Abstract
Objective
There is no standard tool for assessing risk of bias (RoB) in prevalence studies. For the purposes of a living systematic review during the COVID-19 pandemic, we developed a tool to evaluate RoB in studies measuring the prevalence of mental health disorders (RoB-PrevMH) and tested inter-rater reliability.
Methods
We decided on items and signalling questions to include in RoB-PrevMH through iterative discussions. We tested the reliability of assessments by different users with two sets of prevalence studies. The first set included a random sample of 50 studies from our living systematic review. The second set included 33 studies from a systematic review of the prevalence of post-traumatic stress disorders, major depression and generalised anxiety disorder. We assessed the inter-rater agreement by calculating the proportion of agreement and Kappa statistic for each item.
Results
RoB-PrevMH consists of three items that address selection bias and information bias. Introductory and signalling questions guide the application of the tool to the review question. The inter-rater agreement for the three items was 83%, 90% and 93%. The weighted kappa scores were 0.63 (95% CI 0.54 to 0.73), 0.71 (95% CI 0.67 to 0.85) and 0.32 (95% CI −0.04 to 0.63), respectively.
Conclusions
RoB-PrevMH is a brief, user-friendly and adaptable tool for assessing RoB in studies on prevalence of mental health disorders. Initial results for inter-rater agreement were fair to substantial. The tool’s validity, reliability and applicability should be assessed in future projects.
Background
Studies of prevalence provide essential information for estimating the burden of mental health conditions, which can inform research and policy-making.1 The pandemic of COVID-19, a disease first described in 2020,2 rapidly generated a large volume of literature,3 about studies on the prevalence of a wide range of conditions, including those related to mental health. Increased levels of anxiety, depression, psychological distress, as well as an increase in violent behaviour, alcohol and substance use, among others have been described in association with fear of infection and the effects of contamination measures.1 4 Temporary relief from obligations at school or work, or the need to commute, on the other hand, might alleviate stress for some populations.1
A systematic review provides a structured way to gather, assess and synthesise evidence from prevalence studies. One essential step in performing a systematic review is the assessment of risk of bias (RoB) of the included studies5 because the potential biases affect how certain we are about the included evidence and its interpretation.6 There is no agreement on how to assess RoB in prevalence studies,7 despite a 10-fold increase in systematic reviews of prevalence studies in the last decade.7 8 Substantial variability exists in how RoB in prevalence studies have been assessed with more than 30 tools identified and several judged to be inappropriate.9 Notably, some questions/items in existing tools focus on the quality of reporting which makes not possible to assess the biases present in prevalence studies.
To overcome the shortcomings of previous tools, such as distinguishing between RoB and quality of reporting and being adaptable to different questions, the purpose of this paper is to present a RoB tool developed to evaluate RoB in studies measuring the prevalence of mental health disorders (RoB-PrevMH). We describe the steps for developing this tool, its items, and the results of inter-rater agreement obtained by applying the tool to two sets of prevalence studies on mental health disorders.
Methods
RoB-PrevMH was developed within the MHCOVID project (https://mhcovid.ispm.unibe.ch/), a living systematic review assessing the effect of the COVID-19 pandemic and the containment measures on mental health of the population.1 4 10 MHCOVID involves many volunteers recruited through crowdsourcing to help with data extraction and RoB assessment of a large volume of literature (referred to as the MHCOVID Crowd). We prioritised brevity and ease of application in developing the tool, owning to the different backgrounds and levels of experience and expertise of MHCOVID Crowd members in the assessment of RoB.
Development of the tool
We searched Medline and Embase (Ovid) from inception to September 2020 to identify published tools or checklists designed to assess the quality, RoB, and quality of reporting in prevalence studies (online supplemental appendix 1). In addition, we searched the Equator network website (https://www.equator-network.org/) and a database of systematic reviews of prevalence studies.11 One researcher (DBG) screened the search results to identify relevant tools that assessed RoB in prevalence studies.
bmjment-2023-300694supp001.pdf (9.2KB, pdf)
We extracted the items from each tool selected for inclusion and grouped them under the domains of selection bias and information bias. For selection bias, items from the existing tools were separated into those referring to population representativeness or to ‘the proportion of respondents’. For information bias, items from the existing tools were separated into those referring to observer bias, recall bias or misclassification bias. Items not related to the named biases were tagged as ‘other bias’ or ‘reporting’.
