Abstract
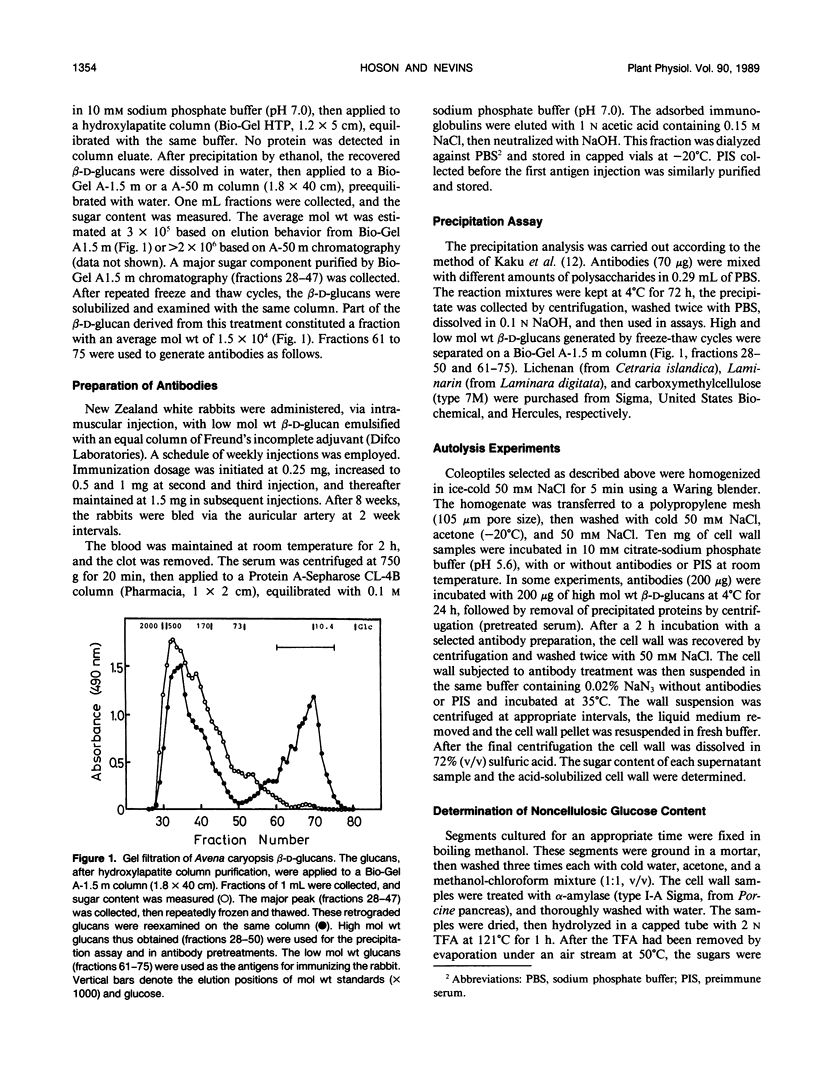
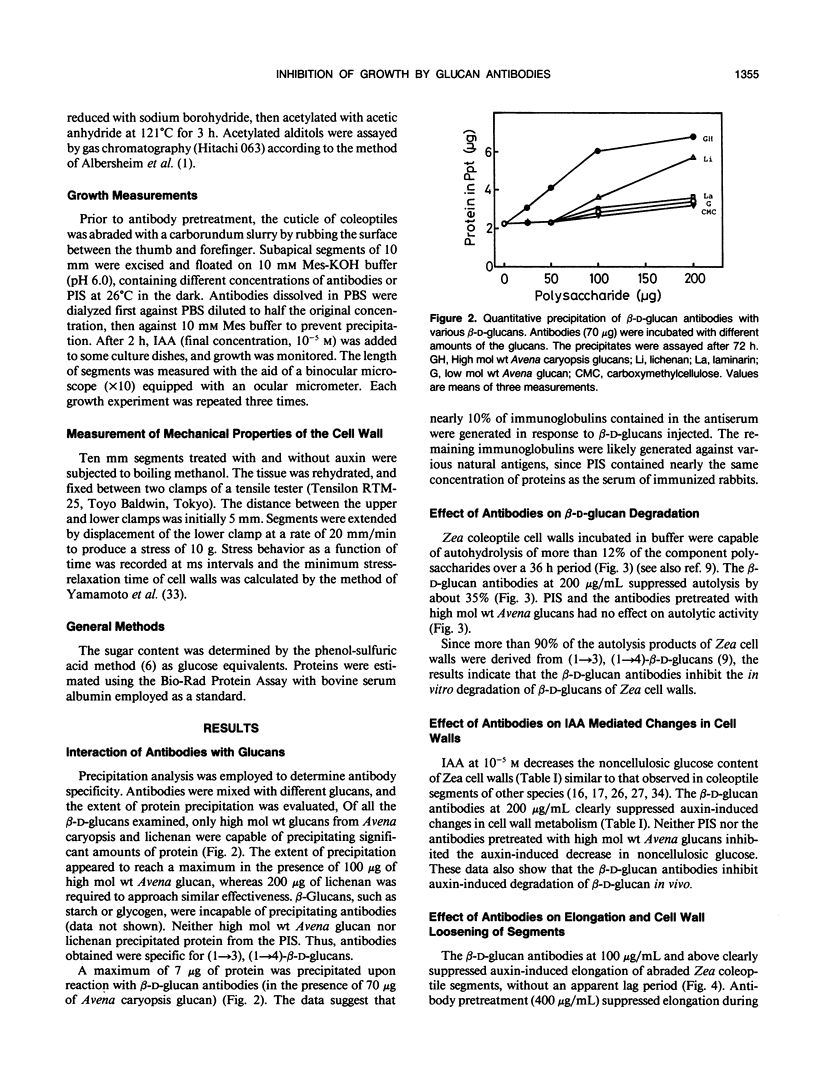
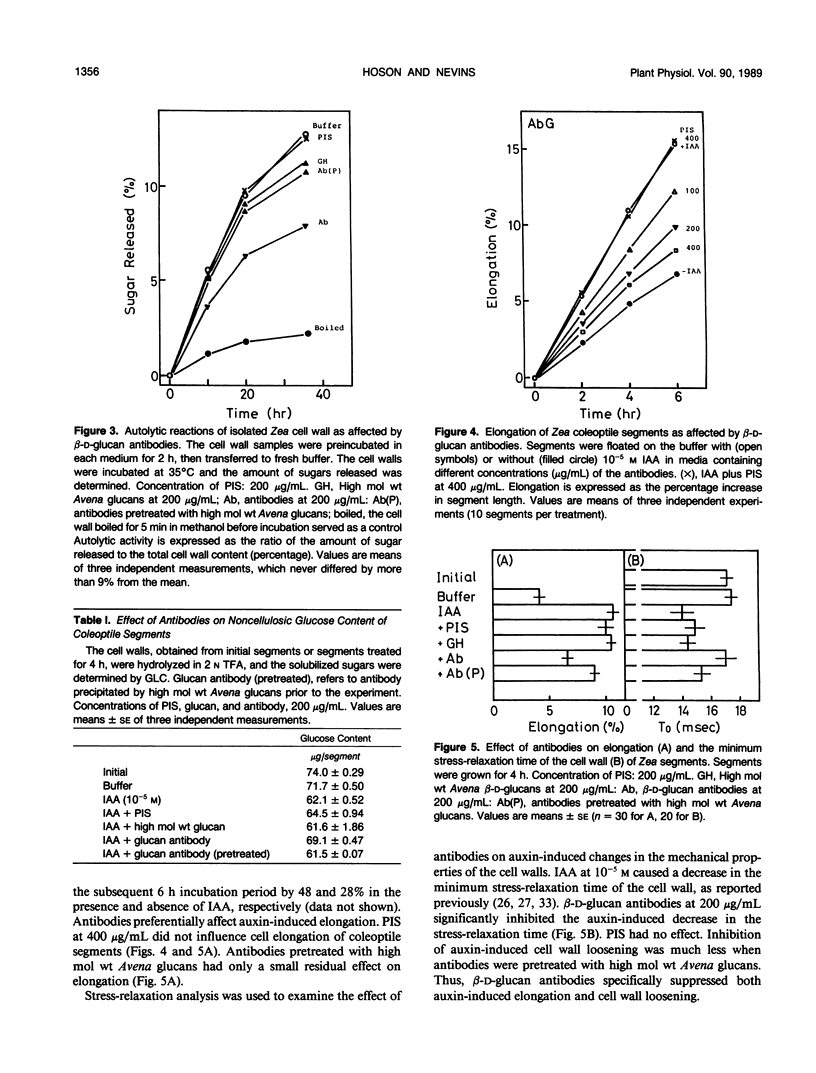
Antiserum was raised against the Avena sativa L. caryopsis β-d-glucan fraction with an average molecular weight of 1.5 × 104. Polyclonal antibodies recovered from the serum after Protein A-Sepharose column chromatography precipitated when cross-reacted with high molecular weight (1→3), (1→4)-β-d-glucans. These antibodies were effective in suppression of cell wall autohydrolytic reactions and auxin-induced decreases in noncellulosic glucose content of the cell wall of maize (Zea mays L.) coleoptiles. The results indicate antibody-mediated interference with in situ β-d-glucan degradation. The antibodies at a concentration of 200 micrograms per milliliter also suppress auxin-induced elongation by about 40% and cell wall loosening (measured by the minimum stress-relaxation time of the segments) of Zea coleoptiles. The suppression of elongation by antibodies was imposed without a lag period. Auxin-induced elongation, cell wall loosening, and chemical changes in the cell walls were near the levels of control tissues when segments were subjected to antibody preparation precipitated by a pretreatment with Avena caryopsis β-d-glucans. These results support the idea that the degradation of (1→3), (1→4)-β-d-glucans by cell wall enzymes is associated with the cell wall loosening responsible for auxin-induced elongation.
