Abstract
Background
The most effective method for encouraging self-management in individuals with pulmonary fibrosis (PF) is unclear. This review aimed to identify common self-management components, the outcome measures used and the impact of these components in PF.
Methods
A scoping review was conducted according to the Joanna Briggs Institute Manual for Evidence Synthesis using Medline, Embase, PsychInfo, CINAHL and the Cochrane Central Register of Controlled Trials. Eligible studies included those with educational, behavioural or support components aimed at facilitating self-management among adults with PF and employed quantitative and/or qualitative methods.
Results
87 studies were included. Common self-management components included education (78%), managing physical symptoms (66%) and enhancing psychosocial wellbeing (54%). Components were predominantly delivered in a pulmonary rehabilitation setting (71%). No studies tested a PF-specific self-management package. Common outcome measures were 6-min walk distance (60%), St George's Respiratory Questionnaire (37%) and the Medical Research Council Dyspnoea scale (34%). Clinically significant improvements in these outcomes were seen in ≥50% of randomised controlled trials. Qualitative data highlighted the importance of healthcare professional and peer support and increased confidence in managing PF.
Conclusion
Self-management components are commonly incorporated into pulmonary rehabilitation programmes rather than being offered as standalone packages. Future research should focus on testing PF-specific self-management packages and employ standardised outcome assessments that include self-efficacy and health-related behaviours.
Tweetable abstract
Self-management components are delivered in various settings. Evaluation of PF self-management packages is needed. More focus on changes in self-efficacy and health behaviours is required when assessing the impact of a PF self-management intervention. https://bit.ly/3r7r0UP
Background
Pulmonary fibrosis (PF) significantly impacts the physical and psychosocial wellbeing of people living with the disease. Common symptoms include persistent cough, breathlessness and fatigue, which often create frustration, anxiety and depression [1]. Until recently, PF was regarded as a disease with no effective treatments and a short survival time; therefore, there was a limited role for self-management. However, the emergence of two antifibrotic drugs shown to delay lung function decline in individuals with a progressive phenotype of PF [2, 3] and increasing evidence supporting nonpharmacological therapies such as pulmonary rehabilitation (PR) for symptom management [4] have provided a more promising outlook for people with PF with a range of underlying diagnoses.
Regardless of the variations in disease trajectory related to the underlying diagnosis, people with PF require support in managing their wellbeing and gaining a better understanding of their disease [5]. Many expressed a sense of personal responsibility for maintaining their health and regarded self-management to be critically important [6]. Self-management involves managing different aspects of health and some individuals are motivated to learn self-management strategies to maintain wellness, gain a sense of control and be better prepared for the future [7, 8]. Self-management interventions can enhance one's ability to manage symptoms, treatments, lifestyle changes and the psychosocial consequences of a disease [9]. Increased knowledge and skill in managing a disease empowers individuals to be more involved in their healthcare and to participate in shared decision-making, which is a key priority in the Australian strategic action plan for lung diseases [10–12]. In COPD and asthma, self-management programmes have been shown to improve health-related quality of life (HRQoL) and reduce hospitalisation [13, 14] and are available to support people with these diseases [15, 16]. These programmes commonly include components such as patient education, managing symptoms, lifestyle adjustments, psychosocial support, self-monitoring, managing exacerbations and medications, action planning, and goal setting [13, 14].
The concept of self-management is new in PF. The development of self-management programmes often adopts approaches used for other lung diseases and are, therefore, not PF-specific. Some self-management components, for example, patient education and psychosocial support, are delivered through settings such as PR programmes. However, the components that should be included in a PF-specific self-management programme remain undefined. This work utilises the scoping review method to gain an overview of what approaches have been used to support people with PF in terms of self-management and to evaluate the associated outcomes. The aims of the study were to 1) identify common components that have been used to support self-management in PF, 2) document the characteristics of studies delivering one or more self-management component, 3) identify the outcome measures used to assess interventions including self-management components and 4) summarise the impact of the interventions on patient outcomes.
Method
A scoping review was conducted according to the Joanna Briggs Institute Manual for Evidence Synthesis framework [17] and reported according to the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) extension for scoping reviews [18]. The protocol was registered with the Open Science Framework database (https://osf.io/euz6s).
Development of a search strategy for scientific literature search
An initial search was conducted using the Medline and Embase databases to identify studies delivering a self-management package or at least one self-management component to people with PF. 20 relevant studies were selected to identify common keywords and index terms used to describe self-management. A librarian was consulted to identify additional keywords representing PF, self-management and self-management components commonly used in other lung diseases. A final search strategy (supplement 1) was developed using the identified keywords and index terms and subsequently tested on the two databases by reviewing a random sample of 50 retrieved studies.
In this review, a “self-management component” refers to an individual component (e.g. education, psychosocial support or self-monitoring) that forms part of an intervention which may improve self-management. A “self-management package” refers to an intervention designed to focus entirely on facilitating self-management that comprises several self-management components.
Eligibility criteria
Self-management components included, but were not limited to, those commonly used in other lung diseases. These components included patient education, breathing techniques, lifestyle adjustment (including exercise, smoking cessation and nutrition), psychosocial support, managing symptoms, acute exacerbations and medications, preventing infections, self-monitoring, action planning, and end-of-life planning.
Studies were included if they 1) described any self-management components (educational, behavioural or support components), 2) involved adults with PF (both idiopathic pulmonary fibrosis (IPF) and non-IPF diagnoses) aged ≥18 years, and 3) employed quantitative, qualitative or mixed methods. We included studies conducted in different settings, including PR, palliative care programmes and other types of programmes that included self-management components. PR programmes were only included if they involved at least one self-management component, such as adoption of an independent home exercise programme or promotion of regular physical activity in daily life. Studies were excluded if they 1) involved a combined group of people with PF and other lung diseases unless results for PF were reported separately, 2) focused on the psychometric properties of tools designed to measure outcomes or 3) were case studies or opinion-driven reports. Systemic non-IPF diagnoses were excluded. Conference abstracts and study protocols were only included during database searches to identify related full-text articles. Reviews and guidelines were also excluded. Non-English publications were excluded. There was no limitation on study design, study setting or time of publication.
Scientific literature search
The previously developed search strategy (supplement 1) was performed on 16 August 2022, using Medline, Embase, PsychInfo, CINAHL and the Cochrane Central Register of Controlled Trials from their inception. The Scopus database and Google Scholar were used to identify original studies associated with conference abstracts and study protocols that met the eligibility criteria. Reference lists of included full-text articles and relevant systematic reviews were hand-searched to identify studies not retrieved during database search. If required, study authors were contacted to obtain further information.
Study selection
Covidence software (Veritas Health Innovation, Melbourne, Australia) was used for title, abstract and full-text screening. Two researchers (C. Malaguti and J.Y.T. Lee) independently screened the titles and abstracts of retrieved studies. Full-text articles were reviewed by two researchers (J.Y.T. Lee and one of the following researchers: A.W. Jones, L. Dowman, M. Hoffman and C.R. Mellerick) for inclusion based on the eligibility criteria. Any disagreements between the researchers were resolved through discussions.
Data charting
The Template for Intervention Description and Replication (TIDieR) checklist [19] guided the data collection process. Information related to the studies (i.e. study design, aim, location and duration, self-management components delivered, materials used, and personnel involved) and participants (i.e. mean age and diagnosis) were collected. Primary and secondary outcomes were documented for studies with quantitative data. All themes were documented for qualitative data. The data collection form was tested on a random sample of eight studies by two researchers (J.Y.T. Lee and one of the following researchers: A.W. Jones, L. Dowman, M. Hoffman and C.R. Mellerick) before each researcher performed data charting independently. Both copies of the charted data were reviewed by J.Y.T. Lee to ensure accuracy. Assessments for risk of bias were not performed since the objective of this review was to provide an overview of studies consisting of self-management components.
Grey literature search
Searches were conducted in July 2022 to identify self-management resources (educational, behavioural or support components) located on public websites. 13 PF-specific websites covering different regions across the world were selected. Only websites published in English were included. The websites searched were as follows: the Thoracic Society of Australia and New Zealand (https://thoracic.org.au); the European Respiratory Society (www.ersnet.org); the American Thoracic Society (www.thoracic.org); the British Thoracic Society (www.brit-thoracic.org.uk); the Pulmonary Fibrosis Foundation (www.pulmonaryfibrosis.org); the Canadian Pulmonary Fibrosis Foundation (https://cpff.ca); the Irish Lung Fibrosis Association (https://ilfa.ie); Action for Pulmonary Fibrosis (www.actionpf.org); Lung Foundation Australia (https://lungfoundation.com.au); Asthma and Lung UK (www.blf.org.uk); the European Lung Foundation (https://europeanlung.org); Breathing Matters (www.breathingmatters.co.uk); and the Chest Foundation (https://foundation.chestnet.org).
Data synthesis and analysis
Scientific studies were reviewed separately from information collected from public websites. Due to the large number of resources, we documented the self-management resources presented on public websites without counting the number of resources available or how often each component was presented. Based on previous studies that described self-management components used in PF and other chronic diseases [20–22], we grouped self-management components identified in each study according to the topics addressed. The number of studies reporting each self-management component and outcome measure are reported as number (%). For the outcome measures most commonly used to assess exercise capacity, HRQoL, symptom burden and psychological wellbeing, post-intervention between-groups mean difference (for randomised controlled trials (RCTs)) and within-group mean change (for non-RCTs) were presented. If the study reported a median value, a mean value was estimated according to the formula provided by Wan et al. [23].
