or captured. We therefore use two edges, one for envelopment and one for complete enclosure itself, to represent this interaction in our networks. We refer to fusion, conforming, envelopment and enclosure interactions as ‘engagement edges’ throughout this study because they add additional physical or geometric links beyond simple attachment, and we interpret them as indicating greater integration among reproductive structures. For detailed descriptions and examples of these interactions, as well as the full list of interactions for all taxa, see electronic supplementary material.
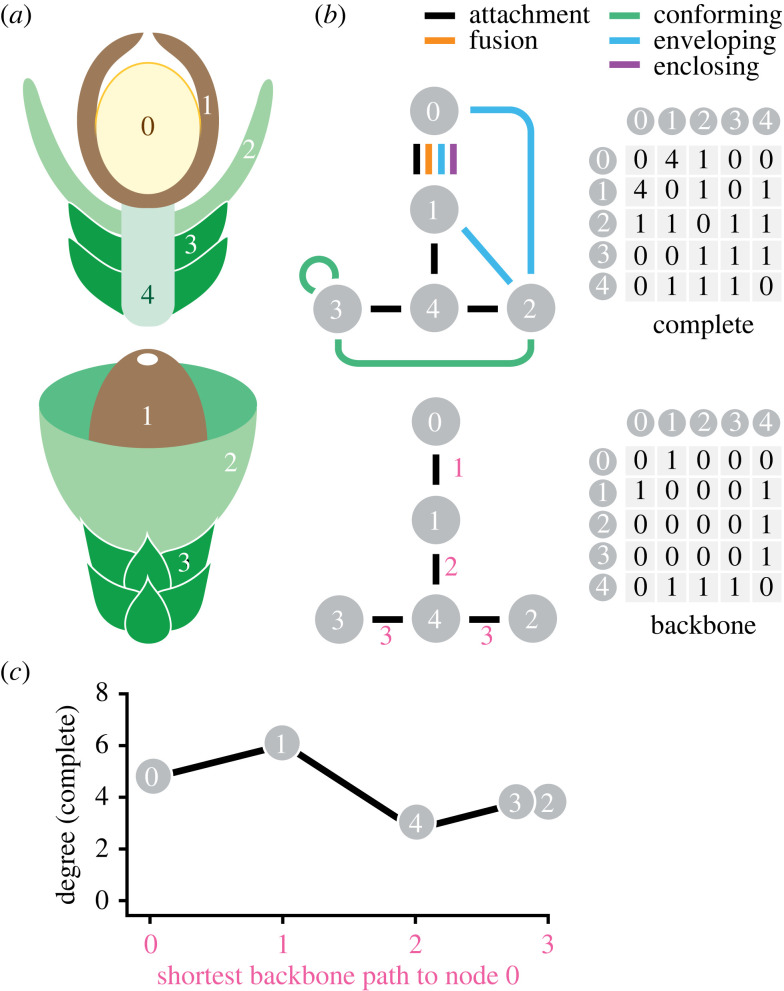
Figure 1.
Network approach to characterizing interactions among reproductive parts. (a) Cross-section and external view of hypothetical reproductive structure with part types numbered from zero (the sporangium). (b) Networks (left) and adjacency matrices (right) based on (a), with part types depicted as numbered nodes and the five types of interactions among them as undirected, unweighted edges. Nodes interacting with themselves indicated by self-loops. Adjacency matrices represent the number of edges connecting pairs of nodes in the networks. For each reproductive structure, we scored a ‘complete’ network depicting all interactions among nodes and a ‘backbone’ network with only attachment interactions; red numbers indicate distance of the nodes in attachment edges from the sporangium. (c) Plot of degree (total edges incident to a node) in complete network from (a, b) as a function of shortest backbone distance from the sporangium.
For each reproductive structure, we used the part types and their interactions to construct two kinds of networks (figure 1b): one with attachment edges only (backbone) and one with attachment plus engagement edges (complete). For both backbone and complete networks, we scored part types that interact with themselves (e.g. sporophylls that are imbricated or sporangia that are fused) as self-loops (figure 1b). We note that edges in our networks represent relationships between part types in aggregate rather than between every individual element belonging to those types; for example, the seed integument in a conifer cone is commonly conformable with adjacent ovuliferous scales and the part types are therefore scored as exhibiting an engagement edge, even though not every ovuliferous scale in a cone produces seeds.
We represented each network by an adjacency matrix, which is a symmetric matrix that records the number of edges among pairs of nodes [39] (figure 1b; all adjacency matrices available on Dryad [44]). In total, we analysed 763 complete adjacency matrices (many reproductive structures are described by the same adjacency matrix) and their backbone equivalents. In each matrix, the sporangium or megasporangium was scored as the 0th node (figure 1a,b); all other parts were numbered arbitrarily. To analyse temporal patterns, we calculated basic metrics [39] including the number of nodes and edges in a network, as well as the maximum and average degree of the network (the degree of a node is the number of edges incident to it [39]). We divided reproductive structures into free-sporing, pollen-producing and seed-producing categories, which correspond to major differences in reproductive biology and function, and binned them into geologic periods, although we separated the Devonian (D) and Cretaceous (K) into D1 (Early; 419–393 Ma), D2 (Middle to Late; 393–359 Ma), K1 (Early; 145–100 Ma) and K2 (Late; 100–66 Ma) intervals to capture major shifts occurring during those periods. Within each time bin, we used the number of engagement edges and average degree for each taxon to calculate minimum, maximum, interquartile range (IQR) and median values. We also performed a bootstrap resampling procedure to calculate 95% confidence intervals on the median number of engagement edges per taxon. For each interval, we resampled the number of engagement edges and average degree with replacement and calculated the median, repeated this 1000 times, and then took the 95% quantile range of this distribution. We also repeated these analyses for complete networks where self-loops had been removed because a self-interaction places two edges incident upon a node and may substantially increase maximum and average degree. The results of these analyses, however, show similar temporal patterns to those that included self-interactions (see electronic supplementary material, figure S7).
For each taxon, we also analysed how the concentration of interaction edges varied throughout the reproductive structure by determining how node degree changes with distance from the sporangium or megasporangium. We recorded distance as the number of attachment edges that separate a given part type from the sporangium. For example, in the hypothetical structure from figure 1, sterile bracts (Part Type 3) are attached to the cone axis (Type 4), which is in turn attached to a seed integument (Type 1) that attaches to the megasporangium (Type 0); the sterile bracts are then two nodes and three attachment edges from the megasporangium (figure 1a,b). Across all reproductive structures, we used backbone networks to assign each part type a distance representing the shortest pathlength in number of attachment edges from that part type to the 0th node, which by convention here is the sporangium or megasporangium (figure 1a,b). We then used the corresponding complete network to calculate degree for all nodes and recorded the maximum degree node at each distance from the sporangium. After combining distance information from the backbone matrix and node degree information from the corresponding complete matrix, each reproductive structure can be represented as a line showing maximum degree for part types that are increasingly distant from the sporangium in terms of their physical attachment (figure 1c). To visualize patterns of node degree with distance from the sporangium or megasporangium in our entire dataset, we also added a slight amount of noise to the maximum degree values at each distance so that lines representing individual reproductive structures did not entirely overlap. This noise is a small fraction of maximum degree values and does not alter any patterns, but it does allow lines from different taxa with the same sequence of values to be visible. These and other network analyses, including computation of summary statistics, used scripts written in Python with Networkx [46]. Statistical procedures for the calculation of temporal patterns were performed using R version 4.1.3 [47]. CSV files for raw data, all adjacency matrices, Python and R scripts are available on Dryad [44].