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Carpita N. C. Cell wall development in maize coleoptiles. Plant Physiol. 1984 Sep;76(1):205–212. doi: 10.1104/pp.76.1.205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carpita N., Sabularse D., Montezinos D., Delmer D. P. Determination of the pore size of cell walls of living plant cells. Science. 1979 Sep 14;205(4411):1144–1147. doi: 10.1126/science.205.4411.1144. [DOI] [PubMed] [Google Scholar]
- Darvill A. G., McNeil M., Albersheim P. Structure of Plant Cell Walls: VIII. A New Pectic Polysaccharide. Plant Physiol. 1978 Sep;62(3):418–422. doi: 10.1104/pp.62.3.418. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huber D. J., Nevins D. J. beta-d-Glucan Hydrolase Activity in Zea Coleoptile Cell Walls. Plant Physiol. 1980 May;65(5):768–773. doi: 10.1104/pp.65.5.768. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kato Y., Nevins D. J. Enzymic Dissociation of Zea Shoot Cell Wall Polysaccharides : II. Dissociation of (1 --> 3),(1 --> 4)-beta-d-Glucan by Purified (1 --> 3),(1 --> 4)-beta-d-Glucan 4-Glucanohydrolase from Bacillus subtilis. Plant Physiol. 1984 Jul;75(3):745–752. doi: 10.1104/pp.75.3.745. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Loescher W. H., Nevins D. J. Turgor-dependent Changes in Avena Coleoptile Cell Wall Composition. Plant Physiol. 