Abstract
Solitary fibrous tumor (SFT) of the central nervous system is a rare fibroblastic tumor of mesenchymal origin. SFTs in the saddle area are much less common. In January 2022, a 43-year-old female patient was admitted with SFT 3 months following partial resection of a microscopic transsphenoidal saddle area tumor at a different hospital. Magnetic resonance imaging indicated that the unresected part of the tumor was significantly enhanced on T1 enhancement, which strongly indicated a recurrence. Subsequently, the patient underwent transnasal endoscopic saddle area tumor resection at our hospital and the tumor was successfully removed. By using postoperative pathology examination, immunohistochemical analysis of Bcl-2, cluster of differentiation 99, STAT6 and vimentin, and a fusion gene test performed by high-throughput sequencing technology, the SFT was definitively diagnosed. Following 3 months of follow-up, the patient was found to have tumor recurrence in the cavernous sinus and absence of tumor growth in the pituitary fossa. Therefore, the patient received proton therapy and tumor growth was controlled effectively.
Keywords: solitary fibrous tumor, central nervous system, neuroendoscopic surgery, proton therapy, sellar
Introduction
Solitary fibrous tumors (SFTs) are considered a type of tumor of mesenchymal origin. Hemangiopericytomas (HPCs) are also rare mesenchymal tumors that exhibit similar clinical, radiological and histological features to SFTs. Intracranial SFT and HPC were initially reported by Carneiro et al (1) and Begg and Garret (2) in 1996 and 1954, respectively. Due to similar histological features, immunophenotype, and the common chromosome 12q13 inversion, nerve growth factor-induced gene A binding protein 2 (NAB2) and STAT6 gene fusion (3), the World Health Organization (WHO) central nervous system (CNS) classification from 2016 used the joint diagnostic terminology ‘Solitary fibrous tumor/hemangiopericytoma’ to describe these disorders and classified them into grades I, II and III (4). However, the distinction between the two types was no longer clinically significant due to the pronounced clinical and histopathological overlap (5). The 2021 WHO classification of CNS tumors changed the previously used term ‘hemangiopericytoma’ to the term SFT (6). SFTs comprise <1% of all primary CNS tumors (7). SFT of the sellar/suprasellar region is even more uncommon. Patients usually present with clinical manifestations of numbness of limbs, headache and different local occupancy compression effects. Tumors in the saddle area tend to cause visual field defects and may be associated with pituitary hormone abnormalities due to their proximity to the pituitary gland (8). SFT in the saddle area is frequently misdiagnosed as a pituitary tumor or meningioma; therefore, preoperative imaging is important for differentiation (9). The treatment of this disease is based on surgery and postoperative adjuvant radiation therapy to reduce recurrence. Proton therapy is a type of radiation therapy that uses beams of protons rather than X-rays to treat cancer, unlike traditional radiotherapy. Protons are positively charged particles that allow precise control of the direction and the depth of the energy release in the body, reducing damage to surrounding normal tissues (10). In the present study, the patient underwent two surgical procedures to achieve complete tumor removal. Due to the proximity of the tumor to the cavernous sinus and the presence of the III–V cranial nerve pair within it, the patient received proton therapy to reduce the risk of nerve damage. At 6 months following treatment, the patient exhibited absence of symptoms of associated nerve damage. To the best of our knowledge, the current study reported the first patient with saddle area SFT who received proton therapy.
