Abstract
Anaerobic oxidation of [1,2-14C]dichloroethene to 14CO2 under Mn(IV)-reducing conditions was demonstrated. The results indicate that oxidative degradation of partially chlorinated solvents like dichloroethene can be significant even under anoxic conditions and demonstrate the potential importance of Mn(IV) reduction for remediation of chlorinated groundwater contaminants.
In chlorinated-ethene-contaminated groundwater systems, production of the Environmental Protection Agency priority pollutant vinyl chloride (VC) by anaerobic biodegradation of dichloroethene (DCE) is well documented (1, 2, 5, 7, 8, 10, 15, 17–19) and typically drives environmental concerns (2, 15). Aerobic oxidation of both compounds without accumulation of reduced intermediates is well-known but of limited relevance because DCE and VC are produced in situ under anaerobic conditions (1, 5, 7, 8, 10, 17–19). It is important, therefore, to determine which of the commonly occurring anaerobic terminal electron acceptors may be energetically sufficient to oxidize compounds like DCE and VC. Anaerobic microbial oxidation of VC with CO2 as the product has been demonstrated in environmental samples under Fe(III)-reducing conditions (3, 4). In contrast, even though low but significant mineralization of DCE under anaerobic conditions has been reported (4, 16), the fact that mineralization involved significant accumulation of VC (4, 16) and was not enhanced by Fe(III) amendment (4) indicates that the initial step was a reduction and that Fe(III) reduction was not sufficient to oxidize DCE directly. These observations suggest that anaerobic oxidation of DCE requires a terminal-electron-accepting process that is more energetically favorable than Fe(III) reduction.
Mn(IV) oxides are common in alluvial and glacial aquifer sediments, and Mn(IV) reduction is more energetically favorable than Fe(III) reduction (9, 11, 14). The natural abundance of Mn(IV) (11, 14), the greater availability for microbial reduction of Mn(IV) oxides [relative to Fe(III) oxides] (14), and the more favorable energetics of Mn(IV) reduction (2, 11, 14) suggest that Mn(IV) reduction may support oxidation of DCE to CO2 under anaerobic conditions without the accumulation of VC. To test this hypothesis, a microcosm study was initiated by using aquifer (shallow and intermediate) and surface sediments collected from a site at the Naval Undersea Warfare Center, Keyport, Washington. Aquifer material was characterized by fine to medium sands with some gravel and by an Mn(IV) oxide content of 0.05 to 0.1% (dry weight). Dissolved-Mn(II) concentrations at the site ranged from 0.04 to 5.7 mg/liter. Water chemistry analyses, field measurements of dissolved-H2 concentrations, and laboratory incubations indicated that Mn(IV) reduction was the predominant terminal-electron-accepting process in the shallow and intermediate aquifers at this site. The surface fill layer was characterized by silt to silty sand and consisted of a mixture of natural material, including glacial till. Water chemistry and H2 concentration data were not available for the surface fill material. However, based on the shallow sample collection depth, periodic fluctuations in the water table with resultant exposure to air, spatial variability in Mn(IV) oxide availability, and Fe(III) oxide staining on the fill material, the predominant terminal-electron-accepting processes in the zone of the surface fill sample appear to vary among O2, Mn(IV), and Fe(III) reduction.
DCE mineralization was evaluated with a neat mixture of [1,2-14C]DCE (29% trans and 71% cis isomers; radiochemical purity ≥ 99.9%; Moravek Biochemicals, Inc., Brea, Calif.). Microcosm preparation, incubation conditions, sampling procedures, and methods for verification of 14CO2 have been described previously (3, 4, 6). In brief, 20-ml microcosms containing 15 g of saturated material each were created with headspaces of air (aerobic treatments) or 100% helium (anaerobic treatments). Some were amended with 1.0 ml of anoxic, sterile distilled water (aerobic and unamended anaerobic treatments), and the rest were amended with 1.0 ml (approximately 0.05 g) of an anoxic, sterile slurry of poorly crystalline MnO2 (12, 13) or Fe(OH)3 (13). Killed controls were autoclaved twice for 1 h each time at 15 lb/in2 and 121°C. The microcosms were preincubated for 5 days and were then spiked with 40,000 dpm of [1,2-14C]DCE (specific activity = 0.6 mCi/mmol). 14CO2 was collected in 3 M KOH and quantified by liquid scintillation counting (3, 4, 6). Recovery of 14CO2 was confirmed in select microcosms as described previously (3, 4, 6).