1973 Sep;52(3):248–251. doi: 10.1104/pp.52.3.248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Loescher W., Nevins D. J. Auxin-induced Changes in Avena Coleoptile Cell Wall Composition. Plant Physiol. 1972 Nov;50(5):556–563. doi: 10.1104/pp.50.5.556. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luttenegger D. G., Nevins D. J. Transient Nature of a (1 --> 3), (1 --> 4)-beta-d-Glucan in Zea mays Coleoptile Cell Walls. Plant Physiol. 1985 Jan;77(1):175–178. doi: 10.1104/pp.77.1.175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McNeil M., Darvill A. G., Fry S. C., Albersheim P. Structure and function of the primary cell walls of plants. Annu Rev Biochem. 1984;53:625–663. doi: 10.1146/annurev.bi.53.070184.003205. [DOI] [PubMed] [Google Scholar]
- Moore P. J., Darvill A. G., Albersheim P., Staehelin L. A. Immunogold localization of xyloglucan and rhamnogalacturonan I in the cell walls of suspension-cultured sycamore cells. Plant Physiol. 1986 Nov;82(3):787–794. doi: 10.1104/pp.82.3.787. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nevins D. J., Huber D. J., Yamamoto R., Loescher W. H. beta-d-Glucan of Avena Coleoptile Cell Walls. Plant Physiol. 1977 Oct;60(4):617–621. doi: 10.1104/pp.60.4.617. [DOI] [PMC free article] [PubMed] [Google Scholar]