Results
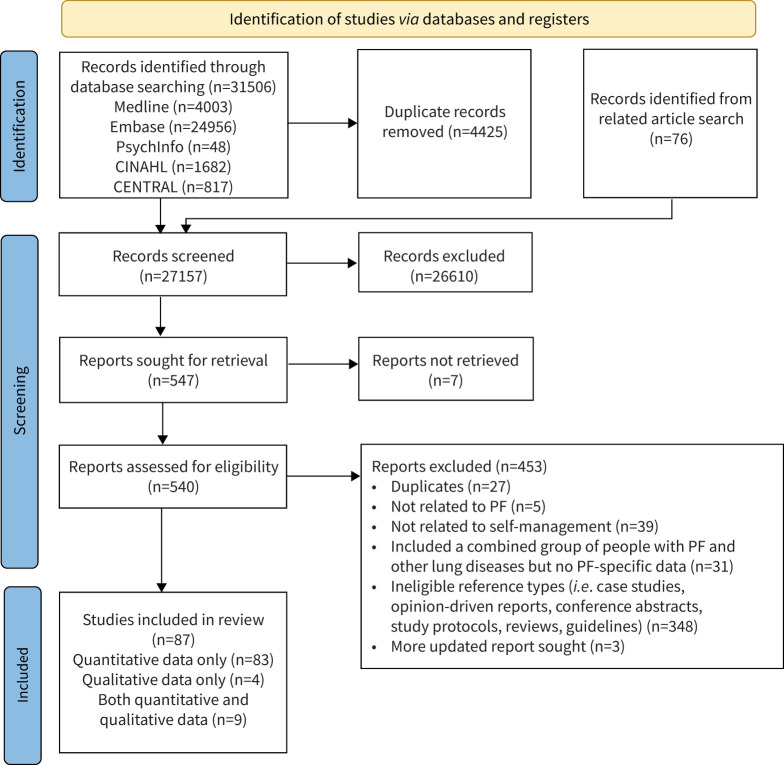
The systematic database search identified 31 506 records. After removing duplicates, 27 157 records were screened and 87 studies were included in the review [24–110] (figure 1). This involved 22 037 participants, of which 1321 were from 26 RCTs (685 in the intervention group including 19 carers, 60 patient/carer dyads; the remaining were people with PF).
FIGURE 1.
PRISMA flow chart. PF: pulmonary fibrosis.
Characteristics of the included studies
Table 1 summarises the characteristics of the 87 studies. The majority were pre-post studies (42%, n=37), followed by RCTs (26%, n=23). Other study designs included longitudinal and cohort studies (9%, n=8), nonrandomised controlled studies (6%, n=5), post-intervention evaluation (5%, n=4), mixed method (8%, n=7) and qualitative studies (4%, n=3). Studies were mostly conducted in Europe and Central Asia (34%). About half were completed in the antifibrotic era (i.e. 2014 onwards; 52%) and included a mixed group of participants with different subtypes of PF (47%), with 31% including those with IPF only. Comprehensive information for each study that reported quantitative and qualitative results are presented in supplement 2.1 and supplement 2.2, respectively.
TABLE 1.
Characteristics of included studies (total, n=87)
| Characteristic | Number of studies, n (%) |
| Study design | |
| Pre-post study | |
| Prospective | 21 (24) |
| Retrospective | 16 (18) |
| RCT | 23 (26) |
| Longitudinal/cohort study | 8 (9) |
| Nonrandomised controlled study | 5 (6) |
| Post-intervention evaluation | 4 (5) |
| Qualitative study | 3 (4) |
| Mixed method | 7 (8)# |
| Origin of study ¶ | |
| Europe and Central Asia | 30 (34) |
| South-East Asia and Pacific | 25 (29) |
| North and South America | 20 (23) |
| Middle East | 8 (9) |
| Multiple countries | 4 (5) |
| Year of study completion | |
| Before 2014 (pre-antifibrotic era) | 24 (27) |
| 2014 onwards | 45 (52) |
| Unclear | 18 (21) |
| Study population | |
| Combined group of various PF subtypes | 41 (47) |
| IPF | 27 (31) |
| Sarcoidosis | 4 (5) |
| Systemic sclerosis | 2 (2) |
| Pneumoconiosis | 2 (2) |
| Silicosis | 1 (1) |
| Interstitial pneumonia | 1 (1) |
| Combined group of PF and other lung/nonlung diseases (with results for PF reported separately) | 9 (11) |
IPF: idiopathic pulmonary fibrosis; PF: pulmonary fibrosis; RCT: randomised controlled trial. #: Studies using mixed methods: RCT and qualitative study (n=3); longitudinal and qualitative study (n=1); pre-post study and qualitative study (n=2); post-intervention evaluation and qualitative study (n=1). ¶: European and Central Asian countries include Italy (n=7), Germany (n=6), France (n=4), Turkey (n=4), UK (n=3), the Netherlands (n=2), Denmark (n=1), Hungary (n=1), Ireland (n=1) and Norway (n=1). South-East Asian and Pacific countries include Japan (n=8), Australia (n=6), China (n=5), India (n=5) and Singapore (n=1). North and South American countries include USA (n=13), Canada (n=6) and Brazil (n=1). Middle East countries include Egypt (n=3), Israel (n=3), Malta (n=1) and Saudi Arabia (n=1). Studies conducted in multiple countries include Austria and UK (n=1), USA and Ireland (n=1), Canada, USA, Australia, Germany and Switzerland (n=1), and Canada, Australia, France, Mexico, Spain, UK and USA (n=1).
Self-management components
Table 2 summarises the self-management components reported across the included studies. The most common components were patient education (78%), managing physical symptoms (66%) and enhancing psychosocial wellbeing (54%). The least common ones were support for goal setting (14%), managing comorbidities (8%) and information regarding sexuality and self-esteem (7%). Behavioural change approaches were used in 46% of studies and included the provision of information or support to encourage positive lifestyle changes related to diet, physical activity or smoking and alcohol cessation. The self-management components delivered in each study are presented in supplement 3.
TABLE 2.
Self-management components delivered in scientific studies (total, n=87)
| Self-management component | Description | Number of studies, n (%) |
| Patient education | Educational sessions covering various topics aimed at facilitating self-management | 68 (78) |
| Managing physical symptoms | Strategies and support to facilitate management of physical symptoms such as breathlessness and cough (including breathing techniques, relaxation, airway clearance, preventive measures and emergency procedures) | 57 (66) |
| Psychosocial support and coping strategies | Support and advice for managing psychosocial wellbeing (including peer support, psychological counselling and strategies for managing mood) | 47 (54) |
| Behavioural modification | Information and support to promote positive behavioural change (including diet, smoking or alcohol cessation, and physical activity) | 40 (46) |
| Managing disease and activities of daily living | Information and support to facilitate management of the disease and daily life activities (including energy conservation techniques, advice for appropriate aids/devices and accessing community resources) | 35 (40) |
| Managing pulmonary fibrosis-related treatments | Information and support to enhance the management of and adherence to both pharmacological and nonpharmacological treatments | 32 (37) |
| Understanding lung disease and clinical tests | Information to promote understanding of lung disease, disease progression, symptoms, prognosis, disease impact on daily life and the clinical tests involved | 32 (37) |
| Home exercise programme | Unsupervised home exercise programme or practices | 29 (33) |
| Understanding treatment options | Information to promote understanding of pharmacological and nonpharmacological treatment options | 21 (24) |
| Preventing infections and managing exacerbation | Information to facilitate early recognition of an exacerbation, strategies to prevent infections and action planning | 20 (23) |
| Self-monitoring | Monitoring of physical activity, symptoms and clinical parameters performed by people with pulmonary fibrosis | 19 (22) |
| Palliation and end-of-life planning | Palliative care and support to facilitate discussion and planning for end-of-life | 18 (21) |
| Goal setting | Support to facilitate setting care goals and patient decision making | 12 (14) |
| Managing comorbidities | Information and support to promote understanding and management of comorbidities (including incontinence, sleep disorders, pain and constipation) | 7 (8) |
| Sexuality and self-esteem | Information and support for maintaining body image and self-esteem | 6 (7) |
Delivery format of self-management components
Our searches did not identify any studies that examined a PF-specific self-management package. Self-management components were implemented in various settings with PR programmes being the most common (71%; table 3). Other settings included palliative care or disease management programmes comprising patient education, care goal setting or end-of-life planning (14%), support programmes for managing medication (4%), home-based self-monitoring programmes (4%), peer support groups (4%), a mindfulness-focused stress reduction programme (1%), a telemedicine service delivered during the coronavirus disease 2019 (COVID-19) pandemic that delivered information on preventing infections and self-monitoring of clinical parameters (1%), a PF-specific educational and support website (1%), and the use of a hand-held fan for managing breathlessness (1%).
TABLE 3.
Delivery format of studies including self-management components (total, n=87)
| Characteristic | Number of studies, n (%) |
| Setting where self-management components are delivered | |
| Rehabilitation programme | 62 (71) |
| Palliative care/disease management programme | 12 (14) |
| Support programme for managing medication | 3 (4) |
| Home-based self-monitoring programme | 3 (4) |
| Peer support group | 3 (4) |
| Mindfulness-based stress reduction programme | 1 (1) |
| Telemedicine service during the COVID-19 pandemic | 1 (1) |
| PF-specific website | 1 (1) |
| Use of hand-held fan | 1 (1) |
| Location | |
| Outpatient | 45 (52) |
| Inpatient | 9 (10) |
| Home-/community-based | 19 (22) |
| Mixed settings# | 14 (16) |
| In-person/remote delivery | |
| In-person (e.g. instructions, supervision, education, clinic/home visits) | 44 (51) |
| Remote (e.g. via telephone/email, unsupervised home programme/practice, self-monitoring) | 11 (13) |
| Mixed | 29 (33) |
| Unclear | 3 (3) |
| Group/individual delivery | |
| Individual | 29 (33) |
| Group | 20 (23) |
| Mixed | 30 (35) |
| Unclear | 8 (9) |
| Self-management components specific to PF | |
| Yes | 33 (38) |
| Partial | 6 (7) |
| No | 12 (14) |
| Unclear | 36 (41) |
| Materials used | |
| Printed educational materials | 23 (26) |
| Exercise/symptom diary | 14 (16) |
| Digital materials to facilitate learning (e.g. audio/video, PowerPoint presentation, compact disc, website) | 13 (15) |
| Electronic devices (e.g. mobile application, tablet, hand-held fan, home spirometer, vital signs monitor) | 13 (15) |
| Personnel involved ¶ | |
| Multi-providers | 46 (53) |
| HCPs specialising in exercise training | 41 (47) |
| Other allied health professionals | 41 (47) |
| Physician | 33 (38) |
| Nurse | 30 (35) |
| Other HCPs | 9 (10) |
| Expert patients | 2 (2) |
| Unclear | 22 (25) |
COVID-19: coronavirus disease 2019; HCP: healthcare professionals; PF: pulmonary fibrosis.