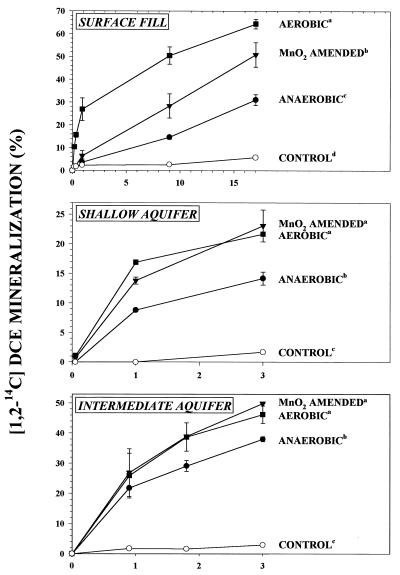
The microorganisms indigenous to all three sample materials were capable of significant DCE mineralization to CO2 under unamended anaerobic conditions (Fig. 1). For aquifer microcosms, mineralization ranged from 14 to 38% over three days. For the surface fill material, approximately 30% mineralization of [1,2-14C]DCE was observed after 17 days. In all cases, DCE mineralization was attributable to biological activity because the final recovery of 14CO2 in killed control microcosms was less than 4%. Mn(IV) reduction was the predominant terminal-electron-accepting process under unamended, anaerobic conditions, as indicated by the lack of significant dissolved O2 ([O2] < 3 μM), NO3 ([NO3] < 0.2 μM), and SO4 ([SO4] < 20 μM); the lack of significant production of CH4 (not detected, i.e., [CH4] < 1 μmol/liter of headspace), dissolved sulfide (not detected, i.e., [HS] < 0.2 μM), and dissolved Fe(II) [2 ± 2 nmol of Fe(II) produced (Table 1)]; and the significant accumulation of Mn(II) (9 ± 1 nmol produced [Table 1]) within these experimental microcosms.
FIG. 1.
Percentage mineralization of [1,2-14C]DCE to 14CO2 in microcosms containing material collected from the surface fill (depth, 1 m), the shallow aquifer (depth, 3 to 5 m) and the intermediate aquifer (depth, 8 to 10 m). Experimental data are means ± standard deviations for duplicate microcosms, and the control data are from a single sterile control microcosm. For each material studied, superscript letters indicate statistically significantly different final mean 14CO2 recoveries according to the Kruskal-Wallis one-way analysis of variance on ranks (P < 0.05).
TABLE 1.
Mn(IV) and Fe(III) reduction during oxidation of [1,2-14C]DCE in intermediate aquifer microcosmsa
| Microcosm | Amt (nmol) of productb
|
|||
|---|---|---|---|---|
| Mn(II)
|
Fe(II)
|
|||
| Exptl | Control | Exptl | Control | |
| Anaerobic | 9 ± 1 | 1 | NS | NS |
| Fe(OH)3 amended | NS | NS | 35 ± 11 | 3 |
| MnO2 amended | 47 ± 6 | NS | NS | NS |
| Aerobic | NS | NS | NS | NS |
Microcosms were vortex mixed and 1.0 ml of sediment supernatant was removed, filtered (0.2-μm pore size), and analyzed colorimetrically with commercially available test kits (Hach Chemical Co.).
The amount of Mn(II) or Fe(II) produced is presented as a qualitative indicator of Mn(IV) or Fe(III) reduction because the Mn(II) or Fe(II) found in the dissolved phase is a small fraction (less than 10%) (14) of the total Mn(II) or Fe(II) present in the system. Experimental data are means ± standard deviations from duplicate microcosms, and control data are from a single sterile control. NS, no significant change.
The bioavailability of Mn(IV) was a major factor affecting anaerobic DCE mineralization for all three sample materials (Fig. 1). Amendment of anaerobic microcosms with poorly crystalline MnO2 resulted in a fivefold increase in Mn(IV) reduction (Table 1). The stimulation of Mn(IV) reduction by MnO2 amendment was associated with increased DCE mineralization and 14CO2 recoveries similar to those under aerobic conditions (Fig. 1). The combined results from the unamended and MnO2-amended microcosm studies demonstrate that, under anaerobic conditions, both Mn(IV) reduction and DCE mineralization were limited by the bioavailability of Mn(IV) and indicate that DCE mineralization was coupled to Mn(IV) reduction.
The results further indicate that, under Mn(IV)-reducing conditions, DCE is efficiently oxidized to CO2 without detectable accumulation of intermediates (Table 2). Following the 3-day incubation period, the headspace of intermediate aquifer microcosms was analyzed for the presence of the daughter products of DCE reductive dechlorination (VC, ethene, and ethane). No volatile organic compounds other than DCE were observed in this study. Moreover, the percent change in headspace DCE concentrations closely corresponded to the percent recoveries of 14CO2 in unamended and MnO2-amended anaerobic microcosms (Table 2). The stoichiometric conversion of DCE to CO2, the lack of accumulation of volatile intermediates, the lack of a detectable lag in the production of 14CO2 (Fig. 1), and the similar degrees of mineralization observed in aerobic and MnO2-amended microcosms (Fig. 1) are consistent with direct oxidation of DCE with CO2 as the end product.