Case report
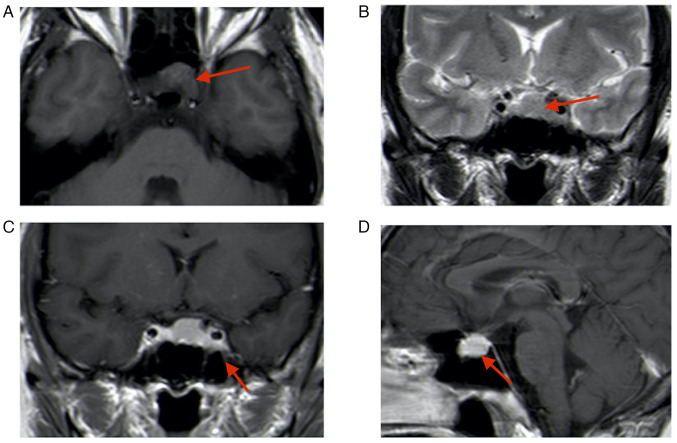
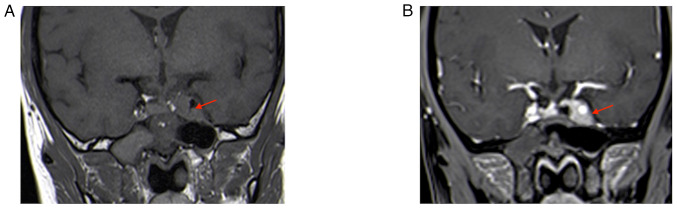
A 43-year-old female patient presented at the Department of Neurosurgery of the Affiliated Suzhou Hospital of Nanjing Medical University (Suzhou, China) three months following a microscopic transsphenoidal saddle area tumor partial resection at a different hospital (January 2022). The patient reported blurred vision in the left eye. The nervous system examination indicated normal eye function. The first magnetic resonance imaging (MRI) performed at the external hospital (October 2021) indicated the presence of a solid tumor sized 20×18×15 mm in the pituitary fossa; this was confirmed by an isodense signal on T1-weighted imaging and T2-weighted imaging (Fig. 1). Enhanced MRI indicated enhancement of the mass and displacement of the pituitary gland to the upper right side. Due to the similar imaging feature to a pituitary tumor, the lesion had been misdiagnosed as a pituitary tumor prior to the first surgery. Subsequently, the patient received a microscopic transsphenoidal saddle area tumor resection; however, only part of the tumor was removed due to the rich blood supply of the tumor and its proximity to the cavernous sinus; intraoperative bleeding from the cavernous sinus was apparent, making it impossible to completely resect the tumor.
Figure 1.
Initial MRI performed at the external hospital (October 2021). (A) T1-MRI; (B) T2-MRI; (C) enhanced T1-MRI coronal image; lesion size, 20×18×15 mm (red arrows indicate lesion areas); (D) enhanced T1-MRI sagittal image. Red arrows indicate lesion areas.
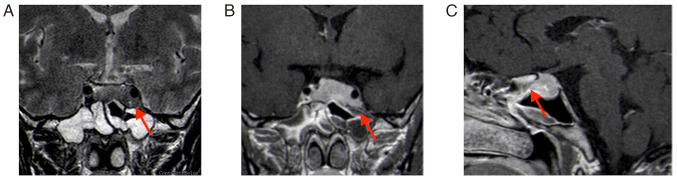
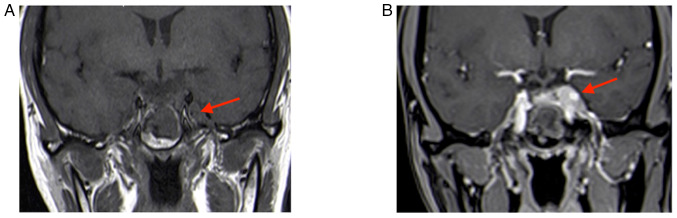
The second MRI (January 2022) performed at the hospital of the present study (the Affiliated Suzhou Hospital of Nanjing Medical University, Suzhou, China) indicated that the tumor grew and was significantly enlarged; it invaded bilateral cavernous sinuses (size, 30×20×18 mm; Fig. 2). Following a thorough evaluation of the patient's condition, a transnasal endoscopic saddle area tumor resection was performed at the hospital of the present study and the tumor was successfully removed. During the operation, the right part of the tumor was soft and could be removed by suction following spatula release; however, the left part was firm and could not be removed by suction; therefore, the tumor was slowly shattered with a curette and removed with tumor removal forceps. The tumor invaded the left cavernous sinus, which was considered to be its origin; bleeding was apparent when the tumor was scraped. Postoperative MRI indicated an extracted cavity with lack of enhancement over the original tumor location, suggesting satisfactory tumor clearance (Fig. 3).
Figure 2.
Review MRI 3 months after the first surgery (January 2022). (A) T2-weighted MRI; (B) enhanced T1-weighted coronal image; lesion size, 30×20×18 mm (red arrows indicate lesion areas); (C) enhanced T1-weighted sagittal image. Red arrows indicate lesion areas.
Figure 3.
MRI at 3 days after the second surgery. (A) T2-MRI (the high signal indicated by the red arrow is the fat tissue filled in the surgery). (B) Enhanced T1-MRI coronal image indicating satisfactory tumor resection with no residual (red arrows indicate operation areas). (C) Enhanced T1-weighted sagittal image (the red arrow indicates the pituitary).
