Abstract
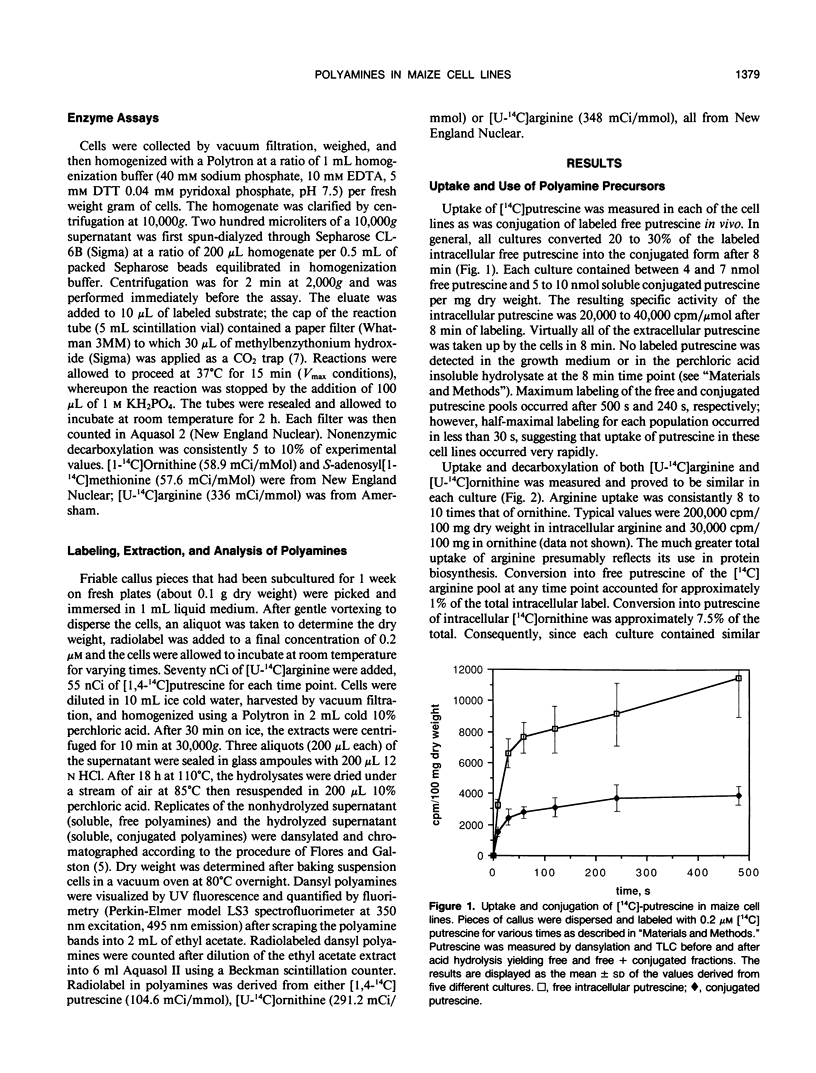
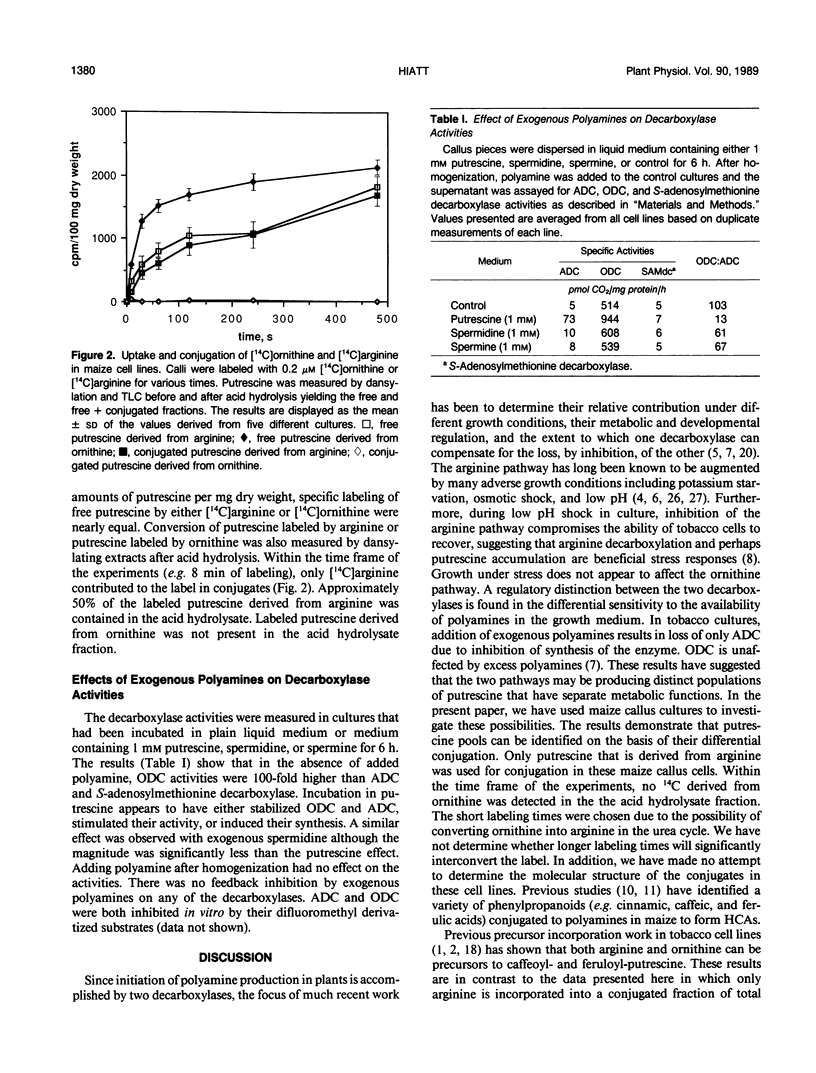
Uptake of [14C]putrescine, [14C]arginine, and [14C]ornithine was measured in five separate callus cell lines of Zea mays. Each precursor was rapidly taken into the intracellular pool in each culture where, on the average, 25 to 50% of the total putrescine was found in a conjugated form, detected after acid hydrolysis. Half-maximal labeling of each culture was achieved in less than 1 minute. Within this time frame of precursor incorporation, only putrescine derived from arginine was conjugated, indicating that putrescine pools derived from arginine may initially be sequestered from ornithine-derived putrescine. The decarboxylase activities were measured in each culture after addition of exogenous polyamine to the growth medium to assess differential regulation of the decarboxylases. Arginine and ornithine decarboxylase activities were augmented by added polyamine, the effect on arginine decarboxylase being eightfold greater than on ornithine decarboxylase. Levels of extractable ornithine decarboxylase were consistently 15- to 100-fold higher than arginine decarboxylase, depending on the titer of extracellular polyamine. Taken as whole the results support the idea that there are distinct populations of polyamine that are initially sequestered after the decarboxylase reactions and that give rise to separate end products and possibly have separate functions.
