Abstract
A method for isolating guard cell protoplasts (GCP) from mechanically prepared epidermis of Vicia faba is described. Epidermis was prepared by homogenizing leaves in a Waring blender in a solution of 10% Ficoll, 5 millimolar CaCl2, and 0.1% polyvinylpyrrolidone 40 (PVP). Attached mesophyll and epidermal cells were removed by shaking epidermis in a solution of Cellulysin, mannitol, CaCl2, PVP, and pepstatin A. Cleaned epidermis was transferred to a solution of mannitol, CaCl2, PVP, pepstatin A, cellulase “Onozuka” RS, and pectolyase Y-23 for the isolation of GCP. Preparations made by this method included both adaxial and abaxial GCP and contained ≤0.017% mesophyll protoplasts, ≤0.6% mesophyll fragments, and no epidermal cell contaminants. Yields averaged 9 × 104 protoplasts/leaflet and 98 to 100% of the GCP excluded trypan blue, concentrated neutral red, and hydrolyzed fluorescein diacetate. Isolated GCP increased in diameter by 2.2 micrometers after incubation in darkness in 10 micromolar fusicoccin, 0.4 molar mannitol, 5 millimolar KCl, and 1 millimolar CaCl2. Illumination of GCP with 800 micromoles per square meter per second of red light resulted in alkalinization of their suspension medium. When 10 micromolar per square meter per second of blue light was superimposed onto the red light background, the medium acidified. Measurements of chlorophyll a fast fluorescence transients from isolated GCP indicated that GCP were capable of electron transport, and slow transients contained the “M” peak usually associated with a functional photosynthetic carbon reduction pathway.
Full text
PDF

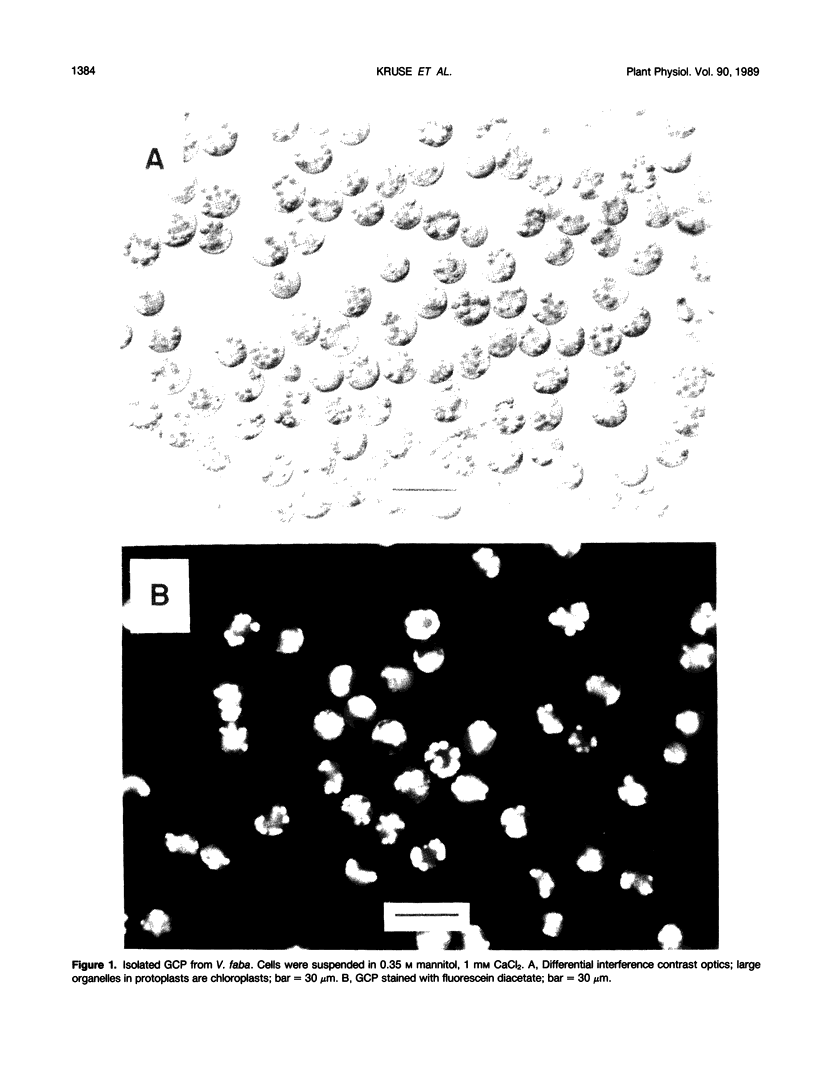
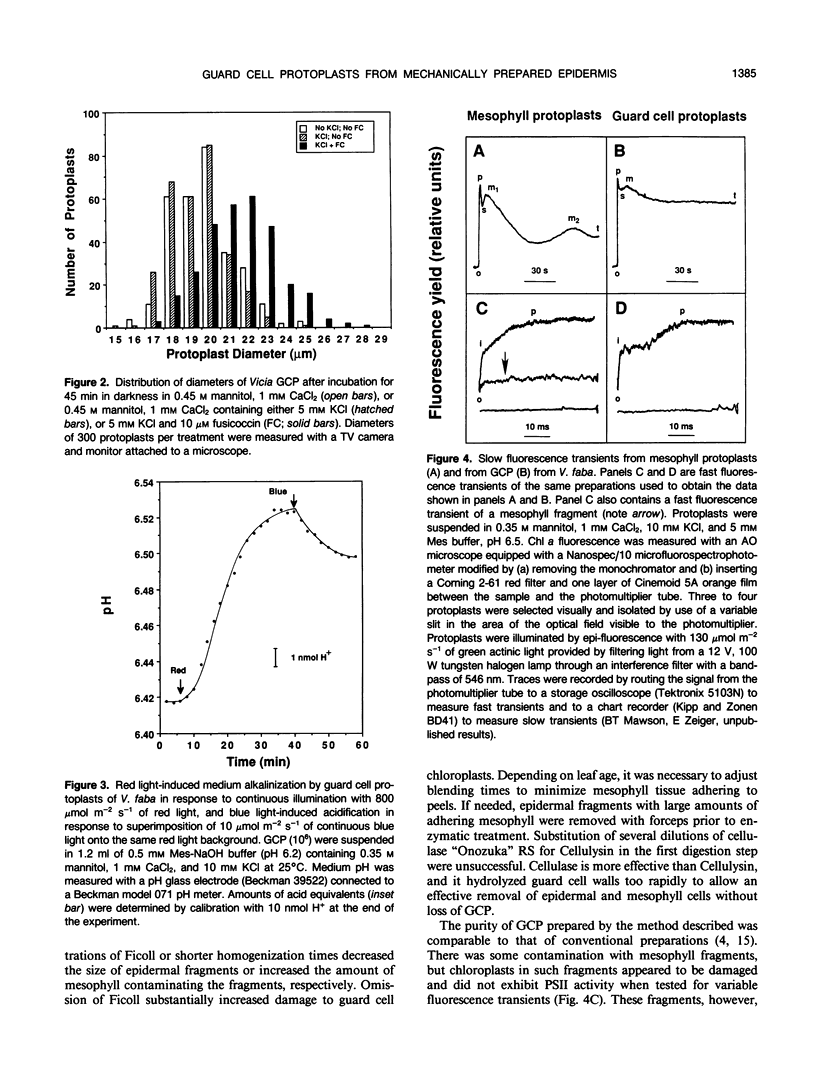

Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Arnon D. I. COPPER ENZYMES IN ISOLATED CHLOROPLASTS. POLYPHENOLOXIDASE IN BETA VULGARIS. Plant Physiol. 1949 Jan;24(1):1–15. doi: 10.1104/pp.24.1.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brown P. H., Outlaw W. H. Effect of Fusicoccin on Dark CO(2) Fixation by Vicia faba Guard Cell Protoplasts. Plant Physiol. 1982 Dec;70(6):1700–1703. doi: 10.1104/pp.70.6.1700. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gotow K., Taylor S., Zeiger E. Photosynthetic Carbon Fixation in Guard Cell Protoplasts of Vicia faba L. : Evidence from Radiolabel Experiments. Plant Physiol. 