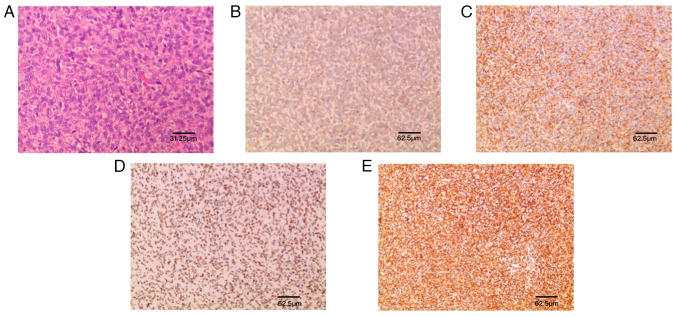
A histopathological examination with immunohistochemistry confirmed that the lesion was SFT, WHO grade II. Under the light microscope, large numbers of typical ‘staghorn-like’ blood vessels and collagen fibroma cells could be observed around the vessels in a wheel-like or concentric circle arrangement, forming a dense or sparse area of cells (Fig. 4). Immunohistochemically, the tumor cells were positive for Bcl-2, cluster of differentiation (CD)99, STAT6 and vimentin, which was consistent with the diagnosis of SFT (Fig. 3). The immunohistochemistry protocol was as follows: At room temperature, formalin-fixed, paraffin-embedded tissue sections (4 µm) were placed on positively charged slides and allowed to dry. Following the removal of paraffin, endogenous peroxidase activity was quenched with hydrogen peroxide in methanol, after which the sections were hydrated with water. The tissue sections were then stained for CD99 (cat. no. GT2123), vimentin (cat. no. GM0725), STAT6 (cat. no. GT2295), Bcl-2 (cat. no. GM0887) (all prediluted by the manufacturer, GeneTech Co., Ltd.).
Figure 4.
Histology and immunohistochemistry. (A) H&E (magnification, ×80; scale bar, 31.25 µm); (B) Bcl-2 (+); (C) CD99 (+); (D) STAT6 (+); (E) Vimentin (+) (scale bars, 62.5 µm).
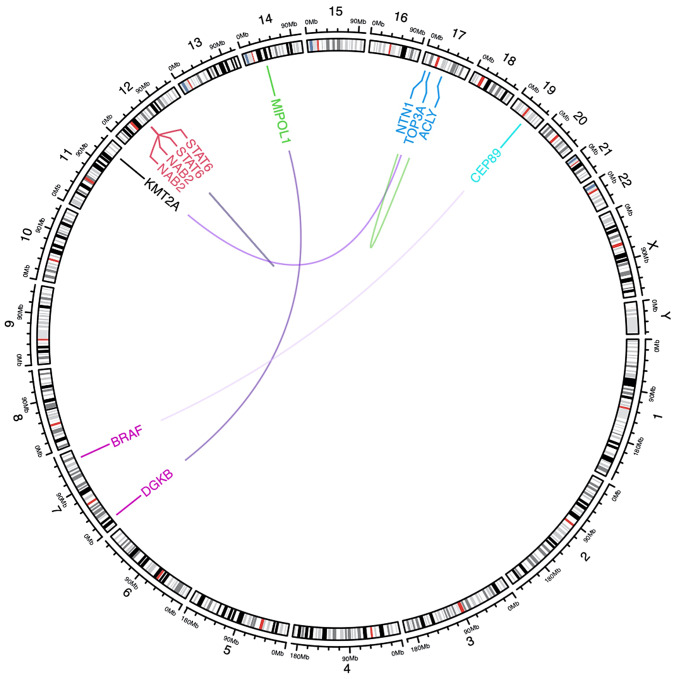
Ki-67 10% suggested an unfavorable prognosis (11). The samples were also examined at Shanghai KR Pharmtech, Inc., Ltd. by high-throughput sequencing technology [using transcriptome sequencing technology (RNA-seq) based on high-throughput sequencing platform (next-generation sequencing), the transcriptome sequences in FFPE samples were comprehensively detected and fusion genes were screened. Sequencing platform: Illumina NovaSeq 6000] to detect the fusion genes. A total of 6 candidate fusion genes were detected in this assay, including NAB2-STAT6 and STAT6-NAB2 fusion genes (Fig. 5 and Table I). The NAB2-STAT6 fusion gene is widely considered a mutation-specific to SFT (12).
Figure 5.
Schematic diagram of gene mapping for gene fusion.
Table I.