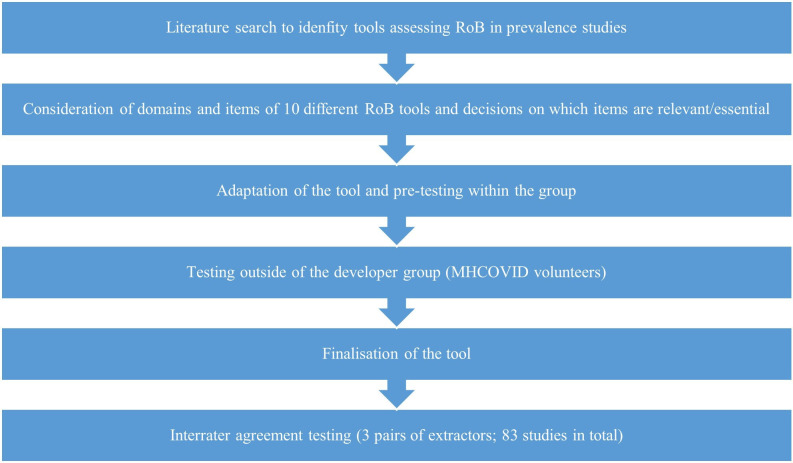
Five researchers (DBG, NL, NLP, GS and TT) individually went through the list of questions in each included tool, excluded duplicated questions, and marked those that were most relevant for prevalence studies for mental health disorders. They then discussed their assessments and reached consensus prior to drafting the first version of the tool and the signalling questions. Figure 1 illustrates the process of developing RoB-PrevMH.
Figure 1.
Process of developing and testing RoB-PrevMH.
Testing and finalisation of the tool
Four members of the team (SL, NLP, GS and TT) pilot tested the first version of the tool and drafted a guidance document. Subsequently, these four researchers and four volunteers from the MHCOVID Crowd (who were not involved in the development of the tool) further tested the tool in a total of eight studies. Based on feedback from this exercise, the guidance document was updated accordingly, including examples and practical advice.
Inter-rater reliability
We tested the reliability of assessments by different users of RoB-PrevMH with two sets of prevalence studies. The first set included 50 prevalence studies (two sets of 25) randomly selected from those identified as potentially relevant for the MHCOVID project during the abstract screening stage. Two pairs of researchers independently applied RoB-PrevMH (team A, 25 studies: CMV and TT; team B 25 studies: DBG and NLP). The second set included 33 studies from a systematic review of the prevalence of post-traumatic stress disorders, major depressive disorder and generalised anxiety disorder in migrants with premigration exposure to armed conflict.12 By using this second set of studies, we examined how RoB-PrevMH performed in a research question that was different from the one it was originally developed for. Two researchers (team C: DBG and CMV) independently applied RoB-PrevMH in this set of studies.
To assess reproducibility, we calculated the unweighted and weighted kappa statistic (with 95% CI). For weighted kappa, the observed and expected proportions of agreement are modified to measure the agreement among the ordered levels of bias (low, unclear, high) by assigning a weight of 0 to complete disagreement (rating low vs high RoB), 1 to perfect agreement and 0.5 for partial disagreement (ratings low vs unclear or high vs unclear).13 14 We also calculated the percentage of agreement between raters (number of agreements/number of assessments x 100). The analysis was conducted in STATA V.15.115 . We followed the interpretation of the kappa statistic proposed by Landis and Koch (1977) and described in the STATA manual where the values below the cut points 0.00, 0.20, 0.40, 0.60, 0.80 and 1.00 approximately define poor, slight, fair, moderate substantial and almost perfect agreement.16
Results
Description of RoB-PrevMH tool
We identified 10 tools that assess RoB in prevalence studies, summarised in table 1.13–22 Following the process mentioned above, we developed RoB-PrevMH which consists of one introductory question and three items (table 2). It also includes signalling questions aimed to help the user reach a judgement; after completing our study we improved and refined the questions associated with two items and these are presented in table 2 alongside the original questions. The elaboration and guidance document is presented in online supplemental appendix 2. RoB for each item can be judged as ‘high’, ‘low’ or ‘unclear’. We instructed users to avoid judging any of the questions as unclear, whenever possible. This recommendation was based on the guidelines to assess the risk bias for Systematic Reviews on Interventions, which states that ‘unclear’ should be only used when the information about the domain is truly unknown.23 The tool does not allow a summary RoB assessment because some aspects of study quality might be more important than others, making aggregated scores problematic.24 25
Table 1.