3. Results
(a) . General patterns in reproductive networks
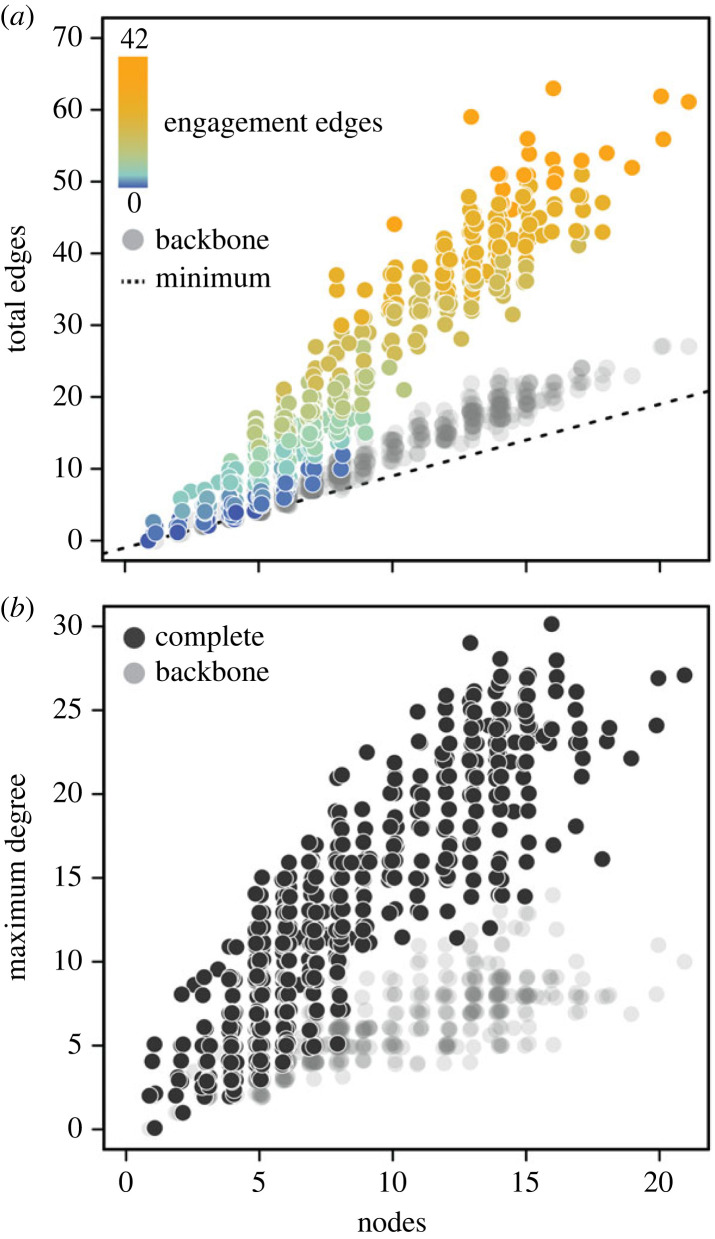
In aggregate, networks from more than 1450 fossil and extant reproductive structures (figure 2a; electronic supplementary material, figure S1) show a proportional increase in edges with increasing nodes, suggesting that complexity as measured by part types is correlated with complexity as measured by interactions among parts. The steep slope of this relationship is due to engagement edges (fusion, conforming, envelopment and enclosure interactions); in networks where these have been removed (the ‘backbone’ networks) the slope is positive but much shallower and more similar to the minimum expected increase for a network of connected parts (n nodes – 1; figure 2a). Maximum degree (the number of edges incident to a node) in reproductive networks also increases sharply with node number (figure 2b), meaning that reproductive structures with many parts often have edges concentrated on specific part types rather than spread diffusely through the network. Again, this effect is driven by engagement edges; backbone networks show a much shallower increase in maximum degree with node number (figure 2b).
Figure 2.
Basic characteristics of vascular plant reproductive networks. (a) Relationship between node number and minimum possible number of edges (n nodes − 1), backbone edges and complete edges across all networks. The number of ‘engagement’ edges (fusion, conforming, enveloping, and enclosure interactions) in complete networks shown as a colour gradient. (b) Relationship between node number and maximum degree across all complete and backbone networks.
(b) . Temporal patterns in part interactions
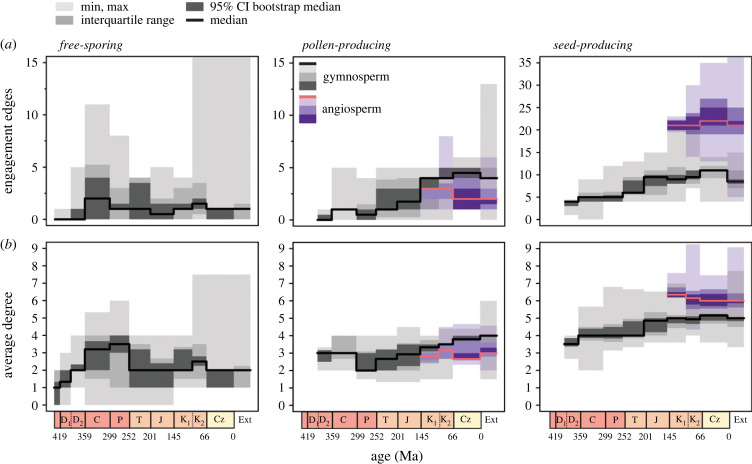
Both average degree and number of engagement edges increased from the Late Silurian through the Late Carboniferous (figure 3). This increase reflects two superimposed processes: (i) a general trend towards the evolution of compact strobili with specialized, tightly packed sporophylls in multiple lineages including lycopsids, horsetails and progymnosperms; and (ii) the evolution of seed plants at the end of the Devonian with their characteristic megasporangium-enveloping organ integument (seed coat). Among free-sporing plants, average measures of complexity after the Carboniferous remains relatively unchanged (figure 3; electronic supplementary material, figure S2), although the appearance of highly specialized aquatic Salviniales ferns [48] in the Late Mesozoic substantially increased maximum complexity (figure 3; note Cretaceous maximum increase in leftmost panels). The apparent decline in average degree over the Mesozoic (figure 3b), which is significant based on resampled median values for some time period comparisons, is primarily due to differences in sampling. Mesozoic intervals are dominated by leptosporangiate ferns, which generally produce simple reproductive structures, although lycopsids and horsetails must also have been present given that they survive to the present day.
Figure 3.
Temporal trends among vascular plant reproductive networks. (a) Engagement edges through time among free-sporing, pollen-producing and seed-producing structures. (b) Average degree of reproductive networks through time. Coloured bars depict maximum and minimum values, interquartile range and 95% confidence intervals (CI) on median values for each geologic time bin. Confidence intervals were calculated from a bootstrap resampling procedure (see Material and methods).
