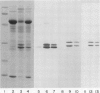
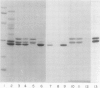
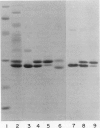
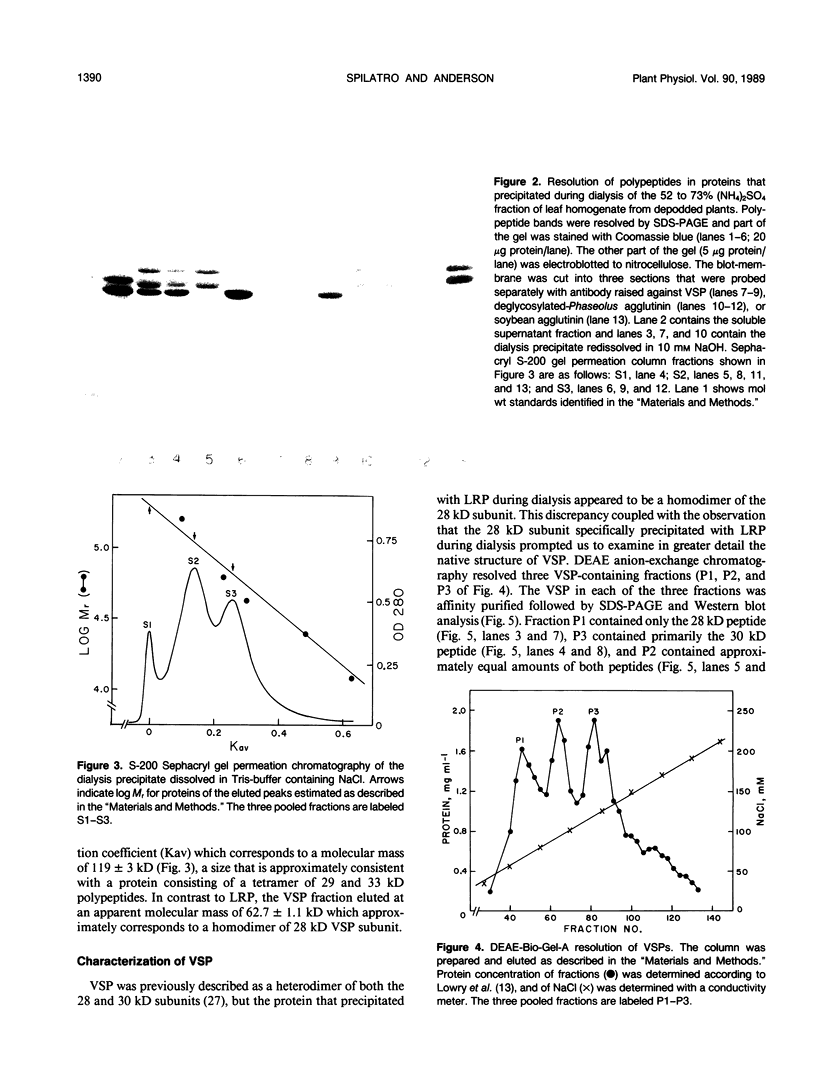
Abstract
Levels of several polypeptides in addition to the vegetative storage protein (VSP) increase in soybean leaves following depodding. Two of these polypeptides interact specifically with antibodies raised against the seed lectins of Phaseolus vulgaris and soybean. The two polypeptides, which had apparent molecular masses of 29,000 daltons and 33,000 daltons, were present in the sink-deprived plants but not in control podded plants and were the subunit polypeptides of a glycoprotein designated lectin-related protein (LRP). Soybean LRP was purified to near homogeneity by a combination of ammonium sulfate precipitation and gel filtration. Dialysis of the resuspended ammonium sulfate precipitate caused LRP to reprecipitate, and LRP was soluble only in the presence of molar NaCl. The native relative molecular mass of LRP was 119,000 daltons, a size consistent with a tetrameric organization of the two polypeptides. LRP precipitated during dialysis in association with a 28,000 dalton polypeptide. The protein coprecipitating with LRP was identified as the dimer of the 28,000 dalton subunit of VSP, one of three native isomeric forms of VSP occurring in leaves of depodded plants. Although the specific association between LRP and VSP was intriguing, an in vivo interaction between LRP and VSP was doubtful. LRP was shown to be immunologically similar to soybean agglutinin but did not have detectable hemagglutinating activity. LRP also was shown to be made up of polypeptides distinct from soybean agglutinin.