Details of the 6 candidate fusion genes detected in this assay.
| Fusion name | Junction read count | 5′Gene | 3′Gene | 5′Gene_BreakPoint_Pos | 3′Gene_BreakPoint_Pos |
|---|---|---|---|---|---|
| NAB2-STAT6 | 32 | NAB2 | STAT6 | chr12:57093598:+ | chr12:57099440:- |
| STAT6-NAB2 | 8 | STAT6 | NAB2 | chr12:57099996:- | chr12:57094612:+ |
| TOP3A-KMT2A | 2 | TOP3A | KMT2A | chr17:18297733:- | chr11:118452490:+ |
| DGKB-MIPOL1 | 1 | DGKB | MIPOL1 | chr7:14283512:- | chr14:37297763:+ |
| CEP89-BRAF | 1 | CEP89 | BRAF | chr19:32877977:- | chr7:140855836:- |
| NTN1-ACLY | 1 | NTN1 | ACLY | chr17:9114030:+ | chr17:41890556:- |
Fusion name, name of the fusion gene, using ‘-’ to separate the two genes at both ends of the breakpoint; junction read count, the number of read segments containing fused breakpoints; 5′Gene, the name of the gene at the 5′ end of the fusion breakpoint; 3′Gene, the name of the gene at the 3′ end of the fusion breakpoint; 5‘Breakpoint/3’Breakpoint, coordinates of the original position of the breakpoint in the genome; Chr, chromosome; pos, position.
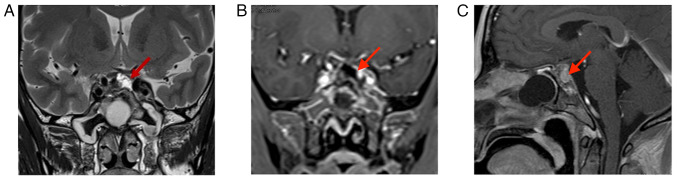
The patient recovered well following surgery without complications. Visual acuity and the visual field condition improved compared with those noted previously. Following surgery, it was explained to the patient that the tumor was recurrent and had a risk of metastasis and routine postoperative radiotherapy was recommended; however, the family refused immediate radiation therapy. At 3 months following surgery, the patient experienced photophobia in the left eye and MRI suggested tumor recurrence in the left cavernous sinus, in the absence of tumor growth in the pituitary fossa (Fig. 6). Therefore, the patient was treated with proton therapy at Shanghai Proton Heavy Ion Hospital (Shanghai, China). The recurrent residual foci were treated with 66 Gray equivalent (GyE)/30 fractions (Fx) and the tumor bed, surgical bed and high-risk area were treated with 54 GyE/30Fx. The process was satisfactory and no radiation therapy-related toxic side effects were observed. At 3 months following the proton therapy, the tumor had slightly receded without any growth (Fig. 7). The patient is being continuously followed up with three-month intervals.
Figure 6.
MRI at 4 months after the second surgery. (A) T1-MRI and (B) enhanced T1-MRI suggest recurrence of the tumor in the left cavernous sinus (red arrows indicate lesion areas). T1-MRI, T1-weighted MRI.
Figure 7.
MRI at three months after proton therapy. (A) T1-MRI; (B) enhanced T1-MRI suggesting the tumor shrank slightly without growth (red arrows indicate lesion areas). T1-MRI, T1-weighted MRI.
Discussion
SFTs are uncommon spindle-cell neoplasms that typically develop in the pleural cavity (13). However, SFTs have been also gradually identified in other parts of the body, such as the meninges (14), retroperitoneum (15), parotid gland (16), salivary gland (17) and soft palate (18). SFTs are usually found in the middle-aged population, with seemingly no differences between genders (19). Intracranial SFTs usually indicate a distinct dural origin and the sites of onset are more commonly in the dura mater, such as the convexity of the brain, the sagittal sinus, the middle fossa of the skull, the falx cerebri and the cerebellar curtain; therefore, they are easily misdiagnosed as meningiomas (20). Solitary non-dural based fibrous tumors in the brain parenchyma are rare. SFTs account for 1% of all primary CNS tumors (21) and SFT located in the saddle or suprasaddle area is even less common.
The clinical features of SFT in the saddle area may be summarized as follows: In a similar way to other saddle area tumors, these tumors often compress the pituitary gland and pituitary stalk, resulting in clinical manifestations, such as headache and dizziness, visual field impairment and hormonal disturbances. In the present case report, although the tumor occupied the pituitary fossa, the patient exhibited a lack of pituitary hormone abnormalities and only developed blurred vision on the left side.
During the initial visit of the patient to a different hospital, she exhibited MRI T1 enhancement and absence of meningeal tail sign. Intraoperatively, the tumor and cavernous sinus were bleeding and partial resection of the tumor was performed. Following 3 months, the patient's MRI T1 enhancement indicated that the tumor was significantly enlarged and had invaded the cavernous sinuses on both sides. The clinical manifestations of SFT in the specific saddle area have not been fully studied and in the clinic, it is easily misdiagnosed by imaging alone, rendering preoperative diagnosis more difficult (22). It has been suggested that the MRI of intracranial grade II and grade III SFT exhibit specific characteristics and the tumor indicates an apparent irregular morphology with multiple nodules of different sizes on the surface, similar to the ‘gathered soap bubble’ sign; a higher the grade is associated with more irregular the morphology, more peripheral lobulation and more significant nodule-like changes (23).