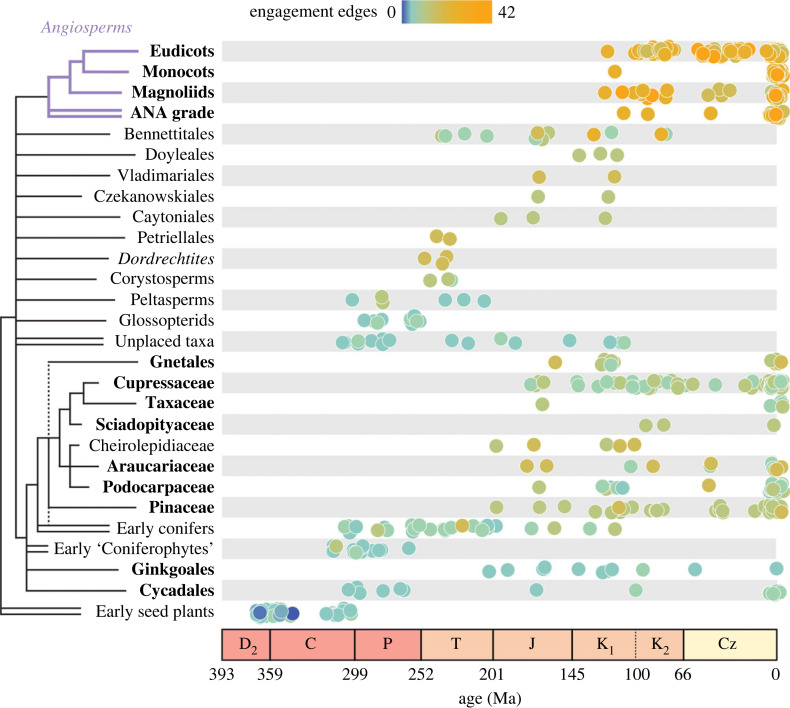
In contrast to free-sporing plants, seed plants show consistent increases in the number of engagement edges through time (figure 3). Engagement edges in their pollen-producing structures rise over the Mesozoic but generally plateau at values similar to those of free-sporing Carboniferous lycopsids and sphenophytes (see electronic supplementary material, figures S2 and S3), reflecting the convergent evolution of a suite of conforming edges between pollen sacs and protective sporophylls (see electronic supplementary material, figure S4). By contrast, seed-producing structures generally produce more parts and engagement edges and reach much higher values over the Mesozoic due to a more diverse set of interactions between seeds and various covering structures. In gymnosperms (here used in a broad sense to refer to all non-angiosperm seed plants), this rise is primarily caused by two different patterns: (i) an increase in the abundance of compact seed cones and therefore conforming edges in some crown conifer clades, and (ii) a greater variety of seed-covering structures creating both conforming and envelopment edges in various ‘seed fern’ groups (e.g. Caytoniales, Doyleales and Petriellales) and some crown conifer clades (Podocarpaceae, Taxaceae) with reduced, fleshy cones (figure 4; electronic supplementary material, figure S4). The median number of nodes in gymnosperm reproductive structures varies little through time, however (electronic supplementary material, figure S5; see also [3]), meaning that the steep slope in the relationship between node number and engagement edges across plants generally (figure 2a) was not initially present in seed plants (electronic supplementary material, figure S6). This stronger relationship emerged only over the later Mesozoic with the appearance of more compact and physically integrated gymnosperm reproductive organs (figures 3 and 4).
Figure 4.
Engagement edges in the seed-producing structures of extinct and extant groups. Reproductive structures from each group are plotted by their average age and coloured by the number of engagement edges. Extant groups are shown in bold and a provisional phylogeny is provided to the left. Double branches leading to taxa indicate either paraphyletic or polyphyletic groups. ANA grade angiosperms refers to the Amborella, Nymphaeales and Austrobaileyales lineages. Phylogenetic relationships among extinct groups are largely unresolved; overall topology primarily reflects [37,49–51].
The relationship between nodes and engagement edges among modern seed plant lineages was further strengthened by the appearance of angiosperms in the Early Cretaceous, whose seed-producing structures (here meaning both pistillate and bisexual flowers) show extremely high numbers of both part types and engagement edges (figure 3; electronic supplementary material, figure S5; see also [3]). Angiosperms are characterized by many fusion, envelopment, and enclosure interactions (note the increase in these interactions in electronic supplementary material, figure S4 coinciding with the diversification of angiosperms) due to developmental and physical integration of seeds, individual carpels and (in many lineages) syncarpous gynoecia. Although some gymnosperms produce reproductive structures that rival average angiosperms in complexity (e.g. certain Gnetales and extinct Bennettitales; figure 4; electronic supplementary material, figure S1), angiosperms reach much higher maximum values than any other group of seed plants (figure 3). These unprecedented maximum values were apparently achieved rapidly by angiosperms, and median values for the group remain similar over their history in our data (figure 3).
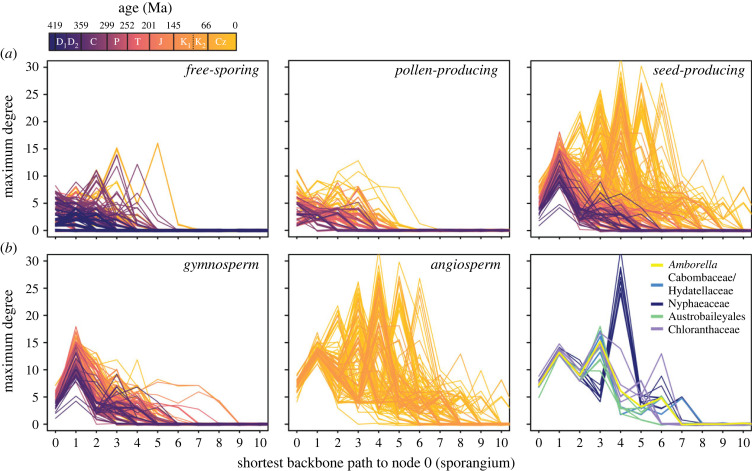
(c) . Spatial patterns in part interactions
The concentration of engagement edges within reproductive networks also shifts through time, generally moving away from the sporangium (figure 5). This change reflects the incorporation of various novel organs into functional relationships with sporangia and later with seeds, which themselves represent a close interaction between a megasporangium (called the nucellus in seed plants) and the integument. Among free-sporing structures, the Palaeozoic rise in edges occurs primarily on the sporangium itself and then to a lesser extent on nodes several attachment edges from it (figure 5a), consistent with sporangia, microsporangia or megasporangia being the locus of physical interactions with closely packed sporophylls. High-degree peaks on more distant part types do occur (figure 5a) but these tend to be found in highly specialized taxa such as the previously mentioned aquatic Salviniales and Late Carboniferous lycopsids that had evolved seed-like structures (e.g. Miadesmia [52]). Pollen-producing structures show a similar overall pattern as free-sporing structures (figure 5a), although as noted earlier (figure 3a), increases in maximum degree occur later in their history when compact pollen cones become more abundant.
Figure 5.