1988 Mar;86(3):700–705. doi: 10.1104/pp.86.3.700. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Outlaw W. H., Mayne B. C., Zenger V. E., Manchester J. Presence of Both Photosystems in Guard Cells of Vicia faba L: IMPLICATIONS FOR ENVIRONMENTAL SIGNAL PROCESSING. Plant Physiol. 1981 Jan;67(1):12–16. doi: 10.1104/pp.67.1.12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rogers C. A. Relationship of Temperature to Stomatal Aperture and Potassium Accumulation in Guard Cells of Vicia faba. Plant Physiol. 1979 Feb;63(2):388–391. doi: 10.1104/pp.63.2.388. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schroeder J. I., Raschke K., Neher E. Voltage dependence of K channels in guard-cell protoplasts. Proc Natl Acad Sci U S A. 1987 Jun;84(12):4108–4112. doi: 10.1073/pnas.84.12.4108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Serrano E. E., Zeiger E., Hagiwara S. Red light stimulates an electrogenic proton pump in Vicia guard cell protoplasts. Proc Natl Acad Sci U S A. 1988 Jan;85(2):436–440. doi: 10.1073/pnas.85.2.436. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shimazaki K., Zeiger E. Red Light-Dependent CO(2) Uptake and Oxygen Evolution in Guard Cell Protoplasts of Vicia faba L.: Evidence for Photosynthetic CO(2) Fixation. Plant Physiol. 1987 May;84(1):7–9. doi: 10.1104/pp.84.1.7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tallman G., Zeiger E. Light quality and osmoregulation in vicia guard cells : evidence for involvement of three metabolic pathways. Plant Physiol. 1988 Nov;88(3):887–895. doi: 10.1104/pp.88.3.887. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Widholm J. M. The use of fluorescein diacetate and phenosafranine for determining viability of cultured plant cells. Stain Technol. 1972 Jul;47(4):189–194. doi: 10.3109/10520297209116483. [DOI] [PubMed] [Google Scholar]
- Zeiger E., Armond P., Melis A. Fluorescence Properties of Guard Cell Chloroplasts: EVIDENCE FOR LINEAR ELECTRON TRANSPORT AND LIGHT-HARVESTING PIGMENTS OF PHOTOSYSTEMS I AND II. Plant Physiol. 1981 Jan;67(1):17–20. doi: 10.1104/pp.67.1.17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zeiger E., Hepler P. K. Light and stomatal function: blue light stimulates swelling of guard cell protoplasts. Science. 1977 May 20;196(4292):887–889. doi: 10.1126/science.196.4292.887. [DOI] [PubMed] [Google Scholar]