In the present study, the imaging characteristics of the patient were not specific regarding the aforementioned evidence and were even consistent with a pituitary tumor presentation, leading to the initial misdiagnosis. Features such as vascular flow-void signal, narrow basal attachment and non-forming meningeal tail sign are of interest in the differential diagnosis (24). He et al (25) used whole-tumor histogram analysis of apparent diffusion coefficient (ADC) maps to distinguish SFT from angiomatous meningioma and mentioned that the minimum ADC (MinADC) value was significantly negatively correlated with the cell proliferation index and may reflect the most proliferative region of the tumor cells. Conventional MRI combined with MinADC is an optimal indicator of grade II and grade III SFT, which may aid the development of a more complete surgical plan prior to surgery.
However, clear pathological and immunohistochemical findings remain the gold standard for diagnosing intracranial SFTs (7). Microscopic histology of SFTs has indicated that their tissues exhibit a proliferation of spindle cells with a variety of growth patterns. SFTs are immunoreactive for Bcl-2 with a positive expression rate of 80–100%. CD99 is also highly expressed in certain cases of SFT, with a positive expression rate of 75–100% (13). In the present case report, the postoperative pathology of the patient indicated typical ‘staghorn-like’ blood vessels and collagen fibroma cells could be observed around the vessels in a wheel-like or concentric circle arrangement, forming a dense or sparse area of cells. According to immunohistochemical analysis, the tumor cells were positive for Bcl-2, CD99, STAT6 and vimentin. Ki-67 levels were indicative of the patient's unfavorable prognosis (11). Berghoff et al (26) reported that SFT in the CNS featured the 12q13 inversion and a fusion of the NAB2 and STAT6 genes. The patient of the present study was also further diagnosed by genetic testing. Currently, no particular antibodies or specific biomarkers are established for diagnosing this kind of tumor. Postoperative pathological examination and immunohistochemistry may be recommended to acquire a definite diagnosis.
Complete surgical resection is the treatment of choice, and total resection or enlarged resection (as much as possible) to reduce the chances of recurrence is the main purpose of treatment for this disease. Due to the tumor's rich blood supply, intraoperative bleeding is likely to occur, particularly for intra-saddle HPC; this leads to a significantly increased risk of bleeding, since the tumor invades the cavernous sinus. Therefore, endoscopic surgical treatment is suggested, since endoscopy has the advantages of a wide field of view, exact hemostasis and optimal tumor resection. The patient was treated with transnasal microscopy during the initial treatment, and only partial resection was performed due to additional bleeding from the left cavernous sinus.
Mena et al (27) indicated that postoperative radiotherapy significantly improved patient survival. Certain studies have reported that chemotherapy may prolong the survival time of patients; however, additional evidence is required to confirm this hypothesis (28).
In the present case report, the patient exhibited a recurrence in the cavernous sinus 3 months later, despite two surgeries and satisfactory resection on postoperative MRI, which indicated a rapid growth rate. It was suspected that the tumor had invaded the cavernous sinus wall and the surgery removed only the pituitary fossa tumor; the tumor cells attached to the cavernous sinus could not be surgically removed. However, the association of the tumor recurrence in a short period of time with the rich blood supply in the cavernous sinus requires further verification. Therefore, early radiation therapy for this tumor in the saddle area is recommended. However, the patient refused to accept radiotherapy immediately after the surgery, consequently resulting in the recurrence of the tumor.
Proton therapy is a type of radiation therapy that uses beams of protons rather than X-rays to treat cancer, unlike traditional radiotherapy. The therapeutic potential of the depth-dose properties of protons was initially reported by Wilson (29) in 1946. Following this study, the first patient was treated with protons in 1954, employing the synchrocyclotron at the University of California, Berkley (USA) (30).
Since then, with the rapid development of accelerator technology, a higher number of patients with malignant tumors are selecting proton therapy. In addition to the advantage of the physics of dose distribution, proton therapy has been used for >60 years due to its low toxicity and the fact that the total dose of radiation to the body is much less than that of photon radiotherapy (10). Proton therapy may deliver higher radiation doses at smaller doses compared with conventional radiotherapy treated with the same factions, which improves the effectiveness of treatment while reducing side effects and complications during treatment (31). Proton therapy significantly reduces the irradiation dose to the normal tissue surrounding the tumor compared with photon therapy, which results in a lower likelihood of normal tissue damage in patients with long-surviving tumors, and in particular, a lower likelihood of inducing a second primary tumor. However, proton therapy still faces significant challenges, mainly in the following areas: a) The cost of proton therapy is high due to limited equipment resources, high equipment cost, complex technology and long construction periods; b) the uncertainty of the proton beam range and of the relative biological effects have not been fully resolved (32); c) the current clinical evidence is relatively insufficient. Although proton therapy has been used for nearly 80 years, only 250,000 oncology patients worldwide have received proton therapy; d) to date, a limited number of randomized controlled trials have been reported, confirming that the physical advantages of protons can be successfully translated into clinical advantages (31).