Location of maximum degree nodes within reproductive structures through time. (a) Maximum degree as a function of the number of attachment edges away from the sporangium for all free-sporing, pollen-producing and seed-producing reproductive structures. (b) maximum degree location within only the seed-producing structures of gymnosperms (left), all angiosperms (centre), and early-diverging angiosperm lineages (left; ANA grade plus Chloranthaceae). Nymphaeaceae are generally resolved as sister to a grade of Cabombaceae and Hydatellaceae. Each line represents an individual reproductive structure colour coded by age. Values at zero distance represent the sporangium itself.
By contrast, the largest number of edges in seed-producing structures occurs on part types other than their megasporangium/nucellus, and the concentration of these edges gradually increases and shifts towards more distant structures over time (figure 5a). The Mesozoic rise in engagement edges among gymnosperms is clearly visible on the seed coat (one edge from the nucellus; figure 5b), as these structures are the locus for physical interactions with surrounding cone scales and cupules that cover them. Angiosperm integuments are comparable to those of later gymnosperms in degree, but the evolution of the carpel creates a new locus of engagement edges at a greater distance from the nucellus (figure 5b; the exact distance varies among taxa due to differences in the number of integuments, funiculus development and differentiation of placental tissues). Additional organs such as enveloping calyces or receptacles may also contribute to peaks at even greater distances. Our sampling within angiosperms is not detailed enough to resolve smaller-scale trends within the group, but derived clades do appear to show a shift towards increasing interactions on their carpels (figure 5b, right panel). In particular, early-diverging extant lineages (with the exception of some derived Nymphaeales) show lower degree carpel peaks (positions 4 and 5 in figure 5b, right panel) than magnoliids, monocots and eudicots. These early groups are notable for their lack of fusion among carpels, resulting in fewer engagement edges, lower degree and generally less complex ovary organization.
4. Discussion
Our plant reproductive networks are independent of homology by design, but a basic understanding of phylogenetic relationships (e.g. [49,50,53]) is nevertheless important when interpreting our results. Namely, increased engagement edges must have arisen independently among many major lineages of free-sporing and seed plants given the morphology of their early members combined with phylogenetic tree topology (figure 4; electronic supplementary material, figures S2 and S3). Although the phylogenetic placement of extinct seed plant lineages is not well resolved due to conflicting topologies and low support (e.g. [37,38,51,54–56]), increased engagement edges in various Mesozoic gymnosperm groups likely evolved independently given that they frequently link different specific organs (e.g. various types of bracts, fertile scales or cupules). At minimum, engagement edges in the flowers of Bennettitales, the cupules of ‘seed ferns’, and the seed cones of crown conifers are almost certainly not homologous given fundamental differences in their construction [25]. Even within crown conifers, increased conformable interactions among cone scales (meaning their bract-scale complexes [57,58]) must have evolved independently because interlocking scales became abundant in the fossil record only in the Jurassic [22], post-dating the divergence of major extant lineages [59].
These results are consistent with some long-standing hypotheses in plant evolution; in particular, the co-option and functional integration of novel organs into reproductive structures is thought to be an important part of their morphological diversification [27,60] and has often been invoked, either explicitly or implicitly, to interpret major patterns among seed plants. For example, the earliest seeds used an elaborate projection of the exposed megasporangium to capture pollen grains [37,61] while later gymnosperms shifted this function to a fully enclosing integument (e.g. [62]). Floral synorganization, or the fusion of carpels, stamens and perianth elements, is a widely recognized trend among derived angiosperms [2,4,13,63] and the group also commonly transfers functional roles in floral protection and display to a wide variety of auxiliary organs [27,64]. Our analyses place these observations in a wider phylogenetic and temporal context, showing that integration, at least as characterized by engagement edges, is common across vascular plant lineages but is expressed most strongly among seed plants and especially among angiosperms.
The correlation between part types and engagement edges in our networks (figure 2a) suggests that morphological complexity is related in some way to the evolution of interactions among parts. For example, differentiated sporophylls in Palaeozoic lycopsids and horsetail strobili, as well as Mesozoic seed plant pollen cones, occur with evidence of greater physical contact (e.g. faceted sporangia or pollen sacs and sporophyll phlanges that closely adhere to them). Seeds and carpels, whose origins represent the largest increases in part types [3], are characterized by their high degree of integration between sporangia and various enclosing organs. These patterns at face value suggest that the evolution of reproductive complexity emerges in the context of specialized interactions among parts. But our results also show that this relationship may not be straightforward; one of the most notable temporal patterns in our data is an increase in engagement edges among Mesozoic seed plants without a change in part type numbers (electronic supplementary material, figures S4–S6). For much of seed plant history, then, the evolution of reproductive structural complexity, at least as measured by part type diversity, was decoupled from changes in physical and functional interactions among these parts.
The gradual emergence of greater integration among seed-producing structures over the Mesozoic could be explained as a response to animal groups that diversified long after seed plant origins. For example, the Jurassic to Early Cretaceous increase in conformable edges among many conifer clades coincides with a shift towards thicker and more heavily armored seed cones [22,65], a pattern that mirrors microevolutionary changes in extant conifer populations experiencing high predation pressure from birds and mammals [66,67]. At the same time, fleshy seed-covering organs and compact fruit-like cones that likely functioned in vertebrate seed dispersal become notable features of other conifer clades [68,69], members of the Gnetales [70] and extinct gymnosperms like Caytonia, whose cupules have been recorded in coprolites [71]. Stronger ecological interactions with vertebrates, particularly as small-bodied birds and mammals radiated from the Jurassic onwards [69,72,73], may have favoured the evolution of more compact reproductive structures better adapted to either protect seeds or function as small edible diaspores [74,75]. These morphologies appear to be largely derived from existing morphological elements, however, rather than new suites of organs, resulting in a diverse range of morphological strategies for covering seeds (e.g. [33,34,56]) but little fundamental change in structural complexity.
Specialized animal pollination syndromes are also thought to have been common over the Mesozoic [76–78] and are associated with high reproductive part type numbers generally [3]. Insect pollination was probably important in the evolution of the most complex gymnosperm reproductive structures; the high part type counts in the bisexual flowers of some extinct bennettitaleans and the staminate strobili of some extant Gnetales (figure 4; electronic supplementary material, figure S3) reflect the presence of both pollen and seed organs as well as enveloping perianth elements. But obvious morphological adaptations to insect pollination were rare among Mesozoic gymnosperms and many extant insect-pollinated gymnosperms like cycads likewise do not show obvious specializations, instead relying on temperature and volatile cues to attract pollinators [79,80]). Although pollination biology may have played an important role in the evolution of extremes among Mesozoic gymnosperms, the general increase in engagement edges appears to have been driven more by the evolution of compact reproductive structures that presumably performed fruit-like functional roles in seed protection and/or dispersal.