RoB tools considered for developing RoB-PrevMH
| ID | Tool | Description | No of items/questions | Validation process |
| 1 | Leboeuf-Yde and Lauritsen17 1995 | A tool designed to assess the quality of prevalence studies on low back pain. | Eleven methodological criteria | Not reported |
| 2 | Loney et al 18 1998 | A critical appraisal tool designed to assess the methodological strengths, results and relevance of articles on prevalence of a health problem. | Eight items with a scoring system | Consensus between two assessors |
| 3 | Boyle19 1998 | A guideline to critically appraise prevalence studies on psychiatric disorders, both in the general population and in specific clinical settings. | Evaluates three main items divided in 11 questions | Not reported |
| 4 | Silva et al 20 2001 | A tool to assess the usefulness of prevalence studies in the context of surveillance activities. | Covers six technical aspects divided in 19 questions with a scoring system | Consensus for the scoring system |
| 5 | Shamliyan et al 13 2010 | A tool for evaluating the quality of studies that examine the prevalence of chronic conditions or risk factors. | Six criteria for external validity and five for internal validity | The tool was tested in four studies of incidence or prevalence. Kappa values showed fair agreement. |
| 6 | Hoy et al 14 2012 | A risk of bias tool for prevalence studies based on Leboeuf-Yde and Lauritsen17 1995. |
Ten items plus a summary assessment. | Overall inter-rater agreement=91% Kappa=0.82 (95% CI 0.76 to 0.86) |
| 7 | Giannakopoulos et al 15 2012 | An instrument for the qualitative assessment of the methodology of prevalence studies. | Ten items with a scoring system | Pilot phase Kappa for the quality score=mean 0.62±0.15 Kappa for individual questions=mean 0.78±0.27 After feedback Kappa for the quality score=range 0.94–1.00 |
| 8 | Munn et al 16 2014 | A critical appraisal tool for assessing studies included in systematic reviews of prevalence. | Ten questions | 5-point Likert scale (one very unacceptable, 5 very acceptable) Ease of tool use=mean 3.63±0.72 Acceptability=mean 4.33±0.49 Timeliness=mean 3.94±0.57 |
| 9 | The Joanna Briggs Institute21 2016 | A tool to assess the methodological quality of a prevalence study and the possibility of bias. | Nine questions with an overall appraisal question. | Not reported |
| 10 | Pega et al 22 2019 | A tool for assessing the risk of bias in prevalence studies of exposure to occupational risk factors. | Eight domains | Using a raw measure of agreement, the tool achieved substantial agreement in six domains (conflict of interest, other bias, lack of blinding of study personnel, exposure misclassification, selective reporting of exposures) and poor agreement in two domains (incomplete exposure data, selection of participants into the study). |
Table 2.
Items included in RoB-PrevMH, suggested rephrasing and guidance
| Item | Question | Elaboration |
| Domain: selection bias | ||
| 1. Representativeness of the sampling frame | Was the sample invited to participate in the study a true or close representation of the target population? | This question is about how well the people invited to participate in the study match the target population in demographic or clinical characteristics that are believed to be associated with the measured condition. |
| 2. Representativeness of the responders | How would you rate the risk of non-response bias? | This question is about the characteristics and assumed presence of the condition in people who were invited but did not respond in a way that enabled the investigators to measure the condition of interest (not necessarily providing complete data). |
| Suggested rephrasing: Was the sample that provided data a true or close representation the sample invited to participate? | ||
| Domain: information bias | ||
| 3. Measurement of the condition | How do you judge the risk of information bias? | This question is about the appropriateness and reliability of the instrument or method used to measure the condition among people who provided the relevant data. Bias might occur when the training of observers of the outcome was not done or the procedures to collect the data are not the same for every participant or every timepoint included in the study. Bias might also occur when questions refer to the past and their answering depends on the condition. |
| Suggested rephrasing: Was the condition measured/detected in an unbiased and reproducible way for all participants? Additional specific questions depending on the context: Was the tool used to measure the condition validated? Were the methods for measuring the condition standardised? Does the measurement of the condition depend on the memory of the participants? | ||
Each of the three items can be given a judgement high, low or unclear.
*This was a requirement for inclusion in the MHCOVID study and hence not included in the current version of the tool.4
bmjment-2023-300694supp002.pdf (176.5KB, pdf)
The introductory question is ‘Was the target population clearly defined?’ By ‘target population’ we refer to the entire population for which we are interested to draw inference. In the first set of studies from the MHCOVID project, the target population of the systematic review was defined as ‘the general population’ or any age or gender-based subgroups of the general population (eg, children only, or men only, or elderly, see online supplemental appendix 2). In the second set of studies, the target population of the systematic review was migrants exposed to armed conflict.25
This introductory question had two response options; ‘yes’ or ‘no’ and has implications for the evaluation of the first RoB item: if the answer is ‘no’, the first item of the tool is automatically assigned an ‘unclear’ risk.