The effective use of proton therapy and the fact that it is an expensive and scarce medical resource that may be used to benefit the patients who need it most, is a key clinical concern. Currently, no clear and uniform methods are available to select patients suitable for proton therapy, i.e., patient selection is often individualized, and more commonly used methods are based on normal tissue complication probability models, cost-effective models, dosimetric comparisons, the use of multidisciplinary consultation or the respect for the patient's decision on the type of therapy they prefer to receive. In the future, it may be possible to combine tumor markers and genetic information to select patients suitable for proton therapy. It also may be possible to combine tumor markers and genetic information for patient selection; proton therapy, as part of the treatment strategy, can be combined with chemotherapy, immunotherapy and surgery to form an improved combined treatment plan.
In the present case report, the patient was treated with proton therapy following a recurrence of the tumor. The patient selected proton beam therapy based on the consideration that it is less damaging than gamma knife or other radiation treatments. To the best of our knowledge, this patient was the first case of intracranial SFT to be treated with proton therapy. The saddle area is close to the cavernous sinus region, which contains several nerves and is prone to nerve damage complications following conventional radiotherapy. This patient exhibited no significant side effects following proton therapy, suggesting that this type of therapy is superior to conventional radiotherapy in terms of treatment accuracy. However, due to the small number of cases, the effectiveness of the specific advantages of proton therapy remains to be verified.
In conclusion, SFT is a rare type of neurological tumor that is poorly understood and is easily misdiagnosed as meningioma in the clinic. Diagnosis must be confirmed by routine postoperative pathology, immunohistochemistry and fusion gene testing. Due to its low prevalence and the inadequate identification of SFT, the preoperative misdiagnosis rate is high, which affects the surgical strategy. In addition, intraoperative bleeding increases the difficulty of surgery. Neuroendoscopy has advantages over the microscope, including a wide field of view, exact hemostasis and optimal tumor resection effect. The disease is prone to recurrence and metastasis. For high-grade SFT, postoperative radiation therapy is highly recommended to prolong the survival time of the patients and reduce the recurrence rate. Proton therapy can deliver higher radiation doses at smaller doses compared with conventional radiotherapy treatment with the same fractions, thus improving the effectiveness of treatment while reducing side effects and complications during treatment (31). Proton therapy appears to have unique advantages in the treatment of this recurrence-prone brain tumor.
Acknowledgements
Not applicable.
Glossary
Abbreviations
- SFT
solitary fibrous tumor
- CNS
central nervous system
- Bcl-2
B-cell lymphoma 2
- STAT6
signal transducer and activator of transcription 6
- HPC
hemangiopericytomas
- MRI
magnetic resonance imaging
Funding Statement
This work was supported by grants from the SuzhouScience and Technology Development Plan (grant nos. sys2020181, sky2021054 and KJXW2022033).
Availability of data and materials
All data generated or analyzed during this study are included in this published article.
Authors' contributions
MM, ZD and YG contributed to the conception of the study. JQ, JW and MM performed the surgery. YG, XT and XH significantly contributed to manuscript preparation, helped collect all data of the patient's proton therapy, and analyzed the figures, including MRI and immunohistochemistry images. MM, JW, ZD and PD helped collect and process the surgical specimens and were involved in constructive discussions. MM and ZD confirm the authenticity of all the raw data. All authors have read and approved the final manuscript.
Ethics approval and consent to participate
The case report was approved by the ethics committee of the Affiliated Suzhou Hospital of Nanjing Medical University (Suzhou, China) and was reported with the consent of the patient according to the tenets of the Declaration of Helsinki.
Patient consent for publication
All images and test results in this case report are published with the consent of the patient and the patient's family. The patient provided written consent for the publication of this manuscript.
Competing interests
The authors declare that they have no competing interests.
