Abstract
Investigating links between nervous system function and behavior requires monitoring neuronal activity at a range of spatial and temporal scales. Here, we summarize recent progress in applying two distinct approaches to the study of network dynamics in the neocortex. Mesoscopic calcium imaging allows simultaneous monitoring of activity across most of the cortex at moderate spatiotemporal resolution. Electrophysiological recordings provide extremely high temporal resolution of neural signals at multiple targeted locations. A number of recent studies have used these tools to reveal novel patterns of activity across distributed cortical subnetworks. This growing body of work strongly supports the hypothesis that the dynamic coordination of spatially distinct regions is a fundamental aspect of cortical function that supports cognition and behavior.
Introduction
Cognitive functions, including perception, attention, and memory are critical for the generation of purposeful behavior and are thought to require interactions between neuronal networks distributed across the neocortex. However, robust characterization of coordinated activity at this scale requires measurements with both high spatial accuracy and temporal fidelity applied simultaneously to large areas of the cortex. Most commonly used methods for assessing cortical activity span a subset of possible spatial and temporal scales, meeting some but not all of these requirements (Figure 1). Here we examine recent work using two highly complementary strategies - mesoscale imaging and electrophysiological recordings. These two approaches have generated exciting new insights into modes of communication present across large-scale cortical networks and links between network dynamics and behavior.
Figure 1.
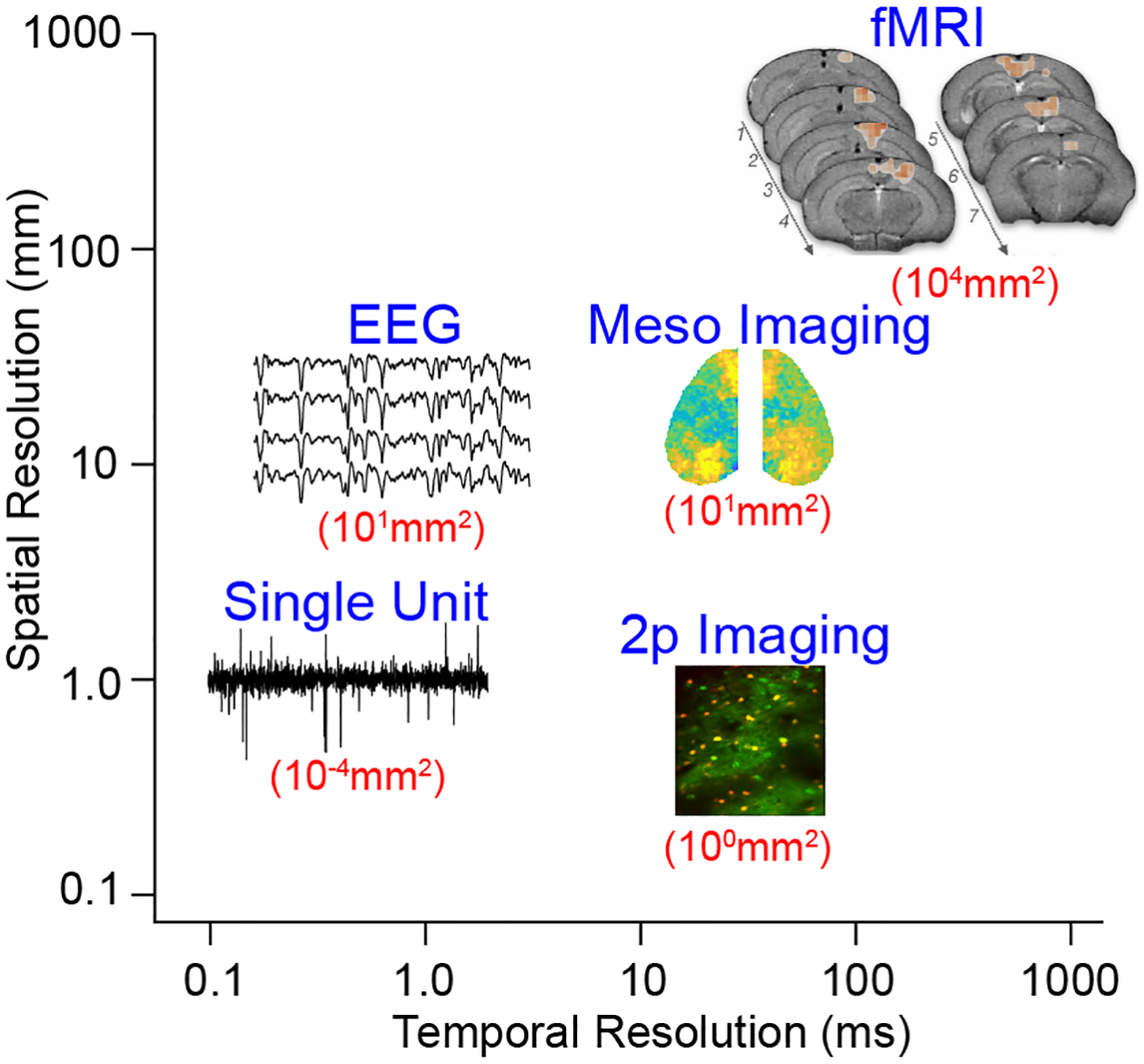
Schematic illustrating the relative spatial and temporal resolution for several methods of monitoring brain dynamics including EEG (electroencephalography), single unit recording, widefield mesoscopic imaging, 2-photon imaging, and fMRI (functional magnetic resonance imaging. Numbers in red indicate approximate spatial scales sampled by each approach.
Spatiotemporal heterogeneity in cortical network dynamics
Over the last twenty years, in vivo fluorescence microscopy has moved from a niche area of research to the dominant method for monitoring neuronal activity, particularly in model systems where genetically encoded indicators are readily applied [1–4]. In particular, protein-based calcium sensors provide a robust though indirect readout of neural firing that is applicable at a range of spatial scales, from single cells to large networks [1,5,6].