Why gymnosperm lineages have rarely evolved highly integrated seed protection/dispersal modules in combination with specialized perianth and staminate organs is unclear, but may relate to their pollination syndromes. Pollinators such as moths and bees, which often interact with highly specialized perianth parts and intricate flower geometries, are thought to have diversified with derived angiosperm clades [78] (although see [81]). By contrast, early-diverging angiosperm lineages generally produce less complex flowers and are primarily visited by ovipositing flies and beetles [82], more akin to proposed Mesozoic pollinators [76,77]. These pollination syndromes are thought to rely less on specialized floral geometries and more on cues like odour or food rewards [83]. But further exploring these potential associations and mechanisms behind them requires a much better sampling of angiosperms and pollination syndromes than provided in this study.
In a broader biological sense, the trend towards increasing integration within some lineages of vascular plants is consistent with patterns in other groups (e.g. [84–87]), particularly vertebrate crania that are perhaps the most well-studied system in this regard (e.g. [88–91]). It is worth noting that this similarity occurs despite fundamental differences in how integration is measured [86]; for example, our engagement edges commonly represent more diffuse geometric and spatial relationships among parts (as do functional interactions in plants generally; see [92]) than size or shape covariation in cranial elements due to a shared joint. The exact relationship between integration and complexity in plants and animals, however, appears to be different. Fusion among vertebrate bones is often regarded as increasing integration and reducing the number of skeletal elements [93,94], whereas plant engagement edges most analogous to bone fusion, such as strong interactions among the parts of seeds and fruits, are associated with an increase in part types when they evolve. Interestingly, network-based analyses that define complexity in terms of interactions among skeletal elements rather than their number reach a conclusion more similar to ours, linking higher integration with increased complexity [95,96].
The approach that we develop here represents a different way to analyse broad macroevolutionary patterns in vascular plants, as well as a means of quantifying the evolution of reproductive complexity with more granularity than analyses of part types alone [3]. Our approach provides a fresh window into functional integration in plants and highlights the key role of major reproductive innovations as the origin of seeds and angiospermy are clearly associated with increased interactions among parts. These results also demonstrate the interplay between close physical integration and the evolution of complexity, as various organs become incorporated into compact, modular structures that in turn become loci for new interactions, ratcheting up structural complexity and functional integration through time. Focused studies of individual plant clades, where the homologies of these part types and phylogenetic histories of interactions among them are better constrained, may provide further tests of whether this complexity ratchet hold true on smaller scales. But the flexibility of networks like those used here means that studies of virtually any group of organisms where anatomy or morphology can be atomized into discrete interacting parts can build on this approach.
Acknowledgements
We thank Ana Andruchow-Colombo and two anonymous reviewers for their comments and suggestions to improve this work. We thank Rebecca Morrison and Carl Simpson for discussions and scripts relating to the ‘distance to zero’ analysis, and we thank Dan McShea for helpful comments on the manuscript.
Ethics
This work did not require ethical approval from a human subject or animal welfare committee.
Data accessibility
Data are available from the Dryad Digital Repository [44] and in the electronic supplementary material [97].
Declaration of AI use
We have not used AI-assisted technologies in creating this article.
Authors' contributions
A.B.L.: conceptualization, data curation, formal analysis, investigation, methodology, project administration, resources, software, supervision, validation, visualization, writing—original draft; L.M.: conceptualization, data curation, formal analysis, investigation, methodology, resources, software, supervision, validation, visualization, writing—original draft.
All authors gave final approval for publication and agreed to be held accountable for the work performed therein.
Conflict of interest declaration
We declare we have no competing interests.
Funding
We received no funding for this study.
References
- 1.Endress PK. 2011. Evolutionary diversification of the flowers in angiosperms. Am. J. Bot. 98, 370-396. ( 10.3732/ajb.1000299) [DOI] [PubMed] [Google Scholar]
- 2.Endress PK. 2016. Development and evolution of extreme synorganization in angiosperm flowers and diversity: a comparison of Apocynaceae and Orchidaceae. Ann. Bot. 117, 749-767. ( 10.1093/aob/mcv119) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Leslie AB, Simpson C, Mander L. 2021. Reproductive innovations and pulsed rise in plant complexity. Science 373, 1368-1372. ( 10.1126/science.abi6984) [DOI] [PubMed] [Google Scholar]
- 4.Endress PK. 2001. Origins of flower morphology. J. Exp. Zool. 291, 105-115. ( 10.1002/jez.1063) [DOI] [PubMed] [Google Scholar]
- 5.Endress PK. 2006. Angiosperm floral evolution: morphological developmental framework. Adv. Bot. Res. 44, 1-61. ( 10.1016/S0065-2296(06)44001-5) [DOI] [Google Scholar]
- 6.Darwin CR. 1862. On the various contrivances by which British and foreign orchids are fertilised by insects. London, UK: John Murray. [PMC free article] [PubMed] [Google Scholar]
- 7.Stebbins GL. 1970. Adaptive radiation of reproductive characteristics in angiosperms, I: pollination mechanisms. Annu. Rev. Ecol. Evol. Syst. 1, 307-326. ( 10.1146/annurev.es.01.110170.001515) [DOI] [Google Scholar]
- 8.Alexandersson R, Johnson SD. 2002. Pollinator-mediated selection on flower-tube length in a hawkmoth-pollinated Gladiolus (Iridaceae). Proc. R. Soc. Lond. B 269, 631-636. ( 10.1098/rspb.2001.1928) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Fenster CB, Armbruster WS, Wilson P, Dudash MR, Thomson JD. 2005. Pollination syndromes and floral specialization. Annu. Rev. Ecol. Evol. Syst. 35, 375-403. ( 10.1146/annurev.ecolsys.34.011802.132347) [DOI] [Google Scholar]