#: Studies delivering in mixed settings: outpatient and home (n=9); inpatient and outpatient (n=4); inpatient, outpatient and home (n=1). ¶: HCPs specialising in exercise training include physiotherapists, physical therapists, sports instructors, exercise physiologists and rehabilitation therapist. Other allied health professionals include dieticians, occupational therapists, speech therapists, psychologists, social workers and pharmacist. Physicians include respiratory physicians, general practitioners, cardiologists and palliative respiratory care experts. Nurses include respiratory specialist nurses, registered nurses, nurse practitioners, palliative care specialist nurses, psychiatric clinical nurse specialists, district nurses and community palliative care nurses. Other HCPs include medical beauticians, ecotrophologists, advance care planning instructors, instructors with training in meditation and mindfulness, palliative care spiritual care providers, and recreational therapists.
The interventions were most commonly delivered in an outpatient location (52%), followed by home-/community-based (22%), mixed (16%) and inpatient (10%). 51% delivered self-management components in person (e.g. to provide instructions, support and education), 13% remotely (e.g. via telephone support, a self-monitoring programme or an unsupervised home programme) and 33% used both methods. A similar number of studies delivered self-management components on an individual basis (33%), in a group setting (23%) or a combination of both (35%). 38% of studies delivered self-management components specific to PF, 7% targeted both PF and other lung diseases (e.g. COPD), 14% targeted lung diseases other than PF, and 41% of studies were unclear. Some studies used printed educational materials (26%) to deliver education, while others provided home exercise or symptom diaries (16%), digital materials to facilitate learning (15%), and electronic devices (15%) such as mobile applications, home spirometers and heart rate monitors for self-monitoring and hand-held fans to manage breathlessness. Less than half of the studies (21 studies out of 44) that delivered in-person teaching provided participants with printed information about the topics covered or instructions on how to perform exercises, of which four provided audio or video resources in addition to printed material. Similarly, in studies involving remote delivery of components, about half (five studies out of 11) provided information in a digital format (e.g. e-learning package, videos or compact discs).
Over half of the studies delivered self-management components using a multidisciplinary approach. Healthcare professionals (HCPs) specialising in exercise training and other allied health professionals were most commonly involved (both 47%). Other HCPs involved, particularly in providing education, were physicians (38%) and nurses (35%). Only two studies involved expert patients (i.e. people who live with PF and can share their disease knowledge and experience with others) [111]. One involved expert patients delivering peer support [99] and, in the other, expert patients were involved in the development of a home monitoring programme [73].
Outcome measures reported in quantitative studies
83 (95%) studies reported quantitative outcome measures, which are summarised in supplement 4. The three most measured outcomes were 6-min walk distance (6MWD) for exercise capacity (60%, n=50 studies), St. George's Respiratory Questionnaire (SGRQ) scores for HRQoL (37%, n=31 studies) and Medical Research Council (MRC) dyspnoea score for symptom burden (34%, n=28 studies). Anxiety and depression were commonly measured using the Hospital Anxiety and Depression Scale (HADS) (24%, n=20 studies). Patient medical records were reviewed to determine the use of PF-related treatments and medication adherence including medication discontinuation or maintenance rate (16%, n=14 studies), healthcare utilisation (24%, n=20 studies) and discussion or completion rate of an advance care plan (ACP) or advance directives (7%, n=6 studies). Physical activity level was measured using wearable devices (5%, n=4 studies) or patient self-reported questionnaires (5%, n=4 studies) including the International Physical Activity Questionnaire (IPAQ) and the Rapid Assessment of Physical Activity Questionnaire (RAPAQ). Disease knowledge was assessed in only two studies using tools developed by the research team due to the lack of PF-validated tools. Self-efficacy was assessed using various methods in five studies (5%). Two used the Self-efficacy for Managing Chronic Disease (SEMCD) six-item scale, one used the COPD self-efficacy scale and two used a numerical rating scale of which one asked about disease preparedness and the other asked about how in control and confident the participants felt about managing their disease.
Effects on disease knowledge and self-efficacy
Two studies demonstrated improved disease knowledge using a tool developed by the research team. One was a community-/home-based rehabilitation programme (pre-post study) [102] and one was a palliative care programme (RCT) [66], both consisted of patient education. The palliative care programme found no difference in numerical rating of disease preparedness following the programme [66]. No change in self-efficacy was also reported in an RCT that examined the impact of a hand-held fan on dyspnoea when measured with the SEMCD six-item scale [63]. Three non-RCT studies reported higher self-efficacy and confidence in managing PF, which include one PR programme [88] that used the SEMCD six-item scale, one palliative care programme [59] that used the COPD self-efficacy scale and one medication support programme [39] that asked participants to rate their confidence in disease management. All three studies included a patient education component.
Effects on physical activity
Two studies (one RCT; one cohort study) reported significant improvement in physical activity. Both were PR programmes that included patient education and a home exercise programme. The RCT used the IPAQ and reported an improvement of median 2164 metabolic equivalent of tasks (MET; min·week−1) (interquartile range=1576 MET min·week−1; p<0.001) in the group that received a 12-week PR programme [103]. The cohort study used the RAPAQ and reported a 0.83-point improvement from baseline following a PR programme across three sites that lasted an average of 7 weeks (95% CI 0.28–1.38, p=0.003) [86]. Two other RCTs also measured physical activity with the IPAQ. One reported higher physical activity level during a PR programme (also consisting of patient education and a home exercise programme) but the improvement was not sustained at the 6-month follow-up [45]. The other RCT reported no change after an education and support programme aimed at improving mental health during the COVID-19 pandemic [98]. Five RCTs measured physical activity using an activity monitor, including one telerehabilitation programme [32], three PR programmes [56, 82, 107] and one testing the effect of using a hand-held fan for dyspnoea [63]. All reported no change following the intervention.
Effects on the use of PF-related treatments
Four studies reported better medication adherence (three medication support programmes [39, 77, 89]; one home monitoring programme [40]). One palliative care programme reported increased use of antifibrotic therapy [58]. Three other palliative care programmes reported increased use of opioids [30, 33, 58]. Two PR programmes reported less use of antibiotics [24, 80], of which one also reported less use of prednisone and short-acting bronchodilator inhalers [24]. One medication support programme [77] and one home monitoring programme [73] reported increased adjustments to medications. Only one study (a home monitoring programme) was an RCT that demonstrated increased adjustment to medications [73].
Effects on healthcare utilisation
Five studies reported no change in hospitalisation and emergency department (ED) visits [24, 33, 55, 73, 80]. Three studies (one cohort study, one post-intervention evaluation and one pre-post study) reported fewer hospitalisation or ED visits. In the cohort study, participants who received a palliative care programme consisting of action planning and care goal setting were 2.32 times and 24.2 times, respectively, less likely to have respiratory-related hospitalisation and ED visits compared to the control group [57]. Evaluation of a telemedicine service during the COVID-19 pandemic that included patient education and self-monitoring reported fewer hospitalisations among people who received the service compared to those who did not in the previous year [95]. The pre-post study reported fewer hospital admission days following a PR programme that included patient education, breathing training and a home exercise programme compared to controls [74].
Two PR programmes [24, 80], one palliative care programme [58] and one home monitoring programme [73] reported the number of physician visits in an outpatient or primary care setting. In the palliative care programme (cohort study), more outpatient visits and fewer general practitioner visits were demonstrated in the group that received the palliative care programme consisting of an action planning component compared to those who received standard specialist or nonspecialist care [58]. The same study also reported that participants in the palliative care group spent less time in an intensive care unit, were more likely to have participated in a PR programme, less likely to be in a long-term care facility and accrued less end-of-life associated costs when compared to the other groups [58]. One of the PR programmes (pre-post study) reported reduced outpatient visits [24], while the other (longitudinal study) reported no change [80]. The home monitoring programme (RCT) also reported no change [73]. Another cohort study reported more referrals to dieticians, social workers and PR among participants who received a palliative care programme consisting of patient education on self-management strategies [33].
Effects on end-of-life planning
One longitudinal study, one cohort study and two RCTs examined the rate of ACP discussion and completion of advance directives in palliative programmes that delivered self-management components; all four studies reported higher rates post-intervention [28, 30, 33, 66].
Effects on exercise capacity
6-min walk test results were reported in 47 (94%) of the 50 studies that measured 6MWD, 15 were RCTs (357 participants included in the intervention group). Almost all PR programmes focused on delivery of physical exercise (n=49), with one RCT focused on breathing exercises. The number of included self-management components varied, ranging between one (patient education or managing physical symptoms only) and 11 components. 12 of the RCTs (80%) reported an improvement that reached the minimal important difference (MID) of 30–33 m [112] when compared to the control group (supplement 5.1.1). In non-RCT studies (n=32), 24 (75%) studies reported an improvement that reached the MID, with one PR programme reporting a slight decline (supplement 5.1.2).
Effects on HRQoL
SGRQ total scores were reported in 29 (94%) of the 31 studies that assessed HRQoL using the SGRQ. The number of self-management components ranged between one (patient education or managing physical symptoms only) and 11 components. 14 were RCTs and 12 studies (269 participants included in the intervention group) reported post-intervention data (10 PR programmes, one palliative care programme and one breathing exercise programme). Six (50%) of the 12 RCTs reported an improvement that reached the MID of seven units [113], with the palliative care programme reporting a decline when compared to control (supplement 5.2.1). In non-RCT studies (n=15; 14 PR programmes and one palliative care programme), six (40%) studies reported an improvement that reached the MID, with one PR programme reporting a worsened score (supplement 5.2.2).
Effects on dyspnoea
MRC dyspnoea results were reported in 27 (96%) of the 28 studies that measured dyspnoea using the MRC dyspnoea scale. The number of self-management components ranged between one (managing physical symptoms only) and 11 components. Nine were RCTs, of which eight (239 participants included in intervention group) reported post-intervention data (seven PR programmes and one involving case conferences). Five (63%) of the eight RCTs reported an improvement that reached the MID of 0.4 unit when compared to control [113], with one PR programme reporting a slightly worsened score (supplement 5.3.1). In non-RCT studies (n=18; 17 PR programmes and one palliative care programme), 13 (72%) reported an improvement that reached the MID, with the palliative care programme reporting an unchanged score and one PR programme reporting a worsened score (supplement 5.3.2).