In the last few years, widefield “mesoscopic” calcium imaging has been rapidly adopted by several laboratories due to its relative ease of application and ability to reveal novel aspects of functional network organization in awake, behaving animals [7]. Mesoscopic imaging represents a compromise among many desirable features, offering extremely large fields of view (~100 mm2) (Figure 1) with moderately high spatial resolution (single camera pixels correspond to spatially averaged activity of neurons over a few tens of microns) and modest temporal resolution (10–100 ms), ultimately resolving local circuit dynamics across the entire dorsal mouse neocortex [7]. Moreover, the availability of transgenic mouse lines and viral vectors for cell type- and circuit-specific expression of indicators enables even further dissection of functional network architecture [1–3,6,8].
Indeed, the recent development of methods for driving widespread expression of genetically encoded indicators using systemic administration of viral vectors has opened the door to mesoscopic imaging in mice without the need for complex breeding strategies [9–11]**. For example, co-injection of two different high titer serotype 9 adenoassociated viral (AAV) vectors driving expression of the red fluorescent calcium sensor jRCaMP1b [12] and the green fluorescence acetylcholine sensor ACh3.0 [13]* enabled us to carry out simultaneous two-color imaging of these signals across the neocortex of awake, behaving mice [14]**. With this strategy, we found that spontaneous variation in behavioral measures of arousal such as locomotion, whisking, and pupil dilation correspond to heterogeneous variation in the magnitude of cortical activity. Indeed, moderate arousal occurred with elevated cholinergic signaling primarily in frontal regions, while stronger arousal was associated with global elevation in neuronal activity [14]**. This work is consistent with that of other labs showing widespread representation of spontaneous motor signals across the cortex [15,16]. Moreover, arousal is associated with an overall increase in large-scale network correlations in cortical activity, but non-monotonic changes in correlations of cholinergic release [14]**. These results suggest a complex interrelationship between cholinergic modulation and cortical functional connectivity that varies across distinct subregions. Looking forward, the rapid emergence of new genetically encoded reporters for a wide variety of neurotransmitter and neuromodulator signals presents a dizzying opportunity for new discoveries in the coming years [8,17].