Full text
PDF

Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Cohen E., Arad S. M., Heimer Y. M., Mizrahi Y. Participation of ornithine decarboxylase in early stages of tomato fruit development. Plant Physiol. 1982 Aug;70(2):540–543. doi: 10.1104/pp.70.2.540. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Flores H. E., Galston A. W. Osmotic stress-induced polyamine accumulation in cereal leaves : I. Physiological parameters of the response. Plant Physiol. 1984 May;75(1):102–109. doi: 10.1104/pp.75.1.102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Flores H. E., Galston A. W. Polyamines and plant stress: activation of putrescine biosynthesis by osmotic shock. Science. 1982 Sep 24;217(4566):1259–1261. doi: 10.1126/science.217.4566.1259. [DOI] [PubMed] [Google Scholar]
- Hiatt A. C., McIndoo J., Malmberg R. L. Regulation of polyamine biosynthesis in tobacco. Effects of inhibitors and exogenous polyamines on arginine decarboxylase, ornithine decarboxylase, and S-adenosylmethionine decarboxylase. J Biol Chem. 1986 Jan 25;261(3):1293–1298. [PubMed] [Google Scholar]
- Hiatt A., Malmberg R. L. Utilization of putrescine in tobacco cell lines resistant to inhibitors of polyamine synthesis. Plant Physiol. 1988 Feb;86(2):441–446. doi: 10.1104/pp.86.2.441. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Malmberg R. L., McIndoo J., Hiatt A. C., Lowe B. A. Genetics of polyamine synthesis in tobacco: developmental switches in the flower. Cold Spring Harb Symp Quant Biol. 1985;50:475–482. doi: 10.1101/sqb.1985.050.01.059. [DOI] [PubMed] [Google Scholar]
- RICHARDS F. J., COLEMAN R. G. Occurrence of putrescine in potassium-deficient barley. Nature. 1952 Sep 13;170(4324):460–460. doi: 10.1038/170460a0. [DOI] [PubMed] [Google Scholar]
- Slocum R. D., Galston A. W. In vivo inhibition of polyamine biosynthesis and growth in tobacco ovary tissues. Plant Cell Physiol. 1985;26(8):1519–1526. [PubMed] [Google Scholar]
- Slocum R. D., Kaur-Sawhney R., Galston A. W. The physiology and biochemistry of polyamines in plants. Arch Biochem Biophys. 1984 Dec;235(2):283–303. doi: 10.1016/0003-9861(84)90201-7. [DOI] [PubMed] [Google Scholar]
- Srivenugopal K. S., Adiga P. R. Enzymic conversion of agmatine to putrescine in Lathyrus sativus seedlings. Purification and properties of a multifunctional enzyme (putrescine synthase). J Biol Chem. 1981 Sep 25;256(18):9532–9541. [PubMed] [Google Scholar]
- Tiburcio A. F., Ingersoll R., Galston A. W. Modified alkaloid pattern in developing tobacco callus. Plant Sci. 1985;38:207–212. doi: 10.1016/0168-9452(85)90040-8. [DOI] [PubMed] [Google Scholar]
- Tiburcio A. F., Kaur-Sawhney R., Ingersoll R. B., Galston A. W. Correlation between polyamines and pyrrolidine alkaloids in developing tobacco callus. Plant Physiol. 1985;78:323–326. doi: 10.1104/pp.78.2.323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Young N. D., Galston A. W. Are polyamines transported in etiolated peas? Plant Physiol. 1983 Dec;73(4):912–914. doi: 10.1104/pp.73.4.912. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Young N. D., Galston A. W. Physiological control of arginine decarboxylase activity in k-deficient oat shoots. Plant Physiol. 1984 Oct;76(2):331–335. doi: 10.1104/pp.76.2.331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Young N. D., Galston A. W. Putrescine and Acid Stress : Induction of Arginine Decarboxylase Activity and Putrescine Accumulation by Low pH. Plant Physiol. 1983 Apr;71(4):767–771. doi: 10.1104/pp.71.4.767. [DOI] [PMC free article] [PubMed] [Google Scholar]