Item 1 selection bias: representativeness of the sampling frame
The first RoB item is related to the representativeness of the sample invited with respect to the target population by asking ‘Was the sample invited to participate in the study a true or close representation of the target population?’ The signalling question for this item asked about the method for recruitment of participants and, based on the response, the instructions guided the user to reach the corresponding RoB judgement (eg, low risk when the total or a randomly selected sample of the target population was invited; high risk for open calls for participation online or quota sampling; and unclear risk when the method to invite participants and the specific context of the sampling was not specified or when the target population was not defined; for more details, see the instructions in online supplemental appendix 2.
Item 2 selection bias: representativeness of the responders
The second item requires a judgement as to whether those who declined the invitation, in relation to those who participated in the study, would introduce bias in the prevalence estimate, ‘How would you rate the risk of non-response bias?’ The reasons for non-participation are instrumental in forming a judgement about RoB. However, these are rarely reported, if ever. We assumed that in our context the decision not to participate is associated, directly or indirectly with the mental health of the persons invited to the study. The signalling question for this item therefore inquires only about the participants providing data as a proportion of the number of people invited to participate. RoB judgement is based on the response.
Item 3 information bias: measurement of the condition
The third item assesses the likelihood of misclassification due to the methods used to measure the target condition, ‘How do you judge the risk of information bias?’ We provided guidance for judging this question for the MHCOVID project (online supplemental appendix 2); for instance, if the tool/method used to measure the condition was not applied properly across time points or across groups of participants, the risk of bias for this item was judged as high.
Inter-rater agreement
Table 3 shows the results of the inter-rater agreement for each item of RoB-PrevMH, including both weighted and unweighted kappa for the 83 included studies. For item 1, the inter-rater agreement was substantial (weighted kappa 0.63, 95% CI 0.54 to 0.73) and overall agreement 83%. For item 2, the agreement was substantial (weighted kappa 0.71, 95% CI 0.67 to 0.85) and overall agreement 90%. For item 3, the weighted kappa was 0.32 (95% CI −0.04 to 0.63; overall agreement 93%), classifying inter-rater agreement as fair.
Table 3.
Results of inter-rater agreement testing
| Item | Unweighted kappa (95% CI) | % agreement | Weighted kappa (95% CI) | % agreement |
| 1. Representativeness of the sampling frame | 0.60 (0.48 to 0.68) | 74.7 | 0.63 (0.54 to 0.73) | 83.1 |
| 2. Representativeness of the responders | 0.69 (0.59 to 0.70) | 81.9 | 0.71 (0.67 to 0.85) | 90.3 |
| 3. Measurement of the condition | 0.28 (0.10 to 0.73) | 89.2 | 0.32 (−0.04 to 0.63) | 93.4 |
There was a total of 45 disagreements out of 249 paired assessments among 83 studies. Most of the disagreements (n=35) were between ‘unclear’ and either ‘high’ or ‘low’. Ten disagreements were between ‘high’ versus ‘low’ assessments.
Discussion
Summary of findings
We developed RoB-PrevMH, a concise RoB tool for prevalence studies in mental health that was designed with the intention to be adaptable to different systematic reviews and consisting of three items: representativeness of the sample, non-response bias and information bias. Our tool showed fair to substantial inter-rater reliability when applied to studies included in two systematic reviews of prevalence studies. All three items from RoB-PrevMH have been considered or included in existing tools.14 18 21 RoB-PrevMH does not contain any item on reporting and does not require an assessment of the overall RoB in a study. For each item, three assessments of RoB are possible (high, unclear and low)
Strengths and limitations
The strengths of RoB-PrevMH include the fact that it was created after a comprehensive review of items identified in previous tools as well as a consensus between researchers. Second, the feedback we received from the MH-COVID Crowd who used the tool suggests that the tool is concise and easy to use. Third, it focuses on RoB only and avoids questions that assess reporting. Fourth, the tool was tested by three pairs of extractors in two sets of studies with different aims. The inter-rater reliability was rated from fair to substantial. Finally, the tool has the potential to be tailored to other research questions.