Full text
PDF





Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Basha S. M., Roberts R. M. The Glycoproteins of Plant Seeds : ANALYSIS BY TWO-DIMENSIONAL POLYACRYLAMIDE GEL ELECTROPHORESIS AND BY THEIR LECTIN-BINDING PROPERTIES. Plant Physiol. 1981 May;67(5):936–939. doi: 10.1104/pp.67.5.936. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cleveland D. W., Fischer S. G., Kirschner M. W., Laemmli U. K. Peptide mapping by limited proteolysis in sodium dodecyl sulfate and analysis by gel electrophoresis. J Biol Chem. 1977 Feb 10;252(3):1102–1106. [PubMed] [Google Scholar]
- Edge A. S., Faltynek C. R., Hof L., Reichert L. E., Jr, Weber P. Deglycosylation of glycoproteins by trifluoromethanesulfonic acid. Anal Biochem. 1981 Nov 15;118(1):131–137. doi: 10.1016/0003-2697(81)90168-8. [DOI] [PubMed] [Google Scholar]
- Franceschi V. R., Wittenbach V. A., Giaquinta R. T. Paraveinal Mesophyll of Soybean Leaves in Relation to Assimilate Transfer and Compartmentation : III. Immunohistochemical Localization of Specific Glycopeptides in the Vacuole after Depodding. Plant Physiol. 1983 Jun;72(2):586–589. doi: 10.1104/pp.72.2.586. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hankins C. N., Kindinger J. I., Shannon L. M. The Lectins of Sophora japonica: II. Purification, Properties, and N-Terminal Amino Acid Sequences of Five Lectins from Bark. Plant Physiol. 1988 Jan;86(1):67–70. doi: 10.1104/pp.86.1.67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hankins C. N., Kindinger J., Shannon L. M. The Lectins of Sophora japonica: I. Purification Properties and N-Terminal Amino Acid Sequences of Two Lectins from Leaves. Plant Physiol. 1987 Apr;83(4):825–829. doi: 10.1104/pp.83.4.825. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- Maliarik M., Roberts D., Goldstein I. J. Lectin from hog peanut, Amphicarpaea bracteata. Methods Enzymol. 1987;138:561–564. doi: 10.1016/0076-6879(87)38051-6. [DOI] [PubMed] [Google Scholar]
- Nsimba-Lubaki M., Peumans W. J. Seasonal Fluctuations of Lectins in Barks of Elderberry (Sambucus nigra) and Black Locust (Robinia pseudoacacia). Plant Physiol. 1986 Mar;80(3):747–751. doi: 10.1104/pp.80.3.747. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pueppke S. G., Bauer W. D. Role of Lectins in Plant-Microorganism Interactions: II. Distribution of Soybean Lectin in Tissues of Glycine max (L.) Merr. Plant Physiol. 1978 May;61(5):779–784. doi: 10.1104/pp.61.5.779. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pueppke S. G. Examination of Le and lele Genotypes of Glycine max (L.) Merr. for Membrane-Bound and Buffer-Soluble Soybean Lectin. Plant Physiol. 1981 Oct;68(4):905–909. doi: 10.1104/pp.68.4.905. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith D. E., Fisher P. A. Identification, developmental regulation, and response to heat shock of two antigenically related forms of a major nuclear envelope protein in Drosophila embryos: application of an improved method for affinity purification of antibodies using polypeptides immobilized on nitrocellulose blots. J Cell Biol. 1984 Jul;99(1 Pt 1):20–28. doi: 10.1083/jcb.99.1.20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spadoro-Tank J. P., Etzler M. E. Heath Shock Enhances the Synthesis of a Lectin-Related Protein in Dolichos biflorus Cell Suspension Cultures. Plant Physiol. 1988 Dec;88(4):1131–1135. doi: 10.1104/pp.88.4.1131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spilatro S. R., Anderson J. M. Carbohydrate Metabolism and Activity of Pyrophosphate: Fructose-6-Phosphate Phosphotransferase in Photosynthetic Soybean (Glycine max, Merr.) Suspension Cells. Plant Physiol. 1988 Nov;88(3):862–868. doi: 10.1104/pp.88.3.862. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Staswick P. E. Developmental regulation and the influence of plant sinks on vegetative storage protein gene expression in soybean leaves. Plant Physiol. 1989 Jan;89(1):309–315. doi: 10.1104/pp.89.1.309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Staswick P. E. Soybean vegetative storage protein structure and gene expression. Plant Physiol. 1988 May;87(1):250–254. doi: 10.1104/pp.87.1.250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Talbot C. F., Etzler M. E. Isolation and characterization of a protein from leaves and stems of Dolichos biflorus that cross reacts with antibodies to the seed lectin. Biochemistry. 1978 Apr 18;17(8):1474–1479. doi: 10.1021/bi00601a018. [DOI] [PubMed] [Google Scholar]
- Vodkin L. O., Raikhel N. V. Soybean lectin and related proteins in seeds and roots of le and le soybean varieties. Plant Physiol. 1986 Jun;81(2):558–565. doi: 10.1104/pp.81.2.558. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wittenbach V. A. Effect of pod removal on leaf photosynthesis and soluble protein composition of field-grown soybeans. Plant Physiol. 1983 Sep;73(1):121–124. doi: 10.1104/pp.73.1.121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wittenbach V. A. Purification and characterization of a soybean leaf storage glycoprotein. Plant Physiol. 1983 Sep;73(1):125–129. doi: 10.1104/pp.73.1.125. [DOI] [PMC free article] [PubMed] [Google Scholar]