Effects on psychological wellbeing
HADS scores were reported in 17 (85%) of the 20 studies that measured anxiety and depression using the HADS. The number of self-management components ranged between two (patient education and managing physical symptoms) and 11 components. Six were RCTs. Three studies (56 participants included in the intervention group) reported post-intervention data (one palliative care programme, one home monitoring programme and one PR programme). Only the PR programme (33%) reported an improvement that reached the MID of 2.4 units for both anxiety and depression [113] (supplement 5.4.1). In non-RCT studies (n=11; nine PR programmes, one palliative care programme and one home monitoring programme), three PR programmes (27%) reported an improvement that reached the MID. One PR programme reported an increase in depression and one home monitoring programme reported an increase in anxiety levels (supplement 5.4.2).
Summary of qualitative results
13 studies reported narrative results (supplement 2.2). The importance of HCPs and peer support was highlighted most often [25, 28, 30, 39, 48, 52, 67, 92, 99]. Support from HCPs was sought in various settings (i.e. in the community or PR, via telephone or video follow-ups) with two studies highlighting the importance of accessibility and reliability of such support [30, 39]. Where interaction between participants was involved (i.e. on PF-specific websites, group educational sessions and peer support programmes), participants generally reported a reduced feeling of isolation [48, 67, 92] and valued the information or experiences shared by their peers [25, 99]. In four studies, participants reported increased confidence in performing unsupervised exercise [48], activities of daily living [69], managing antifibrotic treatment [39] and their condition in general [59]. However, participants in one study still felt anxious about unsupervised exercise after attending a 6-week PR programme that included educational sessions and a home exercise programme [92]. Other themes identified related to better coordination of care, improved understanding of the disease and care options, and the use of personalised coping strategies to manage physical and mental health.
Adverse events (AEs)
62 (71%) studies reported no AEs. 12 (14%) studies reported deaths in the intervention group [29, 35, 43, 45, 49, 52, 54, 66, 74, 79, 86, 99] and 14 (16%) reported other reasons for not completing the intervention [27, 34, 38, 42, 46, 50, 52, 55, 60, 72, 73, 81, 100, 108]; however, no links between the study intervention and death or noncompletion were reported. Reasons for noncompletion included acute exacerbation, other respiratory-related issues, nonrespiratory health issues and personal reasons such as lack of motivation, being called to receive lung transplantation and transportation problems.
Grey literature search
Of the 13 lung disease specific websites, three did not provide patient resources (https://thoracic.org.au, www.ersnet.org and www.brit-thoracic.org.uk). The remaining 10 presented information in various formats, including written materials (e.g. fact sheets and booklets), audio and video resources, webinars, blogs, and weblinks that directed users to other external online information. The information provided covered all components listed in table 3. In addition, three websites covered topics related to strategies in communicating with HCPs or loved ones and two websites addressed the topic of sex when living with PF. Almost all websites provided information to help prepare for an appointment with an HCP and how to get involved (e.g. joining a consumer advisory committee, becoming an advocate, starting a support group and sharing personal stories). Various materials were provided across these websites, including exercise logs, symptom diaries, goal-setting worksheets, self-care plans, smoking cessation workbooks and service directories for lung clinics, exercise programmes and support groups.
Discussion
This review examined 87 scientific studies that described self-management components used in PF. The majority were pre-post studies and RCTs. No studies tested a PF-specific self-management package. Rather, the studies delivered a range of self-management components in various settings with PR programmes being the most common. The self-management components most commonly delivered were patient education, managing physical symptoms and enhancing psychosocial wellbeing. Less common were goal setting, managing comorbidities and information related to sexuality and self-esteem. Self-management components were most often delivered in person and at an outpatient location using a multidisciplinary approach. The outcome measures most frequently reported were 6MWD, SGRQ and MRC dyspnoea scores. Clinically significant improvements were seen in 80%, 50% and 63% of RCTs that reported these outcomes, respectively.
The components delivered in the included studies met many of the support needs in PF; however, a few components previously identified as important were rarely addressed in the scientific studies. Patient education and psychosocial support are key care components in PF [114, 115]. These are commonly delivered across various clinical settings and accessible through grey literature. A recent Delphi study identified essential components for self-management in PF, which addressed a range of aspects, including treatment options, management of medications, breathlessness, fatigue, psychological wellbeing, comorbidities and lifestyle (i.e. physical activity and smoking cessation), accessing support, and communicating with others [116]. Other educational topics identified as being important include regular vaccination, nutrition, managing exacerbations, managing cough and using oxygen therapy [7]. In PF, self-monitoring and end-of-life planning are recognised as important components of care [114], with goal setting being essential for effective self-management [116]. The components identified in this review address the important topics identified previously, except for managing comorbidities and goal setting, which were less commonly seen, and communication strategies, that only appeared in grey literature. Furthermore, information and support for maintaining body image and self-esteem was delivered in only 7% of the scientific studies and two PF-specific websites. In a qualitative study that interviewed participants with PF from the UK, Germany and Italy, 90% of female participants reported a loss of identity as the main family support figure and 15% of male participants reported a detrimental physical and emotional impact on their sex lives [117]. There are considerable variations in the disease experiences, lifestyle and care needs between individuals with PF over the disease course. In addition, individuals may also require varied levels of support in self-management based on language and cultural differences, health literacy and other social determinants of health. Therefore, individualisation remains key when supporting people with PF in self-management [22, 116]. However, only about a third of the included studies delivered self-management components on an individual basis or used a combination of individual and group settings. The involvement of patients in the development and format of future interventions will be critical to ensure the needs of consumers are met. To facilitate individualisation, future studies should consider delivering self-management components based on individual preferences, background, knowledge and circumstances. However, determining an individual's self-management needs remains a challenge that needs addressing.
Self-management components were delivered in various settings; however, a self-management package tailored specifically for PF is currently lacking and the optimal delivery method is yet to be determined. This review showed that half of the studies delivered self-management components in person while about a third used a combination of in-person and remote delivery. However, the most effective approach could not be determined due to the heterogeneity of the programmes. The COVID-19 pandemic has accelerated the use of telehealth, including home monitoring. Whilst telehealth is generally accepted by HCPs and patients, technological and language barriers make it less ideal for some [118]; thus, having the option of face-to-face support remains important [119]. People with PF value information and support from various types of HCPs, in particular having a point of contact when needed [22, 120, 121], although it is not essential for self-management support to be delivered by a multidisciplinary team [116]. The studies included in this review highlighted the under-utilisation of expert patients in the delivery of self-management components. As people with PF often value the support provided and the information shared by others who are also living with the disease [99], future studies should consider engaging expert patients in the development and delivery of components amenable to peer support. These could include managing symptoms, communicating with family and health professionals, and living well with PF. However, adequate training and support should be provided to those involved [99]. In addition, further research is needed to establish the most effective method for delivering self-management components and how best to involve expert patients in delivering components.
The outcome measures used to assess the impact of self-management components were numerous and varied, highlighting the need for a more standardised approach. The goals of self-management programmes are mainly focused on improving disease knowledge, self-efficacy, physical and psychosocial wellbeing, exercise capacity, engagement in positive health behaviours, and healthcare utilisation [122]. Whilst clinical and patient outcomes such as exercise capacity, psychological wellbeing, symptom burden and HRQoL were commonly assessed in studies delivering self-management components, disease knowledge, self-efficacy, level of physical activity and healthcare utilisation were only assessed in a few of them. A validated assessment tool to measure disease or self-care knowledge in PF is currently unavailable, which may explain why studies rarely measured this outcome even though a majority delivered patient education. Tools for measuring self-efficacy are available such as the generalised self-efficacy scale [123], the SEMCD scale [124] and the COPD self-efficacy scale [125], with the latter two being used in some of our included studies. Other approaches for measuring changes in health behaviours such as the use of electronic tools to measure physical activity, goal setting and attainment, and medication adherence should also be considered. Whilst the assessment of patient outcomes remains important, future evaluations of self-management interventions in PF should also focus on assessing changes in outcomes directly related to self-management, such as self-efficacy, disease knowledge, health behaviours and healthcare utilisation.
Establishing the effects of self-management on outcomes was challenging given there were no studies of a PF-specific self-management package and none of the included RCTs specified the contribution of self-management components on outcomes. In studies where self-management components were employed as part of a broader package of care (e.g. PR), clinically significant improvements were seen in 6MWD, SGRQ and MRC dyspnoea scores in ≥50% of RCTs. A majority of the RCTs that reported improvement in these outcomes were studies investigating a PR programme where self-management was specifically encouraged (e.g. an independent home exercise or physical activity programme between sessions). However, it is difficult to interpret these findings in the context of PR, where other core programme elements (particularly supervised exercise training) would be expected to impact on these outcomes, even in the absence of specific self-management components. The positive association between these self-management components and improvements in exercise capacity shown in this review is supported by a Cochrane review [14] examining self-management interventions in COPD, which reported clinically significant improvements in 6MWD when participants received such components. However, this Cochrane review excluded studies that involved a supervised exercise component, a component found in most of the PR programmes included in this review. Future reviews of self-management interventions in PF may consider applying stricter inclusion criteria such as including only studies with a clear aim of facilitating self-management. In addition, self-management components also seemed to have a positive impact on self-efficacy, disease knowledge, medication adherence, hospitalisation or ED visit rates, completion of an ACP and PR participation. However, this review highlights the need for more evidence from RCTs to assess the impact of programmes delivering self-management components on these outcomes.
To our knowledge, this is the first review that systematically investigated the self-management components of interventions delivered to people with PF. The inclusion of studies that consisted of at least one self-management component provided a more wide-reaching review of current evidence, encompassing aspects such as the type of components, their delivery across various settings and impact on health outcomes. However, this also increased study heterogeneity, which limited comparisons between studies. The inclusion of PR studies also made it harder to determine whether improvements seen in exercise capacity and dyspnoea were attributed to the supervised exercise component or the self-management components (e.g. patient education and home exercise programme). However, as a majority of studies delivered self-management components in a PR setting where supervised exercise is a key component, the exclusion of such studies would have greatly limited this scoping review. This review included literature published in English only and only 13 public websites were searched for grey literature; therefore, literature published in other languages or on other PF-specific websites may have been missed.