Our tool also has limitations. First, the team of methodologists and investigators involved in development and testing was small. The tool would have benefited by a wider consultation strategy that involved more mental health experts and investigators who have designed and undertaken prevalence studies, as well as more methodologists. Second, the brevity of the tool could also be considered a limitation. For example, the MHCOVID project only includes studies that used validated tools for measuring mental health outcomes, so we did not include specific items for recall bias and observer bias, which might be important for other questions. Third, even if we assume that RoB-PrevMH would likely be quicker to complete than other tools, we did not formally assess the time required for completion in comparison with other tools. Fourth, the need to tailor the tool for each project and create training material for the people who will apply it might require more time than other tools at the start of a project. Moreover, the inter-rater reliability varied between the three items, with kappa values ranging from 0.32 to 0.71.
An important part of the evaluation of any RoB tool is the assessment of its validity. This is often done indirectly, by contrasting findings from studies judged at low versus high RoB in each domain. For example, randomised trials at high RoB from poor allocation concealment show, on average, larger effects than studies with low RoB.26 Prevalence studies are characterised by large heterogeneity, and it is expected that some of this heterogeneity might be associated with differences in RoB.27 However, RoB-PrevMH was not found to be associated with different study findings in a meta-analysis of the changes of symptoms of depression, anxiety and psychological distress during the pandemic, possibly because other design and population-related factors played a more important role in heterogeneity.4 A large-scale evaluation of the validity of RoB-PrevMH is needed to understand which design and analysis features impact most on the estimation of prevalence.
When we compare our tool’s performance with the available instruments, only the tool proposed by Hoy et al tested the inter-rater agreement and calculated the kappa value with a considerable number of studies on the prevalence of low back and neck pain.14 Even though representativeness of the target population might be difficult to judge objectively, the inter-rater agreement for this item was substantial while in the 54 studies assessed by Hoy et al the inter-rater agreement achieved was higher.14 For the second item on non-response, inter-rater agreement was substantial, but lower than similar items in the Hoy tool.14 The third item on misclassification had the lowest kappa statistic but the highest agreement between raters. In classification tables with great imbalance in the marginal probabilities and a high underlying correct classification rate kappa can be paradoxically low, as was the case of kappa for information bias.28 29 We did not make an overall RoB assessment for each study, which the Hoy tool does14 because of the problems with this approach.24
Application of RoB-PrevMH in future projects
The design of prevalence studies differs substantially depending on the question they intend to answer; as a result, having a universal tool for all types of prevalence studies, like we have for RCTs and some observational studies,23 30 might not be realistic; instead, we need tools that can be tailored to specific research questions.31
Future projects applying RoB-PrevMH might need to improve the questions, and provide a more complete list of signalling questions and considerations to choose from, depending of the context and the nature of the measured prevalence. RoB-PrevMH was conceptualised and developed for the MHCOVID project,4 which required the use of a validated assessment tool. Additional questions about information bias might be needed for projects in which there are no validated diagnostic tools for a condition (eg, cognitive deficits in post-COVID-19) or the project does not impose inclusion criteria. Another example comes from the MHCOVID project itself. In this project we decided to rate RoB for the second and third item at every follow-up time point instead of following the original instructions to give one global rating for each study. Other projects might consider the idea of not having an arbitrary threshold for the proportion of respondents and instead extract the reported proportion and analyse the data by conducting prespecified subgroup analyses, based on this continuum of response rate with meta-regression. Moreover, our chosen arbitrary threshold for response rate might be inappropriate for other studies, as we included studies on the general population, during a pandemic and mostly done online; in other settings a ‘good’ response rate might be higher than 70%.
Evaluating the risk of information bias in prevalence studies of mental health problems requires special attention. The most reliable way to measure the presence of a condition is a diagnostic interview with a trained mental health professional; yet most studies use self-administered screening tools. These are questionnaires aiming to measure symptoms of the condition and the resulting score is used to infer about the presence or not of the condition. This, however, has been shown to overestimate the true prevalence.32 Consequently, care is needed in the interpretation of the prevalence estimated from such studies: the meta-analysis summary result cannot be interpreted as true prevalence of the condition, but rather as the prevalence of symptoms scores above the studied threshold.
Training for the tool should be tailored to a specific project and include relevant examples. For instance, for the MHCOVID project, we developed an educational video and provided online training for the volunteers of the project who extracted data from included studies and conducted RoB assessment (https://mhcovid.ispm.unibe.ch/crowd.html).