Functional parcellation of cortical networks
The ability to readily image activity across the mouse cortex has brought front and center the goal of identifying functional subdivisions that may provide insights into links between neural signaling and behavior. Fundamentally, this is a question of reducing the high dimensionality of data generated by this modality (>200,000 pixels per frame). There is a long history of assigning functional labels to cortical subregions (e.g., visual, somatosensory, association). Such divisions have traditionally derived from cytoarchitectonic and anatomical studies, an approach exemplified by the recent efforts of the Allen Institute to develop the Common Coordinate Framework (CCFv3, [18]). CCFv3 boundaries are easily mapped onto new, functional data sets, providing a standard, uniform parcellation scheme across individual mice and different laboratories and facilitating analysis and comparison of findings across studies.
However, a major drawback to structure-based parcellation is the lack of direct link to dynamic neuronal function. A number of recent studies using mesoscopic calcium imaging in awake, behaving mice have begun to develop strategies for subdividing cortical regions on the basis of activity that are simultaneously driving new understanding of cortical network architecture. For example, Sexena et al (2020) used localized nonnegative matrix factorization to decompose mesoscopic whole-cortex data into functional regions better representative of correlated neural dynamics in the individual animal [19]. However, this approach relied on the Allen CCFv3 as an initial seeding and adhered to corresponding parcel names. In contrast, other approaches have relied strictly on correlations between pixels, using graph-theory-based algorithms to group correlated pixels into functional parcels [20,21]**. These methods provide an entirely data-driven approach to the individual animal, however the resulting parcellations typically do not align well across different individuals, raising challenges to statistical group analyses. Moreover, such functional parcellations do not appear to correspond easily to anatomical-based divisions. Another distinct approach to functional dimensionality reduction was highlighted by MacDowell and Buschman [22]**, who used convolutional nonnegative matrix factorization to identify repeated spatiotemporal motifs. Unlike parcels, these motifs did not tile the cortex, but instead reflected potentially spatially overlapping but temporally dynamic patterns of activity. This method also produces individual, data-driven characterization of cortical networks but does not produce unique labels for discrete structural subdivisions.
Dynamic correlations define cortical subnetworks
Analysis of mesoscopic imaging data has also produced novel insights into cortical network organization that were wholly unpredicted from anatomical divisions. For example, we developed a novel graph-based method to calculate the time-varying changes in pairwise correlations between cortical parcels. Surprisingly, spontaneous fluctuations in motor behaviors were better encoded by these dynamic correlations than by the time-varying changes in parcel activity [21]**. We found that correlation dynamics were organized into two broad anterolateral and posterior subdivisions that did not map onto established anatomical borders [21]**, suggesting distinct organizational principles for functional versus structural data sets. This dissociation was also observed by MacDowell and Buschman (2020), whose identified spatiotemporal motifs in mesoscopic imaging data also correspond poorly to atlas-based parcellation[22]**. Interestingly, we recently combined cortex-wide mesoscopic imaging with simultaneous cellular resolution 2-photon imaging and identified the functional connectivity of single neurons with large-scale networks [9]**. These analyses revealed that neurons were functionally connected with one of two regions that closely corresponded to the same anterolateral and posterior subdivisions derived from large-scale network correlations [9]**.
Carrying out similar imaging studies during task performance also has the potential for revealing network dynamics linked to goal-directed behavior. Mice carrying out a sensory discrimination task exhibit sensory- and strategy-specific activation of distinct regions throughout the cortex [15,23–26]. Overall, these studies reveal the power of mesoscopic imaging to detect coordinated spatial patterns of activity across large-scale networks.