TABLE 2.
Oxidation of [1,2-14C]DCE to 14CO2 in anaerobic, intermediate aquifer microcosmsa
| Microcosm | % of DCE lostb
|
% of 14CO2 recovered
|
||
|---|---|---|---|---|
| Exptl | Control | Exptl | Control | |
| Anaerobic | 37 ± 2 | 15 | 38 ± 1 | 3 |
| MnO2 amended | 42 ± 12 | 5 | 50 ± 0 | 1 |
Experimental data are means ± standard deviations for duplicate microcosms, and control data are from a single sterile microcosm.
DCE concentrations were determined by flame ionization detection-gas chromatography, and DCE loss was estimated from the final headspace DCE concentration and expressed as a percentage of the initial headspace DCE concentration. All DCE loss was as cis-DCE. No significant change in the headspace concentration of trans-DCE was observed.
The oxidation of DCE observed in this study under anaerobic conditions was not attributable to Fe(III) reduction (Table 3). Because others (12) have shown that Fe(III) reduction can proceed under Mn(IV)-reducing conditions without significant accumulation of dissolved Fe(II), we examined the possibility that anaerobic oxidation of DCE was associated with Fe(III) reduction by quantifying the mineralization of [1,2-14C]DCE in anaerobic microcosms amended with poorly crystalline Fe(III) oxide [provided as Fe(OH)3]. Addition of Fe(OH)3 resulted in significant Fe(III) reduction, as indicated by increased accumulation of dissolved Fe(II) (Table 1), but inhibited DCE mineralization (Table 3). The lack of stimulation of DCE mineralization under Fe(III)-amended conditions has been observed previously (4) and indicates that Fe(III)-reducing conditions are not sufficient to support DCE oxidation. Moreover, the apparent inhibitory effect of Fe(III) amendment on DCE oxidation (Table 3) provides additional, compelling evidence that the anaerobic oxidation of DCE observed in the present study was coupled to Mn(IV) reduction.
TABLE 3.
Effect of Fe(OH)3 or MnO2 amendment on the final recovery of 14CO2
| Microcosm | % of 14CO2 recovered from:
|
|||
|---|---|---|---|---|
| Surface filla
|
Intermediate aquiferb
|
|||
| Exptl | Control | Exptl | Control | |
| Anaerobic | 31 ± 2 | 1 ± 0 | 38 ± 1 | 3 |
| Fe(OH)3 amended | 21 ± 6 | 5 ± 1 | 28 ± 1 | 3 |
| MnO2 amended | 51 ± 5 | 6 ± 1 | 50 ± 0 | 1 |
17-day incubation period. Data are means ± standard deviations for triplicate experimental microcosms and duplicate control microcosms.
3-day incubation period. Experimental data are means ± standard deviations for duplicate microcosms, and control data are from a single sterile control microcosm.
This investigation is the first report of anaerobic oxidation of DCE under Mn(IV)-reducing conditions and, to our knowledge, the first report of anaerobic mineralization of DCE to CO2 without the accumulation of reduced intermediates. Mn(IV) oxides are potentially powerful oxidants which are common in aquifer sediments and groundwater systems. Much of the risk associated with DCE contamination in groundwater is due to the potential production of VC. The present results demonstrate that DCE can be oxidized to CO2 under Mn(IV)-reducing conditions without the environmental risk associated with VC accumulation.