Assessment of RoB in prevalence studies applies to any condition. The tools that have been published were mostly developed for specific situations, ranging from low back pain to exposure to occupational risk factors. The methods that we used to develop RoB-PrevMH follow recommended methods for the development of guidelines33 and should be used to further develop an RoB tool that can be applied to any systematic review question that aims to summarise the prevalence of a condition or risk factor. The MHCOVID project has provided the basis for building a network or experts with experience of RoB assessment23 30 and critical appraisal of prevalence studies9 16 to develop a generic framework for tools to assess RoB in prevalence studies.34
Conclusion
RoB-PrevMH is a brief and adaptable tool for assessing RoB in studies on PrevMH disorders. Initial results for inter-rater agreement were fair to substantial. The validity, reliability and applicability of RoB-PrevMH should be further assessed in future projects.
Acknowledgments
The authors acknowledge the contribution of Anna Ceraso, Aoife O'Mahony, Trevor Thompson and Marialena Trivella who tested and gave feedback on the tool; the contribution of Alexander Holloway for technical support and the contribution of Leila Darwish for her support in the MHCOVID project.
Footnotes
Twitter: @ThomyTonia, @dianacarbg, @Toshi_FRKW, @And_Cipriani, @nicolamlow, @Geointheworld
Contributors: GS, NL, TL, TT, NLP, DBG, CMV, TAF, AC and SL designed the study; TT, DBG, NLP and CMV collected data; TAF, GS, DBG and TT performed the statistical analysis; first draft was prepared by TT and DBG; revised and approved by all.
Funding: Swiss National Science Foundation. This study was funded by the National Research Programme 78 COVID-19 of the Swiss National Science Foundation (grant number 198418). AC is supported by the National Institute for Health Research (NIHR) Oxford Cognitive Health Clinical Research Facility, by an NIHR Research Professorship (grant RP-2017-08-ST2-006), by the NIHR Oxford and Thames Valley Applied Research Collaboration and by the NIHR Oxford Health Biomedical Research Centre (grant BRC-1215-20005). DBG is a recipient of the Swiss government excellence scholarship (grant number 2019.0774), the SSPH+ Global PhD Fellowship Programme in Public Health Sciences of the Swiss School of Public Health, and the Swiss National Science Foundation (project number 176233). NL received funding for the COVID-19 Open Access Project from the Swiss National Science Foundation (grant number 176233) and the European Union's Horizon 2020 research and innovation programme—project EpiPose (Grant agreement number 101003688) and acknowledges the contributions of Dr. Leonie Heron and Ms. Hira Imeri. This work reflects only the authors’ view. The European Commission is not responsible for any use that may be made of the information it contains. TL is supported by grant UG1 EY020522 from the National Eye Institute, National Institutes of Health.
Disclaimer: The views expressed are those of the authors and not necessarily those of the Swiss National Science Foundation. The views expressed are those of the authors and not necessarily those of the UK National Health Service, the NIHR, or the UK Department of Health.
Competing interests: TAF reports personal fees from Boehringer-Ingelheim, DT Axis, Kyoto University Original, Shionogi and SONY, and a grant from Shionogi, outside the submitted work; In addition, TAF has patents 2020-548587 and 2022-082495 pending, and intellectual properties for Kokoro-app licensed to Mitsubishi-Tanabe. AC has received research, educational and consultancy fees from INCiPiT (Italian Network for Paediatric Trials), CARIPLO Foundation, Lundbeck and Angelini Pharma. He is the CI/PI of randomised trial about seltorexant in depression, sponsored by Janssen. SL reports personal fees and honoraria from Alkermes, angelini, Lundbeck, Lundbeck Foundation, Otsuka, Angelini, Eisai, Gedeon, Medichem, Merck, Mitsubishi, Otsuka, Recordati, Sanofi-Aventis Recordati, Rovi, Teva.
Provenance and peer review: Not commissioned; externally peer reviewed.
Supplemental material: This content has been supplied by the author(s). It has not been vetted by BMJ Publishing Group Limited (BMJ) and may not have been peer-reviewed. Any opinions or recommendations discussed are solely those of the author(s) and are not endorsed by BMJ. BMJ disclaims all liability and responsibility arising from any reliance placed on the content. Where the content includes any translated material, BMJ does not warrant the accuracy and reliability of the translations (including but not limited to local regulations, clinical guidelines, terminology, drug names and drug dosages), and is not responsible for any error and/or omissions arising from translation and adaptation or otherwise.
Ethics statements
Patient consent for publication
Not applicable.