Electrophysiology reveals fast long-range network dynamics
In comparison to mesoscopic imaging, analysis of electrophysiological brain signals offers several complementary advantages for investigating communication across large-scale cortical networks. Electrical recordings provide sub-millisecond resolution, offering a degree of temporal fidelity not possible via commonly used imaging approaches (Figure 1). The most frequently used electrophysiological technique is extracellular recording, where a single electrode site can measure both local field potentials (LFPs), which largely arise from synchronous synaptic events, and spiking, which arises from the action potentials of individual nearby neurons. A common measure of communication between cortical areas is the correlation, or coherence, between the local field potentials in each area in a specific frequency band such as theta (3–8 Hz), beta (16–35 Hz) or gamma (30–80 Hz). Functional connectivity, which may reflect both direct and indirect influences of one area on another, has also been estimated from measures of coherence and Grainger causality [27–29]**. However, recent work has highlighted the complexity of the relationship between connectivity, power, and coherence. Indeed, coherence may arise from inter-areal communication in accordance with connectivity patterns, and changes in coherence may reflect changes in oscillatory power in synaptic inputs from sender to receiver areas rather than a more direct measure of functional connectivity [29]**. These findings underscore the necessity for laminar recordings and current source density (CSD) measurements in addition to more traditional single-site measurements of field potentials and spiking [30].
Simultaneous recordings across multiple cortical areas have highlighted the potentially distinct roles of specific frequencies of neural activity in inter-areal communication [28,31–33]. Interareal coherence in distinct frequency bands of the LFP during task performance may vary across pairs of visual cortical areas according to relative position in the cortical hierarchy [33], suggesting a selective frequency-based mode of interareal communication. Long-range, frequency-specific interactions have likewise been observed between the hippocampus and prefrontal cortex, with theta and beta frequencies associated with errors and correct responses, respectively [34]. Recent work suggests that functional connectivity supported by these distinct LFP frequency bands may also play a role in predictive coding, with predictable and unpredictable stimuli associated with distinct temporal patterns of activity and changes in functional connectivity among higher-order cortical areas [35].
Dynamic interareal communication
Sampling of signals across the cortical hierarchy reveals functional connectivity, as measured by coherence and Grainger causality, across distinct frequency bands among cortical modules. The overall pattern of this functional connectivity may be related to anatomical connectivity [27,33] and exhibits bidirectional interactions among areas that follow known reciprocal anatomical connections. Functional interactions among large-scale cortical networks exhibit selective feedforward and feedback routing of communication via specific subsets of activity patterns. Feedforward and feedback connectivity, as identified by Grainger causality, may occur in distinct frequency bands [27,33] and rely on distinct patterns of population activity on different timescales [36]. However, frequency-specific interactions are unlikely to represent the sole mechanism for interareal communication. Indeed, fluctuations in activity in higher-order visual areas V2 and V4 are not driven by the largest amplitude fluctuations in their afferent, primary visual cortex (V1), but rather are related to a subset of V1 activity patterns that can be described as a low-dimensional communication subspace [37]*.
Fine temporal modulation of coherence or changes in functional connectivity may further organize information transfer among cortical areas. Functional connectivity across large-scale cortical networks may undergo rapid dynamic changes during behavior. Recent work using a spatial attention task found that the frontal eye field and the lateral intraparietal area exhibited evolving functional interactions characterized by two rhythmically alternating states coordinated by theta phase [38]. Computational modeling suggests that even short bursts of patterned activity can selectively route input signals and shape interareal communication [39]. Indeed, frequency-specific transient synchronization between two cortical areas has been associated with trial-by-trial variations in task performance [40,41]*. Short epochs of rhythmic functional interaction may thus enhance information transmission and contribute to perceptual performance.
Local extracellular recording electrodes often provide both local field and spike signals from the same electrical contact site in the brain. In addition to rhythmic coordination of local field potential signals, fine timescale coordination of spiking events is likewise associated with enhanced perceptual performance. Coordinated spiking between neurons in areas along the cortical hierarchy may be predictive of behavioral performance. In recent work, Shahidi et al. (2019) found that coordinated spiking between V1 and V4 neurons increased in a short epoch following stimulus presentation and was predictive of behavioral performance [42]. Frequency-specific and temporally precise relationships in spiking activity between areas may further enhance encoding and transmission of information in a phase-dependent manner [43]. Similarly, highly dynamic patterns of local and long-range coupling between spike timing and oscillatory phase across frontal cortical areas FEF and LIP were associated with different behavioral outcomes in an attention task [41]*.