- 10.Moyroud E, Glover BJ. 2017. The evolution of diverse floral morphologies. Curr. Biol. 27, R941-R951. ( 10.1016/j.cub.2017.06.053) [DOI] [PubMed] [Google Scholar]
- 11.Armbruster WS, Debevec EM, Willson MF. 2002. Evolution of syncarpy in angiosperms: theoretical and phylogenetic analyses of the effects of carpel fusion on offspring quantity and quality. J. Evol. Biol. 15, 657-672. ( 10.1046/j.1420-9101.2002.00414.x) [DOI] [Google Scholar]
- 12.Rudall PJ, Bateman RM. 2002. Roles of synorganisation, zygomorphy and heterotopy in floral evolution: the gynostemium and labellum of orchids and other lilioid monocots. Biol. Rev. 77, 403-441. ( 10.1017/S1464793102005936) [DOI] [PubMed] [Google Scholar]
- 13.Soltis PS, Brockington SF, Yoo MJ, Piedrahita A, Latvis M, Moore MJ, Chanderbali AS, Soltis DE. 2009. Floral variation and floral genetics in basal angiosperms. Am. J. Bot. 96, 110-128. ( 10.3732/ajb.0800182) [DOI] [PubMed] [Google Scholar]
- 14.Zhong J, Preston JC. 2015. Bridging the gaps: evolution and development of perianth fusion. New Phytol. 208, 330-335. ( 10.1111/nph.13517) [DOI] [PubMed] [Google Scholar]
- 15.Phillips HR, Landis JB, Specht CD. 2020. Revisiting floral fusion: the evolution and molecular basis of a developmental innovation. J. Exp. Bot. 71, 3390-3404. ( 10.1093/jxb/eraa125) [DOI] [PubMed] [Google Scholar]
- 16.Berg RL. 1960. The ecological significance of correlation pleiades. Evolution 14, 171-180. ( 10.2307/2405824) [DOI] [Google Scholar]
- 17.Armbruster WS, Di Stilio VN, Tuxill JD, Flores TC, Velasquez Runk JL. 1999. Covariance and decoupling of floral and vegetative traits in nine Neotropical plants: a re-evaluation of Berg's correlation-pleiades concept. Am. J. Bot. 86, 39-55. ( 10.2307/2656953) [DOI] [PubMed] [Google Scholar]
- 18.Armbruster S, Pélabon C, Hansen T, Mulder C. 2004. Floral integration, modularity, and accuracy: distinguishing complex adaptations from genetic constraints. In Phenotypic integration: studying the ecology and evolution of complex phenotypes (eds Pigliucci M, Preston K), pp. 23-49. Oxford, UK: Oxford University Press. [Google Scholar]
- 19.Ashman TL, Majetic CJ. 2006. Genetic constraints on floral evolution: a review and evaluation of patterns. Heredity 96, 343-352. ( 10.1038/sj.hdy.6800815) [DOI] [PubMed] [Google Scholar]
- 20.Ordano M, Fornoni J, Boege K, Domínguez CA. 2008. The adaptive value of phenotypic floral integration. New Phytol. 179, 1183-1192. ( 10.1111/j.1469-8137.2008.02523.x) [DOI] [PubMed] [Google Scholar]
- 21.Bonacorsi NK, Leslie AB. 2019. Functional diversity and convergence in the evolution of plant reproductive structures. Ann. Bot. 123, 145-152. ( 10.1093/aob/mcy151) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Leslie AB. 2011. Predation and protection in the macroevolutionary history of conifer cones. Proc. R. Soc. B 278, 3003-3008. ( 10.1098/rspb.2010.2648) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Losada JM, Blanco-Moure N, Leslie AB. 2019. Not all ‘pine cones’ flex: functional trade-offs and the evolution of seed release mechanisms. New Phytol. 222, 396-407. ( 10.1111/nph.15563) [DOI] [PubMed] [Google Scholar]
- 24.Edwards D, Feehan J. 1980. Records of Cooksonia-type sporangia from late Wenlock strata in Ireland. Nature 287, 41-42. ( 10.1038/287041a0) [DOI] [Google Scholar]
- 25.Taylor EL, Taylor TN, Krings M. 2009. Paleobotany: the biology and evolution of fossil plants. New York, NY: Academic Press. [Google Scholar]
- 26.Edwards D, Morris JL, Axe L, Duckett JG, Pressel S, Kenrick P. 2022. Piecing together the Eophytes—a new group of ancient plants containing cryptospores. New Phytol. 233, 1440-1455. ( 10.1111/nph.17703) [DOI] [PubMed] [Google Scholar]
- 27.Baum DA, Donoghue MJ. 2002. Transference of function, heterotopy and the evolution of plant development. In Developmental genetics and plant evolution (eds Cronk Q, Bateman R, Hawkins J), pp. 52-69. London, UK: Taylor and Francis. [Google Scholar]
- 28.Mathews S, Kramer EM. 2012. The evolution of reproductive structures in seed plants: a re-examination based on insights from developmental genetics. New Phytol. 194, 910-923. ( 10.1111/j.1469-8137.2012.04091.x) [DOI] [PubMed] [Google Scholar]
- 29.Gillespie WH, Rothwell GW, Scheckler SE. 1981. The earliest seeds. Nature 293, 462-464. ( 10.1038/293462a0) [DOI] [Google Scholar]
- 30.Meade LE, Plackett AR, Hilton J. 2021. Reconstructing development of the earliest seed integuments raises a new hypothesis for the evolution of ancestral seed-bearing structures. New Phytol. 229, 1782-1794. ( 10.1111/nph.16792) [DOI] [PubMed] [Google Scholar]
- 31.Endress PK. 2019. The morphological relationship between carpels and ovules in angiosperms: pitfalls of morphological interpretation. Bot. J. Linn. Soc. 189, 201-227. ( 10.1093/botlinnean/boy083) [DOI] [Google Scholar]
- 32.Stewart WN, Rothwell GW. 1993. Paleobotany and the evolution of plants. Cambridge, UK: Cambridge University Press. [Google Scholar]
- 33.Rothwell GW, Stockey RA. 2016. Phylogenetic diversification of Early Cretaceous seed plants: the compound seed cone of Doylea tetrahedrasperma. Am. J. Bot. 103, 923-937. ( 10.3732/ajb.1600030) [DOI] [PubMed] [Google Scholar]
- 34.Klymiuk AA, Rothwell GW, Stockey RA. 2022. A novel cupulate seed plant, Xadzigacalix quatsinoensis gen. et sp. nov., provides new insight into the Mesozoic radiation of gymnosperms. Am. J. Bot. 109, 966-985. ( 10.1002/ajb2.1853) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.PPG I. 2016. A community-derived classification for extant lycophytes and ferns. J. Syst. Evol. 54, 563-603. ( 10.1111/jse.12229) [DOI] [Google Scholar]
- 36.Kenrick P, Crane PR. 1997. The origin and early diversification of land plants: a cladistic study. Washington, DC: Smithsonian Institution Press. [Google Scholar]
- 37.Hilton J, Bateman RM. 2006. Pteridosperms are the backbone of seed-plant phylogeny. J. Torrey Bot. Soc. 133, 119-168. ( 10.3159/1095-5674(2006)133[119:PATBOS]2.0.CO;2) [DOI] [Google Scholar]
- 38.Doyle JA. 2006. Seed ferns and the origin of angiosperms. J. Torrey Bot. 133, 169-209. ( 10.3159/1095-5674(2006)133[169:SFATOO]2.0.CO;2) [DOI] [Google Scholar]
- 39.Newman M. 2010. Networks: an introduction. Oxford, UK: Oxford Academic Press. [Google Scholar]