References
- 1. Leucht S, Cipriani A, Furukawa TA, et al. A living meta-ecological study of the consequences of the COVID-19 pandemic on mental health. Eur Arch Psychiatry Clin Neurosci 2021;271:219–21. 10.1007/s00406-021-01242-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Liu Y-C, Kuo R-L, Shih S-R. COVID-19: the first documented Coronavirus pandemic in history. Biomed J 2020;43:328–33. 10.1016/j.bj.2020.04.007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Ipekci AM, Buitrago-Garcia D, Meili KW, et al. Outbreaks of publications about emerging infectious diseases: the case of SARS-Cov-2 and Zika virus. BMC Med Res Methodol 2021;21:50. 10.1186/s12874-021-01244-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Salanti G, Peter N, Tonia T, et al. The impact of the COVID-19 pandemic and associated control measures on the mental health of the general population: a systematic review and dose-response meta-analysis. Ann Intern Med 2022;175:1560–71. 10.7326/M22-1507 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Egger E, Higgins JPT, Davey Smith G. Systematic reviews in health research: meta-analysis in context, 3RD edition. In: Systematic Reviews in Health Research. Wiley, 2022. Available: Wileycom [Google Scholar]
- 6. Viswanathan M, Berkman ND, Dryden DM, et al. Assessing risk of Bias and confounding in observational studies of interventions or exposures: further development of the RTI Item bank. Rockville (MD): Agency for Healthcare Research and Quality (US), 2013. [PubMed] [Google Scholar]
- 7. Borges Migliavaca C, Stein C, Colpani V, et al. On behalf of the prevalence estimates reviews – systematic review methodology G: how are systematic reviews of prevalence conducted? A methodological study. BMC Med Res Methodol 2020;20:96. 10.1186/s12874-020-00975-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Hoffmann F, Eggers D, Pieper D, et al. An observational study found large methodological heterogeneity in systematic reviews addressing prevalence and cumulative incidence. J Clin Epidemiol 2020;119:92–9. 10.1016/j.jclinepi.2019.12.003 [DOI] [PubMed] [Google Scholar]
- 9. Borges Migliavaca C, Stein C, Colpani V, et al. Quality assessment of prevalence studies: a systematic review. J Clin Epidemiol 2020;127:59–68. 10.1016/j.jclinepi.2020.06.039 [DOI] [PubMed] [Google Scholar]
- 10. Salanti G, Cipriani A, Furukawa TA, et al. An efficient way to assess the effect of COVID-19 on mental health in the general population. Lancet Psychiatry 2021;8:e14–5. 10.1016/S2215-0366(21)00067-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Buitrago-Garcia D. Meta-análisis de prevalencia: revisión sistemática de los métodos utilizados, propuesta de una herramienta para evaluar la calidad y evaluación de los diferentes métodos estadísticos utilizados para meta analizar prevalencias. Bogotá, Colombia: Universidad Nacional de Colombia, 2018. Available: https://repositorio.unal.edu.co/handle/unal/63768 [Google Scholar]
- 12. Mesa-Vieira C, Haas AD, Buitrago-Garcia D, et al. Mental health of migrants with pre-migration exposure to armed conflict: a systematic review and meta-analysis. Lancet Public Health 2022;7:e469–81. 10.1016/S2468-2667(22)00061-5 [DOI] [PubMed] [Google Scholar]
- 13. Shamliyan TA, Kane RL, Ansari MT, et al. Development quality criteria to evaluate nontherapeutic studies of incidence, prevalence, or risk factors of chronic diseases: pilot study of new Checklists. J Clin Epidemiol 2011;64:637–57. 10.1016/j.jclinepi.2010.08.006 [DOI] [PubMed] [Google Scholar]
- 14. Hoy D, Brooks P, Woolf A, et al. Assessing risk of bias in prevalence studies: modification of an existing tool and evidence of Interrater agreement. J Clin Epidemiol 2012;65:934–9. 10.1016/j.jclinepi.2011.11.014 [DOI] [PubMed] [Google Scholar]
- 15. Giannakopoulos NN, Rammelsberg P, Eberhard L, et al. A new instrument for assessing the quality of studies on prevalence. Clin Oral Investig 2012;16:781–8. 10.1007/s00784-011-0557-4 [DOI] [PubMed] [Google Scholar]