Embedded cortical subnetworks
Although previous work has largely used single electrodes or large fixed arrays to record field potentials and spiking activity, the development of high-density recording arrays and novel strategies for analysis provide new opportunities to examine fast interactions within and among cortical areas. Recent work using multisite high-density recordings across the mouse brain revealed shared spontaneous movement and state representation across areas with cellular resolution [16,44]. Multiple simultaneous high-density recordings from distinct cortical visual areas in mouse cortex further revealed a cortical network of modules of neurons based on functional connectivity [45]**. One module of neurons embedded within the cortical hierarchy may serve feedforward sensory processing, whereas the other may function in recurrent integration of sensory information [45]**. Together, these findings suggest that subnetworks of functionally connected neurons across areas may participate in distinct motor and sensory computations. There is thus a critical need for further high-density recordings with high spatial and temporal resolution to examine how interactions across embedded subnetworks of neurons supports functional connectivity and interareal communication.
General conclusion
The burgeoning array of methodological approaches suitable for monitoring activity with high spatial and temporal resolution across the cortex is driving rapid progress in our understanding of large-scale coordinated neural activity and its relationship to behavior. Both mesoscopic imaging and electrophysiological recordings provide complementary strengths and weaknesses in this regard. The application of genetically encoded indicators targeting specific subsets of cell types opens up the study of functionally distinct circuits that are physically interspersed throughout cortical networks [46–48]. In parallel, novel electrophysiological probes create opportunities for simultaneous recordings of single neurons communicating across distant areas [45]**.
In addition, recent studies are beginning to combine different recording modalities, effectively merging the strengths of each approach to scale diverse spatial and temporal scales. For example, multiple groups have combined 2-photon imaging or electrophysiological recordings of single neurons with simultaneous mesoscopic imaging to investigate the functional connectivity across cells and networks [9,49,50]**. Indeed, a central open question in cortical physiology is the relative role of local versus long-range synaptic connections in linking activity across distant circuits. In the future, such synergistic approaches are likely to open up critical new avenues of exploration and understanding, promoting further discovery of the neural basis of cognition and behavior.
Acknowledgements
This work was supported by funding from the NIH (DP1-EY033975, MH099045 and MH121841 to MJH, EY022951 to JAC, MH113852 to MJH and JAC, EY026878 to the Yale Vision Core), an award from the Kavli Institute of Neuroscience (to JAC and MJH), a Simons Foundation SFARI Research Grant (to JAC and MJH), a Swebilius Foundation award (to JAC and MJH), a Research Grant from Aligning Science Across Parkinson’s (to MJH), and support from the Ludwig Foundation (to JAC).
Footnotes
Conflict of Interest statement
The authors declare no conflict of interest.
References
- 1.Chen TW, Wardill TJ, Sun Y, Pulver SR, Renninger SL, Baohan A, Schreiter ER, Kerr RA, Orger MB, Jayaraman V, et al. : Ultrasensitive fluorescent proteins for imaging neuronal activity. Nature 2013, 499:295–300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Hochbaum DR, Zhao Y, Farhi SL, Klapoetke N, Werley CA, Kapoor V, Zou P, Kralj JM, Maclaurin D, Smedemark-Margulies N, et al. : All-optical electrophysiology in mammalian neurons using engineered microbial rhodopsins. Nat Methods 2014, 11:825–833. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Lin MZ, Schnitzer MJ: Genetically encoded indicators of neuronal activity. Nat Neurosci 2016, 19:1142–1153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Jin L, Han Z, Platisa J, Wooltorton JR, Cohen LB, Pieribone VA: Single action potentials and subthreshold electrical events imaged in neurons with a fluorescent protein voltage probe. Neuron 2012, 75:779–785. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Higley MJ, Sabatini BL: Calcium signaling in dendrites and spines: practical and functional considerations. Neuron 2008, 59:902–913. [DOI] [PubMed] [Google Scholar]
- 6.Daigle TL, Madisen L, Hage TA, Valley MT, Knoblich U, Larsen RS, Takeno MM, Huang L, Gu H, Larsen R, et al. : A Suite of Transgenic Driver and Reporter Mouse Lines with Enhanced Brain-Cell-Type Targeting and Functionality. Cell 2018, 174:465–480 e422. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Cardin JA, Crair MC, Higley MJ: Mesoscopic Imaging: Shining a Wide Light on Large-Scale Neural Dynamics. Neuron 2020, 108:33–43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Wu Z, Lin D, Li Y: Pushing the frontiers: tools for monitoring neurotransmitters and neuromodulators. Nat Rev Neurosci 2022, 23:257–274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- *9.Barson D, Hamodi AS, Shen X, Lur G, Constable RT, Cardin JA, Crair MC, Higley MJ: Simultaneous mesoscopic and two-photon imaging of neuronal activity in cortical circuits. Nat Methods 2020, 17:107–113. [DOI] [PMC free article] [PubMed] [Google Scholar]; Developed simultaneous cellular and mesoscale imaging technology and identified state-dependent and cell type-specific motifs of functional connectivity between single neurons and large-scale cortical networks.