REFERENCES
- 1.Barcelona M J. Symposium on natural attenuation of chlorinated organics in ground water. EPA/540/R-96/509. U.S. Washington, D.C: Environmental Protection Agency; 1996. Case study: Wurtsmith Air Force Base, Michigan; pp. 98–103. [Google Scholar]
- 2.Bouwer E J. Bioremediation of chlorinated solvents using alternate electron acceptors. In: Norris R D, et al., editors. Handbook of bioremediation. Boca Raton, Fla: Lewis Publishers; 1994. pp. 149–175. [Google Scholar]
- 3.Bradley P M, Chapelle F H. Anaerobic mineralization of vinyl chloride in Fe(III)-reducing aquifer sediments. Environ Sci Technol. 1996;30:2084–2086. [Google Scholar]
- 4.Bradley P M, Chapelle F H. Kinetics of DCE and VC mineralization under methanogenic and Fe(III)-reducing conditions. Environ Sci Technol. 1997;31:2692–2696. [Google Scholar]
- 5.Chapelle F H. Symposium on natural attenuation of chlorinated organics in ground water. EPA/540/R-96/509. U.S. Washington, D.C: Environmental Protection Agency; 1996. Identifying redox conditions that favor the natural attenuation of chlorinated ethenes in contaminated ground-water systems; pp. 17–20. [Google Scholar]
- 6.Davis J W, Carpenter C L. Aerobic biodegradation of vinyl chloride in groundwater samples. Appl Environ Microbiol. 1990;56:3878–3880. doi: 10.1128/aem.56.12.3878-3880.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Dupont R R, Gorder K, Sorensen D L, Kemblowski M W, Haas P. Symposium on natural attenuation of chlorinated organics in ground water. EPA/540/R-96/509. U.S. Washington, D.C: Environmental Protection Agency; 1996. Case study: Eielson Air Force Base, Alaska; pp. 104–109. [Google Scholar]
- 8.Ellis D E, Lutz E J, Klecka G M, Pardieck D L, Salvo J J, Heitkamp M A, Gannon D J, Mikula C C, Vogel C M, Sayles G D, Kampbell D H, Wilson J T, Maiers D T. Symposium on natural attenuation of chlorinated organics in ground water. EPA/540/R-96/509. U.S. Washington, D.C: Environmental Protection Agency; 1996. Remediation technology development forum—intrinsic remediation project at Dover Air Force Base, Delaware; pp. 93–97. [Google Scholar]
- 9.Fairbridge R W. Encyclopedia of geochemistry and environmental sciences. New York, N.Y: Van Nostrand Reinhold; 1972. Manganese: element and characteristics; pp. 670–671. [Google Scholar]
- 10.Imbrigiotta T E, Ehlke T A, Wilson B H, Wilson J T. Symposium on natural attenuation of chlorinated organics in ground water. EPA/540/R-96/509. U.S. Washington, D.C: Environmental Protection Agency; 1996. Case study: natural attenuation of a trichloroethene plume at Picatinny Arsenal, New Jersey; pp. 83–89. [Google Scholar]
- 11.Langenhoff A A M, Brouwers-Ceilers D L, Engelberting J H L, Quist J J, Wolkenfelt J G P N, Zehnder A J B, Schraa G. Microbial reduction of manganese coupled to toluene oxidation. Microb Ecol. 1997;22:119–127. [Google Scholar]
- 12.Lovley D R, Phillips E J P. Manganese inhibition of microbial iron reduction in anaerobic sediments. Geomicrobiol J. 1988;6:145–155. [Google Scholar]
- 13.Lovley D R, Phillips E J P. Novel mode of microbial energy metabolism: organic carbon oxidation coupled to dissimilatory reduction of iron or manganese. Appl Environ Microbiol. 1988;54:1472–1480. doi: 10.1128/aem.54.6.1472-1480.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Lovley D R. Dissimilatory Fe(III) and Mn(IV) reduction. Microbiol Rev. 1991;55:259–287. doi: 10.1128/mr.55.2.259-287.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.McCarty P L, Semprini L. Ground-water treatment for chlorinated solvents. In: Norris R D, et al., editors. Handbook of bioremediation. Boca Raton, Fla: Lewis Publishers; 1994. pp. 87–116. [Google Scholar]
- 16.Vogel T M, McCarty P L. Biotransformation of tetrachloroethylene to trichloroethylene, dichloroethylene, vinyl chloride, and carbon dioxide under methanogenic conditions. Appl Environ Microbiol. 1985;49:1080–1083. doi: 10.1128/aem.49.5.1080-1083.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Weaver J W, Wilson J T, Kampbell D H. Symposium on natural attenuation of chlorinated organics in ground water EPA/540/R-96/509. U.S. Washington, D.C: Environmental Protection Agency; 1996. Extraction of degradation rate constants from the St. Joseph, Michigan, trichloroethene site; pp. 69–73. [Google Scholar]
- 18.Weidemeier T H, Wilson J T, Kampbell D H. Symposium on natural attenuation of chlorinated organics in ground water. EPA/540/R-96/509. U.S. Washington, D.C: Environmental Protection Agency; 1996. Natural attenuation of chlorinated aliphatic hydrocarbons at Plattsburg Air Force Base, New York; pp. 74–82. [Google Scholar]
- 19.Wilson J T, Kampbell D H, Weaver J W. Symposium on natural attenuation of chlorinated organics in ground water. EPA/540/R-96/509. U.S. Washington, D.C: Environmental Protection Agency; 1996. Environmental chemistry and the kinetics of biotransformation of chlorinated organic compounds in ground water; pp. 124–127. [Google Scholar]