Points for clinical practice
Self-management components are delivered in various settings with PR being the most common. Depending on the individual's health status and needs, people with PF may be referred to programmes that consist of self-management components in the appropriate setting to learn self-management skills.
Questions for future research
How should the self-management needs of a person with PF be assessed?
What is the optimal method to deliver a PF-specific self-management package?
How would a PF-specific self-management package impact physical and psychosocial wellbeing, self-efficacy, quality of life, use of medication, engagement in positive health behaviours, and healthcare utilisation?
Conclusion
Currently, there is no self-management package designed specifically to support people with PF; however, a range of self-management components are delivered predominantly through PR programmes. Clinical outcomes were commonly assessed (6MWD, SGRQ and MRC scores being the most common), but outcomes related to self-management and behaviour change were rarely assessed. Improvement in 6MWD, SGRQ and MRC dyspnoea scores reached the MID in a majority of studies; however, the contribution of self-management components to these outcomes could not be assessed. Future research should examine the impact of a PF-specific self-management package and consider assessing outcomes including changes in self-efficacy and health behaviours, as well as clinical and patient-reported outcomes.
Supplementary material
Please note: supplementary material is not edited by the Editorial Office, and is uploaded as it has been supplied by the author.
Supplementary 1. Search strategy ERR-0092-2023.SUPPLEMENT (203.1KB, pdf)
Supplement 2.1. Comprehensive information of included studies that reported quantitative results ERR-0092-2023.SUPPLEMENT2 (977KB, pdf)
Supplement 2.2. Comprehensive information of included studies that reported qualitative results ERR-0092-2023.SUPPLEMENT3 (385.9KB, pdf)
Supplement 3. Self-management components delivered in each study ERR-0092-2023.SUPPLEMENT4 (546.4KB, pdf)
Supplement 4. Outcome measures used in studies reporting quantitative results ERR-0092-2023.SUPPLEMENT5 (232.7KB, pdf)
Supplement 5. Mean change data ERR-0092-2023.SUPPLEMENT6 (526.8KB, pdf)
Footnotes
Provenance: Submitted article, peer reviewed.
Author contributions: J.Y.T. Lee: conceptualisation, methodology, data curation, formal analysis, writing – original draft, visualisation, project administration. G. Tikellis: conceptualisation, methodology, formal analysis, writing – review and editing, supervision. L. Dowman: methodology, data curation, formal analysis, writing – review and editing, visualisation. A.W. Jones: methodology, data curation, formal analysis, writing – review and editing, visualisation. M. Hoffman: data curation, formal analysis, writing – review and editing. C.R. Mellerick: data curation, formal analysis, writing – review and editing. C. Malaguti: data curation, formal analysis, writing – review and editing. Y.H. Khor: methodology, formal analysis, writing – review and editing. A.E. Holland: conceptualisation, methodology, formal analysis, writing – review and editing, supervision.
Conflict of interest: J.Y.T. Lee has nothing to disclose. G. Tikellis has nothing to disclose. L. Dowman has nothing to disclose. A.W. Jones reports funding from a Lung Foundation Australia Boehringer Ingelheim Fellowship and unpaid committee positions with Thoracic Society of Australia and New Zealand. M. Hoffman has nothing to disclose. C.R. Mellerick has nothing to disclose. C. Malaguti has nothing to disclose. Y.H. Khor reports grants from National Health and Medical Research Council, Medical Research Future Fund, Lung Foundation Australia, and Austin Medical Research Foundation, and nonfinancial support from Air Liquide Healthcare during the conduct of the study, outside the submitted work. A.E. Holland reports grants from National Health and Medical Research Council and Medical Research Future Fund; nonfinancial support from Air Liquide Healthcare during the conduct of the study, outside the submitted work; and unpaid positions with Thoracic Society of Australia and New Zealand and Lung Foundation Australia.
Support statement: This work is supported by a scholarship provided by the Lung Foundation Australia's Hope Research Fund and Monash University, and financial support from the Centre for Research Excellence in Pulmonary Fibrosis, funded by the National Health and Medical Research Council. Funding information for this article has been deposited with the Crossref Funder Registry.
References
- 1.Swigris J, Cutts K, Male N, et al. The Living with Pulmonary Fibrosis questionnaire in progressive fibrosing interstitial lung disease. ERJ Open Res 2021; 7: 00145-2020. doi: 10.1183/23120541.00145-2020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Richeldi L, du Bois RM, Raghu G, et al. Efficacy and safety of nintedanib in idiopathic pulmonary fibrosis. N Engl J Med 2014; 370: 2071–2082. doi: 10.1056/NEJMoa1402584 [DOI] [PubMed] [Google Scholar]
- 3.King TE, Bradford WZ, Castro-Bernardini S, et al. A phase 3 trial of pirfenidone in patients with idiopathic pulmonary fibrosis. N Engl J Med 2014; 370: 2083–2092. doi: 10.1056/NEJMoa1402582 [DOI] [PubMed] [Google Scholar]
- 4.Dowman L, Hill CJ, May A, et al. Pulmonary rehabilitation for interstitial lung disease. Cochrane Database Syst Rev 2021; 2021: CD006322. 10.1002/14651858.CD006322.pub4 [DOI] [PubMed] [Google Scholar]
- 5.Lee JYT, Tikellis G, Corte TJ, et al. The supportive care needs of people living with pulmonary fibrosis and their caregivers: a systematic review. Eur Respir Rev 2020; 29: 190125. doi: 10.1183/16000617.0125-2019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Burnett K, Glaspole I, Holland A. Understanding the patient's experience of care in idiopathic pulmonary fibrosis. Respirology 2019; 24: 270–277. doi: 10.1111/resp.13414 [DOI] [PubMed] [Google Scholar]
- 7.Holland AE, Watson A, Glaspole I. Comprehensive pulmonary rehabilitation for interstitial lung disease: a consensus approach to identify core education topics. Patient Educ Couns 2019; 102: 1125–1130. doi: 10.1016/j.pec.2019.01.010 [DOI] [PubMed] [Google Scholar]
- 8.Morisset J, Dubé B-P, Garvey C, et al. The unmet educational needs of patients with interstitial lung disease: setting the stage for tailored pulmonary rehabilitation. Ann Am Thorac Soc 2016; 13: 1026–1033. doi: 10.1513/AnnalsATS.201512-836OC [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Richard AA, Shea K. Delineation of self-care and associated concepts. J Nurs Scholarsh 2011; 43: 255–264. [DOI] [PubMed] [Google Scholar]
- 10.Australian Government . National strategic action plan for lung conditions. p. 17. Date last accessed: 2 March 2023. Date last updated: February 2019. www.health.gov.au/sites/default/files/documents/2019/09/national-strategic-action-plan-for-lung-conditions_0.pdf
- 11.Hibbard JH, Stockard J, Mahoney ER, et al. Development of the patient activation measure (PAM): conceptualizing and measuring activation in patients and consumers. Health Serv Res 2004; 39: 1005–1026. doi: 10.1111/j.1475-6773.2004.00269.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Stiggelbout AM, Pieterse AH, De Haes JCJM. Shared decision making: concepts, evidence, and practice. Patient Educ Couns 2015; 98: 1172–1179. doi: 10.1016/j.pec.2015.06.022 [DOI] [PubMed] [Google Scholar]
- 13.Gibson PG, Powell H, Wilson A, et al. Self-management education and regular practitioner review for adults with asthma. Cochrane Database Syst Rev 2003; 2003: CD001117. 10.1002/14651858.CD001117 [DOI] [PubMed] [Google Scholar]
- 14.Schrijver J, Lenferink A, Brusse-Keizer M, et al. Self-management interventions for people with chronic obstructive pulmonary disease. Cochrane Database Syst Rev 2022; 1: CD002990. doi: 10.1002/14651858.CD002990.pub4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.University Hospitals of Leicester NHS trust . SPACE for COPD 2022. Date last accessed: 2 March 2023. www.spaceforcopd.co.uk
- 16.American Lung Association . Breathe well, live well 2022. Date last updated: 25 January 2023. Date last accessed: 2 March 2023. www.lung.org/lung-health-diseases/lung-disease-lookup/asthma/health-professionals-educators/breathe-well-live-well
- 17.Peters MDJ, Godfrey C, McInerney P, et al. Chapter 11: scoping reviews (2020 version). In: Aromataris E, Munn Z, eds. JBI Manual for Evidence Synthesis. Adelaide, JBI, 2020. doi: 10.46658/JBIMES-20-12. [DOI] [Google Scholar]
- 18.Tricco AC, Lillie E, Zarin W, et al. PRISMA extension for scoping reviews (PRISMA-ScR): checklist and explanation. Ann Intern Med 2018; 169: 467–473. doi: 10.7326/M18-0850 [DOI] [PubMed] [Google Scholar]
- 19.Hoffmann TC, Glasziou PP, Boutron I, et al. Better reporting of interventions: template for intervention description and replication (TIDieR) checklist and guide. BMJ 2014; 348: g1687. doi: 10.1136/bmj.g1687 [DOI] [PubMed] [Google Scholar]
- 20.Jonkman NH, Schuurmans MJ, Groenwold RHH, et al. Identifying components of self-management interventions that improve health-related quality of life in chronically ill patients: systematic review and meta-regression analysis. Patient Educ Couns 2016; 99: 1087–1098. doi: 10.1016/j.pec.2016.01.022 [DOI] [PubMed] [Google Scholar]