- 10.Hamodi AS, Martinez Sabino A, Fitzgerald ND, Moschou D, Crair MC: Transverse sinus injections drive robust whole-brain expression of transgenes. Elife 2020, 9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Goertsen D, Flytzanis NC, Goeden N, Chuapoco MR, Cummins A, Chen Y, Fan Y, Zhang Q, Sharma J, Duan Y, et al. : AAV capsid variants with brain-wide transgene expression and decreased liver targeting after intravenous delivery in mouse and marmoset. Nat Neurosci 2022, 25:106–115. [DOI] [PubMed] [Google Scholar]
- 12.Dana H, Mohar B, Sun Y, Narayan S, Gordus A, Hasseman JP, Tsegaye G, Holt GT, Hu A, Walpita D, et al. : Sensitive red protein calcium indicators for imaging neural activity. Elife 2016, 5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- *13.Jing M, Li Y, Zeng J, Huang P, Skirzewski M, Kljakic O, Peng W, Qian T, Tan K, Zou J, et al. : An optimized acetylcholine sensor for monitoring in vivo cholinergic activity. Nat Methods 2020, 17:1139–1146. [DOI] [PMC free article] [PubMed] [Google Scholar]; Developed a novel high signal-to-noise sensor for acetylcholine that is compatible with mesoscale cortical imaging.
- **14.Lohani S, Moberly A, Benisty H, Landa B, Jing M, Li Y, Higley MJ, Cardin JA: Dual color mesoscopic imaging reveals spatiotemporally heterogeneous coordination of cholinergic and neocortical activity. bioRxiv 2020: 10.1101/2020.1112.1109.418632 [DOI] [PMC free article] [PubMed] [Google Scholar]; Demonstrated the potential for simultaneous, dual-color mesoscopic imaging of calcium and acetylcholine in behaving mice and revealed significant spatiotemporal heterogeneity in cholinergic modulation and cortical activity across the neocortex linked to fluctuations in arousal.
- 15.Musall S, Kaufman MT, Juavinett AL, Gluf S, Churchland AK: Single-trial neural dynamics are dominated by richly varied movements. Nat Neurosci 2019, 22:1677–1686. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Stringer C, Pachitariu M, Steinmetz N, Reddy CB, Carandini M, Harris KD: Spontaneous behaviors drive multidimensional, brainwide activity. Science 2019, 364:255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Marvin JS, Scholl B, Wilson DE, Podgorski K, Kazemipour A, Muller JA, Schoch S, Quiroz FJU, Rebola N, Bao H, et al. : Stability, affinity, and chromatic variants of the glutamate sensor iGluSnFR. Nat Methods 2018, 15:936–939. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Wang Q, Ding SL, Li Y, Royall J, Feng D, Lesnar P, Graddis N, Naeemi M, Facer B, Ho A, et al. : The Allen Mouse Brain Common Coordinate Framework: A 3D Reference Atlas. Cell 2020, 181:936–953 e920. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Saxena S, Kinsella I, Musall S, Kim SH, Meszaros J, Thibodeaux DN, Kim C, Cunningham J, Hillman EMC, Churchland A, et al. : Localized semi-nonnegative matrix factorization (LocaNMF) of widefield calcium imaging data. PLoS Comput Biol 2020, 16:e1007791. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Mishne G, Coifman RR, Lavzin M, Schiller J: Automated cellular structure extraction in biological images with applications to calcium imaging data. bioRxiv 2018. [Google Scholar]
- **21.Benisty H, Moberly AH, Lohani S, Barson D, Coifman RR, Mishne G, Cardin JA, Higley MJ: Rapid fluctuations in functional connectivity of cortical networks encode spontaneous behavior. bioRxiv 2021: 10.1101/2021.1108.1115.456390 [DOI] [PMC free article] [PubMed] [Google Scholar]; Developed a novel graph-of-graphs approach to quantifying dynamic correlations in activity across cortical regions, revealed subnetworks defined by functional connectivity not predicted by conventional atlas-based parcellation.