- 40.Rasskin-Gutman D, Esteve-Altava B. 2014. Connecting the dots: anatomical network analysis in morphological EvoDevo. Biol. Theory 9, 178-193. ( 10.1007/s13752-014-0175-x) [DOI] [Google Scholar]
- 41.Rawson JR, Esteve-Altava B, Porro LB, Dutel H, Rayfield EJ. 2022. Early tetrapod cranial evolution is characterized by increased complexity, constraint, and an offset from fin-limb evolution. Sci. Adv. 8, eadc8875. ( 10.1126/sciadv.adc8875) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Roopnarine PD, Angielczyk KD, Wang SC, Hertog R. 2007. Trophic network models explain instability of Early Triassic terrestrial communities. Proc. R. Soc. B 274, 2077-2086. ( 10.1098/rspb.2007.0515) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Huang Y, Chen ZQ, Roopnarine PD, Benton MJ, Zhao L, Feng X, Li Z. 2023. The stability and collapse of marine ecosystems during the Permian-Triassic mass extinction. Curr. Biol. 33, 1059-1070. ( 10.1016/j.cub.2023.02.007) [DOI] [PubMed] [Google Scholar]
- 44.Leslie AB, Mander L. 2023. Data from: Quantifying the complexity of plant reproductive structures reveals a history of morphological and functional integration [Dataset]. Dryad Digital Repository. ( 10.5061/dryad.n02v6wx36) [DOI] [PMC free article] [PubMed]
- 45.The Angiosperm Phylogeny Group. 2016. An update of the Angiosperm Phylogeny Group classification for the orders and families of flowering plants: APG IV. Bot. J. Linn. Soc. 181, 1-20. ( 10.1111/boj.12385) [DOI] [Google Scholar]
- 46.Hagberg A, Swart P, Chult DS. 2008. Exploring network structure, dynamics, and function using NetworkX. In Proc. of the 7th Python in Science Conf., Pasadena, USA, pp. 11-15. [Google Scholar]
- 47.R Core Team. 2022. R: a language and environment for statistical computing. Vienna, Austria: R Foundation for Statistical Computing. [Google Scholar]
- 48.Nagalingum NS, Schneider H, Pryer KM. 2007. Molecular phylogenetic relationships and morphological evolution in the heterosporous fern genus Marsilea. Syst. Bot. 32, 16-25. ( 10.1600/036364407780360256) [DOI] [Google Scholar]
- 49.Wickett NJ, et al. 2014. Phylotranscriptomic analysis of the origin and early diversification of land plants. Proc. Natl Acad. Sci. USA 111, E4859-E4868. ( 10.1073/pnas.1323926111) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.One Thousand Plant Transcriptomes Initiative. 2019. One thousand plant transcriptomes and the phylogenomics of green plants. Nature 574, 679-685. ( 10.1038/s41586-019-1693-2) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Doyle JA. 2008. Integrating molecular phylogenetic and paleobotanical evidence on origin of the flower. Int. J. Plant Sci. 169, 816-843. ( 10.1086/589887) [DOI] [Google Scholar]
- 52.Benson MX. 1908. Miadesmia membranacea, Bertand; a new Palaeozoic lycopod with a seed-like structure. Phil. Trans. R. Soc. B 199, 409-425. [Google Scholar]
- 53.Pryer KM, Schuettpelz E, Wolf PG, Schneider H, Smith AR, Cranfill R. 2004. Phylogeny and evolution of ferns (monilophytes) with a focus on the early leptosporangiate divergences. Am. J. Bot. 91, 1582-1598. ( 10.3732/ajb.91.10.1582) [DOI] [PubMed] [Google Scholar]
- 54.Nixon KC, Crepet WL, Stevenson D, Friis EM. 1994. A reevaluation of seed plant phylogeny. Ann. Mo. Bot. Gard. 81, 484-533. ( 10.2307/2399901) [DOI] [Google Scholar]
- 55.Rothwell GW, Serbet R. 1994. Lignophyte phylogeny and the evolution of spermatophytes: a numerical cladistic analysis. Syst. Bot. 19, 443-482. ( 10.2307/2419767) [DOI] [Google Scholar]
- 56.Shi G, Herrera F, Herendeen PS, Clark EG, Crane PR. 2021. Mesozoic cupules and the origin of the angiosperm second integument. Nature 594, 223-226. ( 10.1038/s41586-021-03598-w) [DOI] [PubMed] [Google Scholar]
- 57.Axsmith BJ, Taylor TN, Taylor EL. 1998. A new fossil conifer from the Triassic of North America: implications for models of ovulate cone scale evolution. Int. J. Plant Sci. 159, 358-366. ( 10.1086/297557) [DOI] [Google Scholar]
- 58.Spencer AR, Mapes G, Bateman RM, Hilton J, Rothwell GW. 2015. Middle Jurassic evidence for the origin of Cupressaceae: a paleobotanical context for the roles of regulatory genetics and development in the evolution of conifer seed cones. Am. J. Bot. 102, 942-961. ( 10.3732/ajb.1500121) [DOI] [PubMed] [Google Scholar]
- 59.Leslie AB, Beaulieu J, Holman G, Campbell CS, Mei W, Raubeson LR, Mathews S. 2018. An overview of extant conifer evolution from the perspective of the fossil record. Am. J. Bot. 105, 1531-1544. ( 10.1002/ajb2.1143) [DOI] [PubMed] [Google Scholar]
- 60.Corner EJ. 1958. Transference of function. Zool. J. Linn. Soc. 44, 33-40. ( 10.1111/j.1096-3642.1958.tb01706.x) [DOI] [Google Scholar]
- 61.Rothwell GW, Scheckler SE. 1988. Origin and evolution of gymnosperms, pp 85-113. New York, NY: Columbia University Press. [Google Scholar]
- 62.Owens JN, Takaso T, Runions CJ. 1998. Pollination in conifers. Trends Plant Sci. 3, 479-485. ( 10.1016/S1360-1385(98)01337-5) [DOI] [Google Scholar]
- 63.Bessey CE. 1915. The phylogenetic taxonomy of flowering plants. Ann. Mo. Bot. Gard. 2, 109-164. ( 10.2307/2990030) [DOI] [Google Scholar]
- 64.Donoghue MJ, Bell CD, Winkworth RC. 2003. The evolution of reproductive characters in Dipsacales. Int. J. Plant Sci. 164, S453-S464. ( 10.1086/376874) [DOI] [Google Scholar]
- 65.Leslie AB. 2011. Shifting functional roles and the evolution of conifer pollen-producing and seed-producing cones. Paleobiology 37, 587-602. ( 10.1666/10049.1) [DOI] [Google Scholar]
- 66.Benkman CW. 1995. The impact of tree squirrels (Tamiasciurus) on limber pine seed dispersal adaptations. Evolution 49, 585-592. [DOI] [PubMed] [Google Scholar]
- 67.Parchman TL, Benkman CW. 2002. Diversifying coevolution between crossbills and black spruce on Newfoundland. Evolution 56, 1663-1672. [DOI] [PubMed] [Google Scholar]
- 68.Reymanówna M. 1987. A Jurassic podocarp from Poland. Rev. Palaeobot. Palyno 51, 133-143. ( 10.1016/0034-6667(87)90026-1) [DOI] [Google Scholar]
- 69.Leslie AB, Benson RBJ. 2022. Molecular and fossil congruence in the evolution of Podocarpaceae (Coniferales) reproductive morphology. Front. Ecol. Evol. 10, 1058746. ( 10.3389/fevo.2022.1058746) [DOI] [Google Scholar]
- 70.Yang Y, Wang Q. 2013. The earliest fleshy cone of Ephedra from the early cretaceous Yixian formation of Northeast China. PLoS ONE 8, e53652. ( 10.1371/journal.pone.0053652) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Harris TM. 1964. The Yorkshire Jurassic flora. II. Caytoniales, Cycadales & pteridosperms. London, UK: Trustees of the British Museum. [Google Scholar]