- 16. Munn Z, Moola S, Riitano D, et al. The development of a critical appraisal tool for use in systematic reviews addressing questions of prevalence. Int J Health Policy Manag 2014;3:123–8. 10.15171/ijhpm.2014.71 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Leboeuf-Yde C, Lauritsen JM. The prevalence of low back pain in the literature. A structured review of 26 Nordic studies from 1954 to 1993. Spine 1995;20:2112–8. 10.1097/00007632-199510000-00009 [DOI] [PubMed] [Google Scholar]
- 18. Loney PL, Chambers LW, Bennett KJ, et al. Critical appraisal of the health research literature: prevalence or incidence of a health problem. Chronic Dis Can 1998;19:170–6. [PubMed] [Google Scholar]
- 19. Boyle MH. Guidelines for evaluating prevalence studies. Evid Based Ment Health 1998;1:37–9. 10.1136/ebmh.1.2.37 [DOI] [Google Scholar]
- 20. Silva LC, Ordúñez P, Paz Rodríguez M, et al. A tool for assessing the usefulness of prevalence studies done for surveillance purposes: the example of hypertension. Rev Panam Salud Publica 2001;10:152–60. 10.1590/s1020-49892001000900002 [DOI] [PubMed] [Google Scholar]
- 21. Joanna Briggs I. The Joanna Briggs institute critical appraisal tools for use in JBI systematic reviews checklist for prevalence studies. Joanna Briggs Institute, 2017. [Google Scholar]
- 22. Pega F, Norris SL, Backes C, et al. Rob-SPEO: A tool for assessing risk of bias in studies estimating the prevalence of exposure to occupational risk factors from the WHO/ILO joint estimates of the work-related burden of disease and injury. Environ Int 2020;135:105039. 10.1016/j.envint.2019.105039 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Higgins JPT, Altman DG, Gøtzsche PC, et al. The Cochrane collaboration's tool for assessing risk of bias in randomised trials. BMJ 2011;343:d5928. 10.1136/bmj.d5928 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Jüni P, Witschi A, Bloch R, et al. The hazards of scoring the quality of clinical trials for meta-analysis. JAMA 1999;282:1054–60. 10.1001/jama.282.11.1054 [DOI] [PubMed] [Google Scholar]
- 25. Stroup DF, Berlin JA, Morton SC, et al. Meta-analysis of observational studies in epidemiology: a proposal for reporting meta-analysis of observational studies in epidemiology (MOOSE) group. JAMA 2000;283:2008–12. 10.1001/jama.283.15.2008 [DOI] [PubMed] [Google Scholar]
- 26. Wood L, Egger M, Gluud LL, et al. Empirical evidence of bias in treatment effect estimates in controlled trials with different interventions and outcomes: meta-epidemiological study. BMJ 2008;336:601–5. 10.1136/bmj.39465.451748.AD [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Migliavaca CB, Stein C, Colpani V, et al. Meta-analysis of prevalence: I2 statistic and how to deal with heterogeneity. Res Synth Methods 2022;13:363–7. 10.1002/jrsm.1547 [DOI] [PubMed] [Google Scholar]
- 28. Cicchetti DV, Feinstein AR. High agreement but low Kappa: II. Resolving the paradoxes. J Clin Epidemiol 1990;43:551–8. 10.1016/0895-4356(90)90159-m [DOI] [PubMed] [Google Scholar]
- 29. Feinstein AR, Cicchetti DV. High agreement but low Kappa: I. The problems of two paradoxes. J Clin Epidemiol 1990;43:543–9. 10.1016/0895-4356(90)90158-l [DOI] [PubMed] [Google Scholar]
- 30. Sterne JA, Hernán MA, Reeves BC, et al. ROBINS-I: a tool for assessing risk of bias in non-randomised studies of interventions. BMJ 2016:i4919. 10.1136/bmj.i4919 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Buitrago-Garcia D, Salanti G, Low N. Studies of prevalence: how a basic epidemiology concept has gained recognition in the COVID-19 pandemic. BMJ Open 2022;12:e061497. 10.1136/bmjopen-2022-061497 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Thombs BD, Kwakkenbos L, Levis AW, et al. Addressing overestimation of the prevalence of depression based on self-report screening questionnaires. CMAJ 2018;190:E44–9. 10.1503/cmaj.170691 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Moher D, Schulz KF, Simera I, et al. Guidance for developers of health research reporting guidelines. PLoS Med 2010;7:e1000217. 10.1371/journal.pmed.1000217 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Buitrago-Garcia D. Development of a risk of bias tool for prevalence studies. 2023. Available: https://osf.io/b4qt9 [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
bmjment-2023-300694supp001.pdf (9.2KB, pdf)
bmjment-2023-300694supp002.pdf (176.5KB, pdf)