- *22.MacDowell CJ, Buschman TJ: Low-Dimensional Spatiotemporal Dynamics Underlie Cortex-wide Neural Activity. Curr Biol 2020, 30:2665–2680 e2668. [DOI] [PMC free article] [PubMed] [Google Scholar]; Used convolutional non-negative matrix factorization to reveal spatiotemporal motifs in cortical networks reflecting coordinated flow of activity across functionally related areas.
- 23.Gallero-Salas Y, Han S, Sych Y, Voigt FF, Laurenczy B, Gilad A, Helmchen F: Sensory and Behavioral Components of Neocortical Signal Flow in Discrimination Tasks with Short-Term Memory. Neuron 2021, 109:135–148 e136. [DOI] [PubMed] [Google Scholar]
- 24.Gilad A, Gallero-Salas Y, Groos D, Helmchen F: Behavioral Strategy Determines Frontal or Posterior Location of Short-Term Memory in Neocortex. Neuron 2018, 99:814–828 e817. [DOI] [PubMed] [Google Scholar]
- 25.Pinto L, Rajan K, DePasquale B, Thiberge SY, Tank DW, Brody CD: Task-Dependent Changes in the Large-Scale Dynamics and Necessity of Cortical Regions. Neuron 2019, 104:810–824 e819. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Makino H, Ren C, Liu H, Kim AN, Kondapaneni N, Liu X, Kuzum D, Komiyama T: Transformation of Cortex-wide Emergent Properties during Motor Learning. Neuron 2017, 94:880–890 e888. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Vezoli J, Vinck M, Bosman CA, Bastos AM, Lewis CM, Kennedy H, Fries P: Brain rhythms define distinct interaction networks with differential dependence on anatomy. Neuron 2021, 109:3862–3878 e3865. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Gregoriou GG, Gotts SJ, Zhou H, Desimone R: High-frequency, long-range coupling between prefrontal and visual cortex during attention. Science 2009, 324:1207–1210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- **29.Schneider M, Broggini AC, Dann B, Tzanou A, Uran C, Sheshadri S, Scherberger H, Vinck M: A mechanism for inter-areal coherence through communication based on connectivity and oscillatory power. Neuron 2021, 109:4050–4067 e4012. [DOI] [PMC free article] [PubMed] [Google Scholar]; Proposed an alternative mechanism for interareal coherence based on afferent synaptic input rather than spiking entrainment.