- 72.Wing SL, Tiffney BH. 1987. The reciprocal interaction of angiosperm evolution and tetrapod herbivory. Rev. Palaeobot. Palynol. 50, 179-210. ( 10.1016/0034-6667(87)90045-5) [DOI] [Google Scholar]
- 73.Tiffney BH. 2004. Vertebrate dispersal of seed plants through time. Annu. Rev. Ecol. Evol. Syst. 35, 1-29. ( 10.1146/annurev.ecolsys.34.011802.132535) [DOI] [Google Scholar]
- 74.Contreras DL, Duijnstee IA, Ranks S, Marshall CR, Looy CV. 2017. Evolution of dispersal strategies in conifers: functional divergence and convergence in the morphology of diaspores. Perspect. Plant Ecol. Evol. Syst. 24, 93-117. ( 10.1016/j.ppees.2016.11.002) [DOI] [Google Scholar]
- 75.Leslie AB, Beaulieu JM, Mathews S. 2017. Variation in seed size is structured by dispersal syndrome and cone morphology in conifers and other nonflowering seed plants. New Phytol. 216, 429-437. ( 10.1111/nph.14456) [DOI] [PubMed] [Google Scholar]
- 76.Labandeira CC, Kvaček J, Mostovski MB. 2007. Pollination drops, pollen, and insect pollination of Mesozoic gymnosperms. Taxon 56, 663-695. ( 10.2307/25065852) [DOI] [Google Scholar]
- 77.Peris D, Pérez-de la Fuente R, Penalver E, Delclos X, Barron E, Labandeira CC. 2017. False blister beetles and the expansion of gymnosperm-insect pollination modes before angiosperm dominance. Curr. Biol. 27, 897-904. ( 10.1016/j.cub.2017.02.009) [DOI] [PubMed] [Google Scholar]
- 78.Labandeira CC, et al. 2016. The evolutionary convergence of mid-Mesozoic lacewings and Cenozoic butterflies. Proc. R. Soc. B 283, 20152893. ( 10.1098/rspb.2015.2893) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Terry I, Walter GH, Moore C, Roemer R, Hull C. 2007. Odor-mediated push-pull pollination in cycads. Science 318, 70. ( 10.1126/science.1145147) [DOI] [PubMed] [Google Scholar]
- 80.Toon A, Terry LI, Tang W, Walter GH, Cook LG. 2020. Insect pollination of cycads. Aust. Ecol. 45, 1033-1058. ( 10.1111/aec.12925) [DOI] [Google Scholar]
- 81.Asar Y, Ho SY, Sauquet H. 2022. Early diversifications of angiosperms and their insect pollinators: were they unlinked? Trends Plant Sci. 27, 858-869. ( 10.1016/j.tplants.2022.04.004) [DOI] [PubMed] [Google Scholar]
- 82.Luo SX, Zhang LJ, Yuan S, Ma ZH, Zhang DX, Renner SS. 2018. The largest early-diverging angiosperm family is mostly pollinated by ovipositing insects and so are most surviving lineages of early angiosperms. Proc. R. Soc. B 285, 20172365. ( 10.1098/rspb.2017.2365) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Gottsberger G, 1977. Some aspects of beetle pollination in the evolution of flowering plants. In Flowering plants. Plant systematics and evolution / entwicklungsgeschichte und systematik der pflanzen, vol 1 (ed. Kubitzki K), pp. 211-226. Vienna, Austria: Springer. [Google Scholar]
- 84.Olson EC, Miller RL. 1958. Morphological integration. Chicago, IL: University of Chicago Press. [Google Scholar]
- 85.Hallgrímsson B, Willmore K, Hall BK. 2002. Canalization, developmental stability, and morphological integration in primate limbs. Am. J. Phys. Anthropol. 119, 131-158. ( 10.1002/ajpa.10182) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Klingenberg CP. 2008. Morphological integration and developmental modularity. Annu. Rev. Ecol. Evol. Syst. 39, 115-132. ( 10.1146/annurev.ecolsys.37.091305.110054) [DOI] [Google Scholar]
- 87.Goswami A, Smaers JB, Soligo C, Polly PD. 2014. The macroevolutionary consequences of phenotypic integration: from development to deep time. Phil. Trans. R. Soc. B 369, 20130254. ( 10.1098/rstb.2013.0254) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Cheverud JM. 1982. Phenotypic, genetic, and environmental morphological integration in the cranium. Evolution 36, 499-516. ( 10.2307/2408096) [DOI] [PubMed] [Google Scholar]
- 89.Goswami A. 2006. Morphological integration in the carnivoran skull. Evolution 60, 169-183. ( 10.1111/j.0014-3820.2006.tb01091.x) [DOI] [PubMed] [Google Scholar]
- 90.Porto A, de Oliveira FB, Shirai LT, De Conto V, Marroig G. 2009. The evolution of modularity in the mammalian skull I: morphological integration patterns and magnitudes. Evol. Biol. 36, 118-135. ( 10.1007/s11692-008-9038-3) [DOI] [Google Scholar]
- 91.Marroig G, Shirai LT, Porto A, de Oliveira FB, De Conto V. 2009. The evolution of modularity in the mammalian skull II: evolutionary consequences. Evol. Biol. 36, 136-148. ( 10.1007/s11692-009-9051-1) [DOI] [Google Scholar]
- 92.Leslie AB, Beaulieu JM, Crane PR, Knopf P, Donoghue MJ. 2015. Integration and macroevolutionary patterns in the pollination biology of conifers. Evolution 69, 1573-1583. ( 10.1111/evo.12670) [DOI] [PubMed] [Google Scholar]
- 93.Gregory WK. 1935. ‘Williston's law’ relating to the evolution of skull bones in the vertebrates. Am. J. Phys. Anthropol. 20, 123-152. ( 10.1002/ajpa.1330200202) [DOI] [Google Scholar]
- 94.Sidor CA. 2001. Simplification as a trend in synapsid cranial evolution. Evolution 55, 1419-1442. [DOI] [PubMed] [Google Scholar]
- 95.Esteve-Altava B, Marugán-Lobón J, Botella H, Rasskin-Gutman D. 2013. Structural constraints in the evolution of the tetrapod skull complexity: Williston's law revisited using network models. Evol. Biol. 40, 209-219. ( 10.1007/s11692-012-9200-9) [DOI] [Google Scholar]
- 96.Esteve-Altava B, Marugán-Lobón J, Botella H, Rasskin-Gutman D. 2014. Random loss and selective fusion of bones originate morphological complexity trends in tetrapod skull networks. Evol. Biol. 41, 52-61. ( 10.1007/s11692-013-9245-4) [DOI] [Google Scholar]
- 97.Leslie AB, Mander L. 2023. Quantifying the complexity of plant reproductive structures reveals a history of morphological and functional integration. Figshare. ( 10.6084/m9.figshare.c.6888270) [DOI] [PMC free article] [PubMed]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Citations
- Leslie AB, Mander L. 2023. Data from: Quantifying the complexity of plant reproductive structures reveals a history of morphological and functional integration [Dataset]. Dryad Digital Repository. ( 10.5061/dryad.n02v6wx36) [DOI] [PMC free article] [PubMed]
- Leslie AB, Mander L. 2023. Quantifying the complexity of plant reproductive structures reveals a history of morphological and functional integration. Figshare. ( 10.6084/m9.figshare.c.6888270) [DOI] [PMC free article] [PubMed]
Data Availability Statement
Data are available from the Dryad Digital Repository [44] and in the electronic supplementary material [97].