References
- 1.Carneiro SS, Scheithauer BW, Nascimento AG, Hirose T, Davis DH. Solitary fibrous tumor of the meninges: A lesion distinct from fibrous meningioma. A clinicopathologic and immunohistochemical study. Am J Clin Pathol. 1996;106:217–224. doi: 10.1093/ajcp/106.2.217. [DOI] [PubMed] [Google Scholar]
- 2.Begg CF, Garret R. Hemangiopericytoma occurring in the meninges: Case report. Cancer. 1954;7:602–606. doi: 10.1002/1097-0142(195405)7:3<602::AID-CNCR2820070319>3.0.CO;2-A. [DOI] [PubMed] [Google Scholar]
- 3.Gengler C, Guillou L. Solitary fibrous tumour and haemangiopericytoma: Evolution of a concept. Histopathology. 2006;48:63–74. doi: 10.1111/j.1365-2559.2005.02290.x. [DOI] [PubMed] [Google Scholar]
- 4.Louis DN, Perry A, Reifenberger G, von Deimling A, Figarella-Branger D, Cavenee WK, Ohgaki H, Wiestler OD, Kleihues P, Ellison DW. The 2016 World Health Organization classification of tumors of the central nervous system: A summary. Acta Neuropathol. 2016;131:803–820. doi: 10.1007/s00401-016-1545-1. [DOI] [PubMed] [Google Scholar]
- 5.Lin Q, Zhu J, Zhang X. Solitary fibrous tumor of the central nervous system invading and penetrating the skull: A case report. Oncol Lett. 2023;25:81. doi: 10.3892/ol.2023.13667. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Louis DN, Perry A, Wesseling P, Brat DJ, Cree IA, Figarella-Branger D, Hawkins C, Ng HK, Pfister SM, Reifenberger G, et al. The 2021 WHO classification of tumors of the central nervous system: A summary. Neuro Oncol. 2021;23:1231–1251. doi: 10.1093/neuonc/noab106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Li Q, Zhang C, Li Z. Delayed pulmonary metastasis and recurrence of intracranial malignant solitary fibrous tumor/hemangiopericytoma: Case report and literature review. Oncol Lett. 2022;24:255. doi: 10.3892/ol.2022.13375. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Thapa S, Fujio S, Kitazono I, Yonenaga M, Masuda K, Kuroki S, Bajagain M, Yatsushiro K, Yoshimoto K. Solitary fibrous tumor or hemangiopericytoma of the sella in an older patient treated with partial removal followed by fractionated gamma knife radiosurgery. NMC Case Rep J. 2021;8:697–703. doi: 10.2176/nmccrj.cr.2021-0103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Yang X, Jiang Q, Yu B. Solitary fibrous tumor located in the sella turcica: A report of two cases and review of the literature. Oncol Lett. 2015;10:354–358. doi: 10.3892/ol.2015.3162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Jones B, McMahon SJ, Prise KM. The radiobiology of proton therapy: Challenges and opportunities around relative biological effectiveness. Clin Oncol (R Coll Radiol) 2018;30:285–292. doi: 10.1016/j.clon.2018.01.010. [DOI] [PubMed] [Google Scholar]
- 11.Yamamoto Y, Hayashi Y, Murakami I. Recurrence of solitary fibrous tumor/hemangiopericytoma could be predicted by Ki-67 regardless of its origin. Acta Med Okayama. 2020;74:335–343. doi: 10.18926/AMO/60372. [DOI] [PubMed] [Google Scholar]
- 12.Olson NJ, Linos K. Dedifferentiated solitary fibrous tumor: A concise review. Arch Pathol Lab Med. 2018;142:761–766. doi: 10.5858/arpa.2016-0570-RS. [DOI] [PubMed] [Google Scholar]
- 13.Wang ZY, Qiu K, Ma YH, Wang XT, Bao JJ, Zhang ZF, Liu XZ. Intracranial solitary fibrous tumors: A report of two cases and a review of the literature. Oncol Lett. 2015;11:1057–1060. doi: 10.3892/ol.2015.3985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Yan H, Luo K, Liu B, Kang J. A solitary fibrous tumor with concurrent meningioma at the same site: A case report and review of the literature. Oncol Lett. 2016;11:3655–3659. doi: 10.3892/ol.2016.4486. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Liu L, Chen S, Wang L. Retroperitoneal malignant solitary fibrous tumor with second recurrence and lymphatic metastases: A case report. Oncol Lett. 2022;25:57. doi: 10.3892/ol.2022.13643. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Chen D, Xuan J, Sun M, Guan H. Lipomatous hemangiopericytoma (adipocytic variant of solitary fibrous tumor) of the parotid gland: A case report and review of the literature. Oncol Lett. 2013;6:1380–1382. doi: 10.3892/ol.2013.1538. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Zhou Y, Zheng J, Zhu Q, Xia W, Bhagat SK. Solitary fibrous tumor of the salivary gland: A case report. Oncol Lett. 2016;11:901–903. doi: 10.3892/ol.2015.3948. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Li XM, Yu JQ, Xu GH. Solitary fibrous tumor of the soft palate: A report of two cases. Oncol Lett. 2014;7:1975–1977. doi: 10.3892/ol.2014.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Ge W, Yu DC, Chen G, Ding YT. Clinical analysis of 47 cases of solitary fibrous tumor. Oncol Lett. 2016;12:2475–2480. doi: 10.3892/ol.2016.4967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Yip CM, Hsu SS, Liao WC, Liu SH, Lin YS, Hsu YH, Hsu HI, Cheng YW, Wu YL. Intracranial solitary fibrous tumor/hemangiopericytoma-a case series. Surg Neurol Int. 2020;11:414. doi: 10.25259/SNI_490_2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Jalali R, Srinivas C, Nadkarni TD, Rajasekharan P. Suprasellar haemangiopericytoma-challenges in diagnosis and treatment. Acta Neurochir (Wien) 2008;150:67–71. doi: 10.1007/s00701-007-1474-9. [DOI] [PubMed] [Google Scholar]
- 22.Metellus P, Bouvier C, Guyotat J, Fuentes S, Jouvet A, Vasiljevic A, Giorgi R, Dufour H, Grisoli F, Figarella-Branger D. Solitary fibrous tumors of the central nervous system: Clinicopathological and therapeutic considerations of 18 cases. Neurosurgery. 2007;60:715–722. doi: 10.1227/01.NEU.0000255418.93678.AD. [DOI] [PubMed] [Google Scholar]
- 23.Nakasu S, Nakajima M, Matsumura K, Nakasu Y, Handa J. Meningioma: Proliferating potential and clinicoradiological features. Neurosurgery. 1995;37:1049–1055. doi: 10.1227/00006123-199512000-00003. [DOI] [PubMed] [Google Scholar]
- 24.Yang BT, Song ZL, Wang YZ, Dong JY, Wang ZC. Solitary fibrous tumor of the sinonasal cavity: CT and MR imaging findings. AJNR Am J Neuroradiol. 2013;34:1248–1251. doi: 10.3174/ajnr.A3485. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.He W, Xiao X, Li X, Guo Y, Guo L, Liu X, Xu Y, Zhou J, Wu Y. Whole-tumor histogram analysis of apparent diffusion coefficient in differentiating intracranial solitary fibrous tumor/hemangiopericytoma from angiomatous meningioma. Eur J Radiol. 2019;112:186–191. doi: 10.1016/j.ejrad.2019.01.023. [DOI] [PubMed] [Google Scholar]
- 26.Berghoff AS, Kresl P, Bienkowski M, Koelsche C, Rajky U, Hainfellner JA, Preusser M. Validation of nuclear STAT6 immunostaining as a diagnostic marker of meningeal solitary fibrous tumor (SFT)/hemangiopericytoma. Clin Neuropathol. 2017;36:56–59. doi: 10.5414/NP300993. [DOI] [PubMed] [Google Scholar]
- 27.Mena H, Ribas JL, Pezeshkpour GH, Cowan DN, Parisi JE. Hemangiopericytoma of the central nervous system: A review of 94 cases. Hum Pathol. 1991;22:84–91. doi: 10.1016/0046-8177(91)90067-Y. [DOI] [PubMed] [Google Scholar]
- 28.Lottin M, Escande A, Peyre M, Sevestre H, Maurage CA, Chauffert B, Penel N. What's new in the management of meningeal solitary fibrous tumor/hemangiopericytoma? Bull Cancer. 2020;107:1260–1273. doi: 10.1016/j.bulcan.2020.09.011. (In French) [DOI] [PubMed] [Google Scholar]
- 29.Wilson RR. Radiological use of fast protons. Radiology. 1946;47:487–491. doi: 10.1148/47.5.487. [DOI] [PubMed] [Google Scholar]
- 30.Lawrence JH, Tobias CA, Born JL, Mccombs RK, Roberts JE, Anger HO, Low-Beer BV, Huggins CB. Pituitary irradiation with high-energy proton beams: A preliminary report. Cancer Res. 1958;18:121–134. [PubMed] [Google Scholar]
- 31.Mohan R, Grosshans D. Proton therapy-present and future. Adv Drug Deliv Rev. 2017;109:26–44. doi: 10.1016/j.addr.2016.11.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Paganetti H. Relative biological effectiveness (RBE) values for proton beam therapy. Variations as a function of biological endpoint, dose, and linear energy transfer. Phys Med Biol. 2014;59:R419–R472. doi: 10.1088/0031-9155/59/22/R419. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
All data generated or analyzed during this study are included in this published article.