- 21.Barlow J, Wright C, Sheasby J, et al. Self-management approaches for people with chronic conditions: a review. Patient Educ Couns 2002; 48: 177–187. doi: 10.1016/S0738-3991(02)00032-0 [DOI] [PubMed] [Google Scholar]
- 22.Lee JYT, Tikellis G, Glaspole I, et al. Self-management for pulmonary fibrosis: insights from people living with the disease and healthcare professionals. Patient Educ Couns 2022; 105: 956–964. doi: 10.1016/j.pec.2021.07.005 [DOI] [PubMed] [Google Scholar]
- 23.Wan X, Wang W, Liu J, et al. Estimating the sample mean and standard deviation from the sample size, median, range and/or interquartile range. BMC Med Res Methodol 2014; 14: 135. doi: 10.1186/1471-2288-14-135 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Al Moamary MS. Impact of a pulmonary rehabilitation programme on respiratory parameters and health care utilization in patients with chronic lung diseases other than COPD. East Mediterr Health J 2012; 18: 120–126. doi: 10.26719/2012.18.2.120 [DOI] [PubMed] [Google Scholar]
- 25.Albright K, Walker T, Baird S, et al. Seeking and sharing: why the pulmonary fibrosis community engages the web 2.0 environment. BMC Pulm Med 2016; 16: 4. doi: 10.1186/s12890-016-0167-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Archibald N, Bakal JA, Richman-Eisenstat J, et al. Early integrated palliative care bundle impacts location of death in interstitial lung disease: a pilot retrospective study. Am J Hosp Palliat Care 2021; 38: 104–113. doi: 10.1177/1049909120924995 [DOI] [PubMed] [Google Scholar]
- 27.Arizono S, Taniguchi H, Sakamoto K, et al. Pulmonary rehabilitation in patients with idiopathic pulmonary fibrosis: comparison with chronic obstructive pulmonary disease. Sarcoidosis Vasc Diffuse Lung Dis 2017; 34: 283–289. doi: 10.36141/svdld.v34i4.5549 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Bajwah S, Ross JR, Wells AU, et al. Palliative care for patients with advanced fibrotic lung disease: a randomised controlled phase II and feasibility trial of a community case conference. Thorax 2015; 70: 830–839. doi: 10.1136/thoraxjnl-2014-206583 [DOI] [PubMed] [Google Scholar]
- 29.Bassi I, Guerrieri A, Carpano M, et al. Feasibility and efficacy of a multidisciplinary palliative approach in patients with advanced interstitial lung disease. A pilot randomised controlled trial. Pulmonology 2021; in press [ 10.1016/j.pulmoe.2021.11.004]. doi: 10.1016/j.pulmoe.2021.11.004 [DOI] [PubMed] [Google Scholar]
- 30.Bischoff KE, Choi S, Su A, et al. Better together: a mixed-methods study of palliative care co-management for patients with interstitial lung disease. J Palliat Med 2021; 24: 1823–1832. doi: 10.1089/jpm.2020.0787 [DOI] [PubMed] [Google Scholar]
- 31.Brunetti G, Malovini A, Maniscalco M, et al. Pulmonary rehabilitation in patients with interstitial lung diseases: correlates of success. Respir Med 2021; 185: 106473. doi: 10.1016/j.rmed.2021.106473 [DOI] [PubMed] [Google Scholar]
- 32.Cerdán-de-las-Heras J, Balbino F, Løkke A, et al. Tele-rehabilitation program in idiopathic pulmonary fibrosis – a single-center randomized trial. Int J Environ Res Public Health 2021; 18: 10016. doi: 10.3390/ijerph181910016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Chai GT, Neo HY, Abisheganaden J, et al. Impact of palliative care in end-of-life of fibrotic interstitial lung disease patients. Am J Hosp Palliat Care 2022; 39: 1443–1451. doi: 10.1177/10499091221083575 [DOI] [PubMed] [Google Scholar]
- 34.Chéhère B, Bougault V, Chenivesse C, et al. Cardiorespiratory adaptation during 6-minute walk test in fibrotic idiopathic interstitial pneumonia patients who did or did not respond to pulmonary rehabilitation. Eur J Phys Rehabil Med 2019; 55: 103–112. doi: 10.23736/S1973-9087.18.05093-1 [DOI] [PubMed] [Google Scholar]
- 35.da Fontoura FF, Berton DC, Watte G, et al. Pulmonary rehabilitation in patients with advanced idiopathic pulmonary fibrosis referred for lung transplantation. J Cardiopulm Rehabil Prev 2018; 38: 131–134. doi: 10.1097/HCR.0000000000000315 [DOI] [PubMed] [Google Scholar]
- 36.Deniz S, Sahin H, Yalniz E. Does the severity of interstitial lung disease affect the gains from pulmonary rehabilitation? Clinical Respir J 2018; 12: 2141–2150. doi: 10.1111/crj.12785 [DOI] [PubMed] [Google Scholar]
- 37.Devani P, Pinto N, Jain P, et al. Effect of pulmonary rehabilitation (PR) program in patients with interstitial lung disease (ILD) – Indian scenario. J Assoc Physicians India 2019; 67: 28–33. [PubMed] [Google Scholar]
- 38.Dowman LM, McDonald CF, Hill CJ, et al. The evidence of benefits of exercise training in interstitial lung disease: a randomised controlled trial. Thorax 2017; 72: 610–619. doi: 10.1136/thoraxjnl-2016-208638 [DOI] [PubMed] [Google Scholar]
- 39.Duck A, Pigram L, Errhalt P, et al. IPF Care: a support program for patients with idiopathic pulmonary fibrosis treated with pirfenidone in Europe. Adv Ther 2015; 32: 87–107. doi: 10.1007/s12325-015-0183-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Edwards C, Costello E, Cassidy N, et al. Use of the patientMpower app with home-based spirometry to monitor the symptoms and impact of fibrotic lung conditions: longitudinal observational study. JMIR Mhealth Uhealth 2020; 8: e16158. doi: 10.2196/16158 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Elganady A, El Hoshy M, Eshmawey H, et al. Value of pulmonary rehabilitation in interstitial lung diseases. Egypt J Chest Dis Tuberc 2020; 69: 542–548. doi: 10.4103/ejcdt.ejcdt_165_19 [DOI] [Google Scholar]
- 42.Ferreira A, Garvey C, Connors GL, et al. Pulmonary rehabilitation in interstitial lung disease: benefits and predictors of response. Chest 2009; 135: 442–447. doi: 10.1378/chest.08-1458 [DOI] [PubMed] [Google Scholar]
- 43.Ferreira G, Feuerman M, Spiegler P. Results of an 8-week, outpatient pulmonary rehabilitation program on patients with and without chronic obstructive pulmonary disease. J Cardiopulm Rehabil 2006; 26: 54–60. doi: 10.1097/00008483-200601000-00011 [DOI] [PubMed] [Google Scholar]
- 44.Fuschillo S, De Felice A, Elia A, et al. Effect of pulmonary rehabilitation on functional exercise capacity and hypoxemia in patients with interstitial lung diseases: a retrospective study. Sarcoidosis Vasc Diffuse Lung Dis 2018; 35: 245–251. doi: 10.36141/svdld.v35i3.6828 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Gaunaurd IA, Gomez-Marin OW, Ramos CF, et al. Physical activity and quality of life improvements of patients with idiopathic pulmonary fibrosis completing a pulmonary rehabilitation program. Respir Care 2014; 59: 1872–1879. doi: 10.4187/respcare.03180 [DOI] [PubMed] [Google Scholar]
- 46.Grongstad A, Spruit MA, Oldervoll LM, et al. Pulmonary rehabilitation in patients with pulmonary sarcoidosis: impact on exercise capacity and fatigue. Respiration 2020; 99: 289–297. doi: 10.1159/000506295 [DOI] [PubMed] [Google Scholar]
- 47.Guler SA, Hur SA, Stickland MK, et al. Survival after inpatient or outpatient pulmonary rehabilitation in patients with fibrotic interstitial lung disease: a multicentre retrospective cohort study. Thorax 2022; 77: 589–595. doi: 10.1136/thoraxjnl-2021-217361 [DOI] [PubMed] [Google Scholar]
- 48.Hoffman M, Mellerick C, Symons K, et al. Pulmonary rehabilitation for interstitial lung disease: referral and patient experiences. Chron Respir Dis 2021; 18: 14799731211046022. doi: 10.1177/14799731211046022 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Holland AE, Hill CJ, Conron M, et al. Short term improvement in exercise capacity and symptoms following exercise training in interstitial lung disease. Thorax 2008; 63: 549–554. doi: 10.1136/thx.2007.088070 [DOI] [PubMed] [Google Scholar]
- 50.Holland AE, Hill CJ, Glaspole I, et al. Predictors of benefit following pulmonary rehabilitation for interstitial lung disease. Respir Med 2012; 106: 429–435. doi: 10.1016/j.rmed.2011.11.014 [DOI] [PubMed] [Google Scholar]
- 51.Huppmann P, Sczepanski B, Boensch M, et al. Effects of inpatient pulmonary rehabilitation in patients with interstitial lung disease. Eur Respir J 2013; 42: 444–453. doi: 10.1183/09031936.00081512 [DOI] [PubMed] [Google Scholar]
- 52.Igai Y, Porter SE. Development and applicability of a dignity-centred palliative care programme for people with idiopathic pulmonary fibrosis: a qualitative-driven mixed methods study. Nurs Open 2022; 10: 8–23. doi: 10.1002/nop2.1274 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Igarashi A, Iwanami Y, Sugino K, et al. Using 6-min walk distance expressed as a percentage of reference to evaluate the effect of pulmonary rehabilitation in elderly patients with interstitial lung disease. J Cardiopulm Rehabil Prev 2018; 38: 342–347. doi: 10.1097/HCR.0000000000000305 [DOI] [PubMed] [Google Scholar]
- 54.Jackson RM, Gómez-Marín OW, Ramos CF, et al. Exercise limitation in IPF patients: a randomized trial of pulmonary rehabilitation. Lung 2014; 192: 367–376. doi: 10.1007/s00408-014-9566-9 [DOI] [PubMed] [Google Scholar]
- 55.Janssen K, Rosielle D, Wang Q, et al. The impact of palliative care on quality of life, anxiety, and depression in idiopathic pulmonary fibrosis: a randomized controlled pilot study. Respir Res 2020; 21: 2. doi: 10.1186/s12931-019-1266-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Jarosch I, Schneeberger T, Gloeckl R, et al. Short-term effects of comprehensive pulmonary rehabilitation and its maintenance in patients with idiopathic pulmonary fibrosis: a randomized controlled trial. J Clin Med 2020; 9: 1567. doi: 10.3390/jcm9051567 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Kalluri M, Claveria F, Ainsley E, et al. Beyond idiopathic pulmonary fibrosis diagnosis: multidisciplinary care with an early integrated palliative approach is associated with a decrease in acute care utilization and hospital deaths. J Pain Symptom Manage 2018; 55: 420–426. doi: 10.1016/j.jpainsymman.2017.10.016 [DOI] [PubMed] [Google Scholar]