- 30.Fernandez-Ruiz A, Oliva A, Soula M, Rocha-Almeida F, Nagy GA, Martin-Vazquez G, Buzsaki G: Gamma rhythm communication between entorhinal cortex and dentate gyrus neuronal assemblies. Science 2021, 372. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Gregoriou GG, Gotts SJ, Zhou H, Desimone R: Long-range neural coupling through synchronization with attention. Prog Brain Res 2009, 176:35–45. [DOI] [PubMed] [Google Scholar]
- 32.Gregoriou GG, Gotts SJ, Desimone R: Cell-type-specific synchronization of neural activity in FEF with V4 during attention. Neuron 2012, 73:581–594. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Bastos AM, Vezoli J, Bosman CA, Schoffelen JM, Oostenveld R, Dowdall JR, De Weerd P, Kennedy H, Fries P: Visual areas exert feedforward and feedback influences through distinct frequency channels. Neuron 2015, 85:390–401. [DOI] [PubMed] [Google Scholar]
- 34.Brincat SL, Miller EK: Frequency-specific hippocampal-prefrontal interactions during associative learning. Nat Neurosci 2015, 18:576–581. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Bastos AM, Lundqvist M, Waite AS, Kopell N, Miller EK: Layer and rhythm specificity for predictive routing. Proc Natl Acad Sci U S A 2020, 117:31459–31469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Semedo JD, Jasper AI, Zandvakili A, Krishna A, Aschner A, Machens CK, Kohn A, Yu BM: Feedforward and feedback interactions between visual cortical areas use different population activity patterns. Nat Commun 2022, 13:1099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Semedo JD, Zandvakili A, Machens CK, Yu BM, Kohn A: Cortical Areas Interact through a Communication Subspace. Neuron 2019, 102:249–259 e244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- *38.Fiebelkorn IC, Pinsk MA, Kastner S: A Dynamic Interplay within the Frontoparietal Network Underlies Rhythmic Spatial Attention. Neuron 2018, 99:842–853 e848. [DOI] [PMC free article] [PubMed] [Google Scholar]; Identified precise spike timing in frontal cortical areas as a key mechanism underlying enhanced sensory processing during focused attention.
- 39.Palmigiano A, Geisel T, Wolf F, Battaglia D: Flexible information routing by transient synchrony. Nat Neurosci 2017, 20:1014–1022. [DOI] [PubMed] [Google Scholar]
- 40.Rohenkohl G, Bosman CA, Fries P: Gamma Synchronization between V1 and V4 Improves Behavioral Performance. Neuron 2018, 100:953–963 e953. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Fiebelkorn IC, Kastner S: Spike Timing in the Attention Network Predicts Behavioral Outcome Prior to Target Selection. Neuron 2021, 109:177–188 e174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Shahidi N, Andrei AR, Hu M, Dragoi V: High-order coordination of cortical spiking activity modulates perceptual accuracy. Nat Neurosci 2019, 22:1148–1158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Voloh B, Oemisch M, Womelsdorf T: Phase of firing coding of learning variables across the fronto-striatal network during feature-based learning. Nat Commun 2020, 11:4669. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Allen WE, Chen MZ, Pichamoorthy N, Tien RH, Pachitariu M, Luo L, Deisseroth K: Thirst regulates motivated behavior through modulation of brainwide neural population dynamics. Science 2019, 364:253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- **45.Jia X, Siegle JH, Durand S, Heller G, Ramirez TK, Koch C, Olsen SR: Multi-regional module-based signal transmission in mouse visual cortex. Neuron 2022. [DOI] [PubMed] [Google Scholar]; Used high-density multisite recordings to identify multiple embedded subnetworks of functionally distinct neurons across the visual cortical hierarchy.
- 46.Matho KS, Huilgol D, Galbavy W, JHe M, Kim G, An X, Lu J, Wu P, Di Bella DJ, Shetty AS, et al. : Genetic dissection of glutamatergic neuron subpopulations and developmental trajectories in the cerebral cortex. bioRxiv. [Google Scholar]
- 47.Tang L, Higley MJ: Layer 5 Circuits in V1 Differentially Control Visuomotor Behavior. Neuron 2020, 105:346–354 e345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Musall S, Sun XR, Mohan H, An X, Gluf S, Drewes R, Osten P, Churchland A: Pyramidal cell types drive functionally distinct cortical activity patterns during decisionmaking. bioRxiv 2021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- *49.Clancy KB, Orsolic I, Mrsic-Flogel TD: Locomotion-dependent remapping of distributed cortical networks. Nat Neurosci 2019. [DOI] [PMC free article] [PubMed] [Google Scholar]; Combined electrophysiology and mesoscale cortical imaging to reveal state-dependent coupling of individual neurons to local and large-scale networks.
- 50.Peters AJ, Fabre JMJ, Steinmetz NA, Harris KD, Carandini M: Striatal activity topographically reflects cortical activity. Nature 2021, 591:420–425. [DOI] [PMC free article] [PubMed] [Google Scholar]