- 58.Kalluri M, Lu-Song J, Younus S, et al. Health care costs at the end of life for patients with idiopathic pulmonary fibrosis. Evaluation of a pilot multidisciplinary collaborative interstitial lung disease clinic. Ann Am Thorac Soc 2020; 17: 706–713. doi: 10.1513/AnnalsATS.201909-707OC [DOI] [PubMed] [Google Scholar]
- 59.Kalluri M, Younus S, Archibald N, et al. Action plans in idiopathic pulmonary fibrosis: a qualitative study—“I do what I can do”. BMJ Support Palliat Care 2021; in press [ 10.1136/bmjspcare-2020-002831] [DOI] [PubMed] [Google Scholar]
- 60.Kaymaz D, Ergun P, Candemir I, et al. Pulmonary rehabilitation in interstitial lung diseases. Tuberk Toraks 2013; 61: 295–302. doi: 10.5578/tt.6291 [DOI] [PubMed] [Google Scholar]
- 61.Kerti M, Kelemen K, Varga J. The effectiveness of pulmonary rehabilitation in comparison interstitial lung diseases and idiopathic pulmonary fibrosis. J Pulm Respir Med 2018; 8: 1000475. doi: 10.4172/2161-105X.1000475 [DOI] [Google Scholar]
- 62.Keyser RE, Woolstenhulme JG, Chin LMK, et al. Cardiorespiratory function before and after aerobic exercise training in patients with interstitial lung disease. J Cardiopulm Rehabil Prev 2015; 35: 47–55. doi: 10.1097/HCR.0000000000000083 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Khor YH, Saravanan K, Holland AE, et al. A mixed-methods pilot study of handheld fan for breathlessness in interstitial lung disease. Sci Rep 2021; 11: 6874. doi: 10.1038/s41598-021-86326-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Kozu R, Senjyu H, Jenkins SC, et al. Differences in response to pulmonary rehabilitation in idiopathic pulmonary fibrosis and chronic obstructive pulmonary disease. Respiration 2011; 81: 196–205. doi: 10.1159/000315475 [DOI] [PubMed] [Google Scholar]
- 65.Ku V, Janmeja AK, Aggarwal D, et al. Pulmonary rehabilitation in patients with interstitial lung diseases in an outpatient setting: a randomised controlled trial. Indian J Chest Dis Allied Sci 2017; 59: 75–80. [Google Scholar]
- 66.Lindell KO, Klein SJ, Veatch MS, et al. Nurse-led palliative care clinical trial improves knowledge and preparedness in caregivers of patients with idiopathic pulmonary fibrosis. Ann Am Thorac Soc 2021; 18: 1811–1821. doi: 10.1513/AnnalsATS.202012-1494OC [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Lindell KO, Olshansky E, Song M-K, et al. Impact of a disease-management program on symptom burden and health-related quality of life in patients with idiopathic pulmonary fibrosis and their care partners. Heart Lung 2010; 39: 304–313. doi: 10.1016/j.hrtlng.2009.08.005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Lingner H, Buhr-Schinner H, Hummel S, et al. Short-term effects of a multimodal 3-week inpatient pulmonary rehabilitation programme for patients with sarcoidosis: the ProKaSaRe study. Respiration 2018; 95: 343–353. doi: 10.1159/000486964 [DOI] [PubMed] [Google Scholar]
- 69.Lo HYLK, Luo XY, Lau CMJ, et al. Pilot project in developing community rehabilitation service for migrant workers suffering from pneumoconiosis in Mainland China. Work 2007; 30: 33–38. [PubMed] [Google Scholar]
- 70.Magnani D, Lenoci G, Balduzzi S, et al. Effectiveness of support groups to improve the quality of life of people with idiopathic pulmonary fibrosis a pre-post test pilot study. Acta Biomed 2017; 88: 5–12. doi: 10.23750/abm.v88i5-S.6870 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Matsuo S, Okamoto M, Ikeuchi T, et al. Early intervention of pulmonary rehabilitation for fibrotic interstitial lung disease is a favorable factor for short-term improvement in health-related quality of life. J Clin Med 2021; 10: 3153. doi: 10.3390/jcm10143153 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Moor CC, Leuven SIv, Wijsenbeek MS, et al. Feasibility of online home spirometry in systemic sclerosis–associated interstitial lung disease: a pilot study. Rheumatol 2021; 60: 2467–2471. doi: 10.1093/rheumatology/keaa607 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Moor CC, Mostard RLM, Grutters JC, et al. Home monitoring in patients with idiopathic pulmonary fibrosis: a randomized controlled trial. Am J Respir Crit Care Med 2020; 202: 393–401. doi: 10.1164/rccm.202002-0328OC [DOI] [PubMed] [Google Scholar]
- 74.Naji NA, Connor MC, Donnelly SC, et al. Effectiveness of pulmonary rehabilitation in restrictive lung disease. J Cardiopulm Rehabil 2006; 26: 237–243. doi: 10.1097/00008483-200607000-00007 [DOI] [PubMed] [Google Scholar]
- 75.Nasrat SA, El-Hady AAA, Hafiz HA, et al. The efficacy of pulmonary rehabilitation combined with threshold inspiratory muscle training and upper extremities exercises in patients with interstitial lung diseases. Syst Rev Pharm 2021; 12: 527–533. doi: 10.31838/srp.2021.3.77 [DOI] [Google Scholar]
- 76.Naz I, Ozalevli S, Ozkan S, et al. Efficacy of a structured exercise program for improving functional capacity and quality of life in patients with stage 3 and 4 sarcoidosis: a randomized controlled trial. J Cardiopulm Rehabil Prev 2018; 38: 124–130. doi: 10.1097/HCR.0000000000000307 [DOI] [PubMed] [Google Scholar]
- 77.Near AM, Burudpakdee C, Viswanathan S, et al. Effect of a patient support program for idiopathic pulmonary fibrosis patients on medication persistence: a retrospective database analysis. Adv Ther 2021; 38: 3888–3899. doi: 10.1007/s12325-021-01768-w [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Nishiyama O, Kondoh Y, Kimura T, et al. Effects of pulmonary rehabilitation in patients with idiopathic pulmonary fibrosis. Respirology 2008; 13: 394–399. doi: 10.1111/j.1440-1843.2007.01205.x [DOI] [PubMed] [Google Scholar]
- 79.Nolan CM, Polgar O, Schofield SJ, et al. Pulmonary rehabilitation in idiopathic pulmonary fibrosis and COPD: a propensity-matched real-world study. Chest 2022; 161: 728–737. doi: 10.1016/j.chest.2021.10.021 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Ochmann U, Kotschy-Lang N, Raab W, et al. Long-term efficacy of pulmonary rehabilitation in patients with occupational respiratory diseases. Respiration 2012; 84: 396–405. doi: 10.1159/000337271 [DOI] [PubMed] [Google Scholar]
- 81.Ozalevli S, Karaali HK, Ilgin D, et al. Effect of home-based pulmonary rehabilitation in patients with idiopathic pulmonary fibrosis. Multidiscip Respir Med 2010; 5: 31–37. doi: 10.1186/2049-6958-5-1-31 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Perez-Bogerd S, Wuyts W, Barbier V, et al. Short and long-term effects of pulmonary rehabilitation in interstitial lung diseases: a randomised controlled trial. Respir Res 2018; 19: 182. doi: 10.1186/s12931-018-0884-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Prajapat B, Sandhya AS, Menon B, et al. Effect of pulmonary rehabilitation on systemic inflammation muscle mass and functional status in interstitial lung diseases. Int J Sci Res 2016; 5: 760–766. [Google Scholar]
- 84.Rammaert B, Leroy S, Cavestri B, et al. Home-based pulmonary rehabilitation in idiopathic pulmonary fibrosis. Rev Mal Respir 2011; 28: e52–e57. doi: 10.1016/j.rmr.2011.06.006 [DOI] [PubMed] [Google Scholar]
- 85.Rifaat N, Anwar E, Ali YM, et al. Value of pulmonary rehabilitation in patients with idiopathic pulmonary fibrosis. Egypt J Chest Dis Tuberc 2014; 63: 1013–1017. doi: 10.1016/j.ejcdt.2014.06.004 [DOI] [Google Scholar]
- 86.Ryerson CJ, Cayou C, Topp F, et al. Pulmonary rehabilitation improves long-term outcomes in interstitial lung disease: a prospective cohort study. Respir Med 2014; 108: 203–210. doi: 10.1016/j.rmed.2013.11.016 [DOI] [PubMed] [Google Scholar]
- 87.Salhi B, Troosters T, Behaegel M, et al. Effects of pulmonary rehabilitation in patients with restrictive lung diseases. Chest 2010; 137: 273–279. doi: 10.1378/chest.09-0241 [DOI] [PubMed] [Google Scholar]
- 88.Sanchez-Ramirez DC. Impact of pulmonary rehabilitation services in patients with different lung diseases. J Clin Med 2022; 11: 407. doi: 10.3390/jcm11020407 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Satsuma Y, Ikesue H, Kusuda K, et al. Effectiveness of pharmacist-physician collaborative management for patients with idiopathic pulmonary fibrosis receiving pirfenidone. Front Pharmacol 2020; 11: 529654. doi: 10.3389/fphar.2020.529654 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Sciriha A, Lungaro-Mifsud S, Fsadni P, et al. Pulmonary rehabilitation in patients with interstitial lung disease: the effects of a 12-week programme. Respir Med 2019; 146: 49–56. doi: 10.1016/j.rmed.2018.11.007 [DOI] [PubMed] [Google Scholar]
- 91.Sgalla G, Cerri S, Ferrari R, et al. Mindfulness-based stress reduction in patients with interstitial lung diseases: a pilot, single-centre observational study on safety and efficacy. BMJ Open Respir Res 2015; 2: e00065. doi: 10.1136/bmjresp-2014-000065 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Sharp C, McCabe M, Hussain MJ, et al. Duration of benefit following completion of pulmonary rehabilitation in interstitial lung disease-an observational study. QJM 2017; 110: 17–22. doi: 10.1093/qjmed/hcw105 [DOI] [PubMed] [Google Scholar]
- 93.Shen L, Zhang Y, Su Y, et al. New pulmonary rehabilitation exercise for pulmonary fibrosis to improve the pulmonary function and quality of life of patients with idiopathic pulmonary fibrosis: a randomized control trial. Ann Palliat Med 2021; 10: 7289–7297. doi: 10.21037/apm-21-71 [DOI] [PubMed] [Google Scholar]
- 94.Shimoda M, Takao S, Kokutou H, et al. In-hospital pulmonary rehabilitation after completion of primary respiratory disease treatment improves physical activity and ADL performance: a prospective intervention study. Medicine 2021; 100: e28151. doi: 10.1097/MD.0000000000028151 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Stanziola AA, Salzano A, D'Angelo R, et al. Idiopathic pulmonary fibrosis telemedicine management during COVID-19 outbreak. Open Med 2022; 17: 689–693. doi: 10.1515/med-2022-0466 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Swigris JJ, Fairclough DL, Morrison M, et al. Benefits of pulmonary rehabilitation in idiopathic pulmonary fibrosis. Respir Care 2011; 56: 783–789. doi: 10.4187/respcare.00939 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Talwar A, Sahni S, John S, et al. Effects of pulmonary rehabilitation on fatigue severity scale in patients with lung disease. Pneumonol Alergol Pol 2014; 82: 534–540. [DOI] [PubMed] [Google Scholar]
- 98.Thombs BD, Kwakkenbos L, Levis B, et al. Effects of a multi-faceted education and support programme on anxiety symptoms among people with systemic sclerosis and anxiety during COVID-19 (SPIN-CHAT): a two-arm parallel, partially nested, randomised, controlled trial. Lancet Rheumatol 2021; 3: e427–e437. doi: 10.1016/S2665-9913(21)00060-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Tikellis G, Lee JYT, Corte TJ, et al. Peer connect service for people with pulmonary fibrosis in Australia: participants’ experiences and process evaluation. Respirology 2020: 25: 1053–1059. doi: 10.1111/resp.13807 [DOI] [PubMed] [Google Scholar]
- 100.Tonelli R, Cocconcelli E, Lanini B, et al. Effectiveness of pulmonary rehabilitation in patients with interstitial lung disease of different etiology: a multicenter prospective study. BMC Pulm Med 2017; 17: 130. doi: 10.1186/s12890-017-0476-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Trivedi SJ. Effect of pulmonary rehabilitation on functional capacity of lungs and dyspnea in patient with silicosis in Anand district of Gujarat. Indian J Physiother Occup Ther 2017; 11: 207–209. doi: 10.5958/0973-5674.2017.00105.8 [DOI] [Google Scholar]
- 102.Tsang EW, Kwok H, Chan AKY, et al. Outcomes of community-based and home-based pulmonary rehabilitation for pneumoconiosis patients: a retrospective study. BMC Pulm Med 2018; 18: 133. doi: 10.1186/s12890-018-0692-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Vainshelboim B, Fox BD, Kramer MR, et al. Short-term improvement in physical activity and body composition after supervised exercise training program in idiopathic pulmonary fibrosis. Arch Phys Med Rehabil 2016; 97: 788–797. doi: 10.1016/j.apmr.2016.01.018 [DOI] [PubMed] [Google Scholar]
- 104.Vainshelboim B, Oliveira J, Fox BD, et al. Long-term effects of a 12-week exercise training program on clinical outcomes in idiopathic pulmonary fibrosis. Lung 2015; 193: 345–354. doi: 10.1007/s00408-015-9703-0 [DOI] [PubMed] [Google Scholar]
- 105.Vainshelboim B, Oliveira J, Yehoshua L, et al. Exercise training-based pulmonary rehabilitation program is clinically beneficial for idiopathic pulmonary fibrosis. Respiration 2014; 88: 378–388. doi: 10.1159/000367899 [DOI] [PubMed] [Google Scholar]
- 106.Wallaert B, Duthoit L, Drumez E, et al. Long-term evaluation of home-based pulmonary rehabilitation in patients with fibrotic idiopathic interstitial pneumonias. ERJ Open Res 2019; 5: 00045-2019. doi: 10.1183/23120541.00045-2019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Wallaert B, Kyheng M, Labreuche J, et al. Long-term effects of pulmonary rehabilitation on daily life physical activity of patients with stage IV sarcoidosis: a randomized controlled trial. Respir Med Res 2020; 77: 1–7. doi: 10.1016/j.resmer.2019.10.003 [DOI] [PubMed] [Google Scholar]
- 108.Zaki S, Moiz JA, Mujaddadi A, et al. Does inspiratory muscle training provide additional benefits during pulmonary rehabilitation in people with interstitial lung disease? A randomized control trial. Physiother Theory Pract 2022; 39: 518–528. doi: 10.1080/09593985.2021.2024311 [DOI] [PubMed] [Google Scholar]
- 109.Zhang Z, Tian Y, Yang J. Effects of pulmonary rehabilitation therapy combined with conventional drugs on BODE and pulmonary function indexes in elderly patients with interstitial pneumonia. Pak J Med Sci 2022; 38: 1703–1707. doi: 10.12669/pjms.38.6.5748 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Zhou M, Zhang H, Li F, et al. Pulmonary Daoyin as a traditional Chinese medicine rehabilitation programme for patients with IPF: a randomized controlled trial. Respirology 2021; 26: 360–369. doi: 10.1111/resp.13972 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Swift TL, Dieppe PA. Using expert patients’ narratives as an educational resource. Patient Educ Couns 2005; 57: 115–121. doi: 10.1016/j.pec.2004.05.004 [DOI] [PubMed] [Google Scholar]
- 112.Holland AE, Spruit MA, Troosters T, et al. An official European Respiratory Society/American Thoracic Society technical standard: field walking tests in chronic respiratory disease. Eur Respir J 2014; 44: 1428–1446. doi: 10.1183/09031936.00150314 [DOI] [PubMed] [Google Scholar]
- 113.Kim JW, Clark A, Birring SS, et al. Psychometric properties of patient reported outcome measures in idiopathic pulmonary fibrosis. Chron Respir Dis 2021; 18: 14799731211033925. doi: 10.1177/14799731211033925 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Kreuter M, Bendstrup E, Russell A-M, et al. Palliative care in interstitial lung disease: living well. Lancet Respir Med 2017; 5: 968–980. doi: 10.1016/S2213-2600(17)30383-1 [DOI] [PubMed] [Google Scholar]
- 115.Wijsenbeek MS, Holland AE, Swigris JJ, et al. Comprehensive supportive care for patients with fibrosing interstitial lung disease. Am J Respir Crit Care Med 2019; 200: 152–159. doi: 10.1164/rccm.201903-0614PP [DOI] [PubMed] [Google Scholar]
- 116.Lee JYT, Tikellis G, Khor YH, et al. Developing a self-management package for pulmonary fibrosis: an international Delphi study. ERJ Open Res 2022; 8: 00349-2022. doi: 10.1183/23120541.00349-2022 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Russell A-M, Ripamonti E, Vancheri C. Qualitative European survey of patients with idiopathic pulmonary fibrosis: patients’ perspectives of the disease and treatment. BMC Pulm Med 2016; 16: 10. doi: 10.1186/s12890-016-0171-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Nakshbandi G, Moor CC, Johannson KA, et al. Worldwide experiences and opinions of healthcare providers on eHealth for patients with interstitial lung diseases in the COVID-19 era. ERJ Open Res 2021; 7: 00405-2021. doi: 10.1183/23120541.00405-2021 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Gillett M, Hope-Gill B. Telephone clinics during the COVID-19 pandemic – the experiences of interstitial lung disease patients and their carers. J Patient Exp 2022; 9: 23743735221133638. doi: 10.1177/23743735221133638 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.McLean AEB, Webster SE, Fry M, et al. Priorities and expectations of patients attending a multidisciplinary interstitial lung disease clinic. Respirology 2021; 26: 80–86. doi: 10.1111/resp.13913 [DOI] [PubMed] [Google Scholar]
- 121.Graney BA, He C, Marll M, et al. Essential components of an interstitial lung disease clinic: results from a Delphi survey and patient focus group analysis. Chest 2021; 159: 1517–1530. doi: 10.1016/j.chest.2020.09.256 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Effing TW, Vercoulen JH, Bourbeau J, et al. Definition of a COPD self-management intervention: international expert group consensus. Eur Respir J 2016; 48: 46–54. doi: 10.1183/13993003.00025-2016 [DOI] [PubMed] [Google Scholar]
- 123.Schwarzer R, Jerusalem M. Generalized self-efficacy scale. In: Weinman J, Wright S, Johnston M, eds. Measures in Health Psychology: a User's Portfolio Causal and Control Beliefs. Windsor, UK, NFER-NELSON, 1995; pp. 35–37. [Google Scholar]
- 124.Ritter PL, Lorig K. The English and Spanish self-efficacy to manage chronic disease scale measures were validated using multiple studies. J Clin Epidemiol 2014; 67: 1265–1273. doi: 10.1016/j.jclinepi.2014.06.009 [DOI] [PubMed] [Google Scholar]
- 125.Wigal JK, Creer TL, Kotses H. The COPD self-efficacy scale. Chest 1991; 99: 1193–1196. doi: 10.1378/chest.99.5.1193 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Please note: supplementary material is not edited by the Editorial Office, and is uploaded as it has been supplied by the author.
Supplementary 1. Search strategy ERR-0092-2023.SUPPLEMENT (203.1KB, pdf)
Supplement 2.1. Comprehensive information of included studies that reported quantitative results ERR-0092-2023.SUPPLEMENT2 (977KB, pdf)
Supplement 2.2. Comprehensive information of included studies that reported qualitative results ERR-0092-2023.SUPPLEMENT3 (385.9KB, pdf)
Supplement 3. Self-management components delivered in each study ERR-0092-2023.SUPPLEMENT4 (546.4KB, pdf)
Supplement 4. Outcome measures used in studies reporting quantitative results ERR-0092-2023.SUPPLEMENT5 (232.7KB, pdf)
Supplement 5. Mean change data ERR-0092-2023.SUPPLEMENT6 (526.8KB, pdf)