Abstract
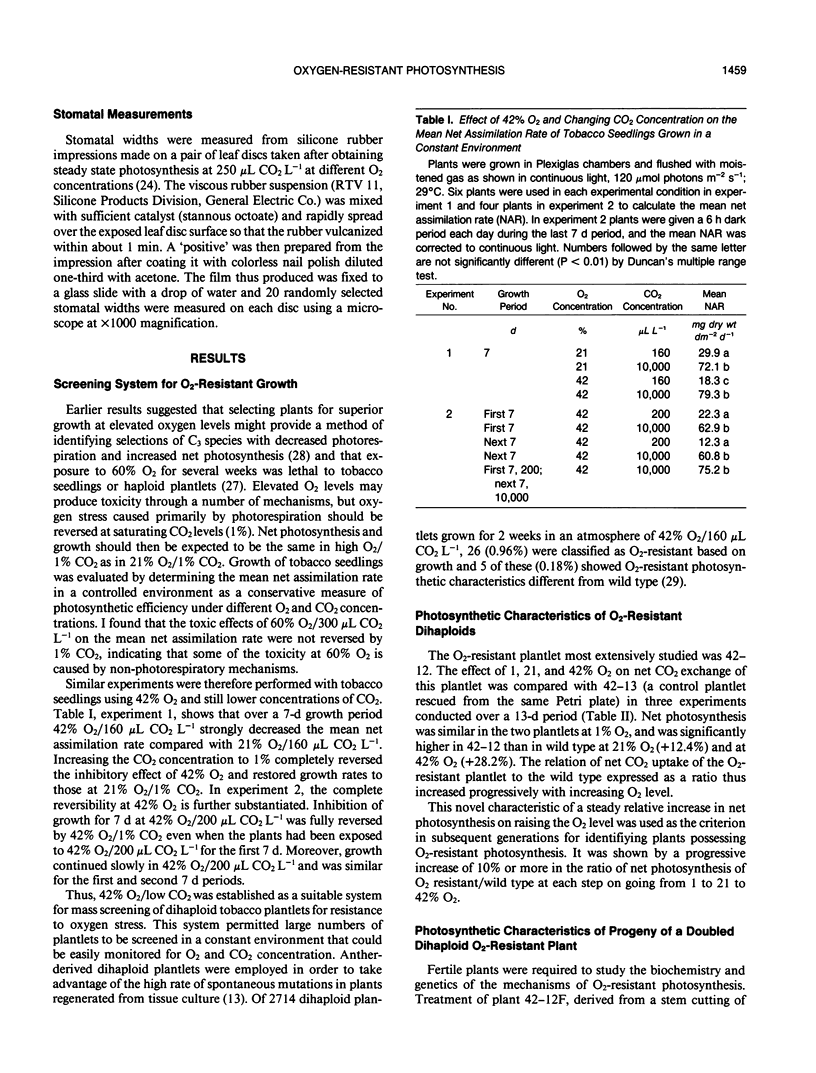
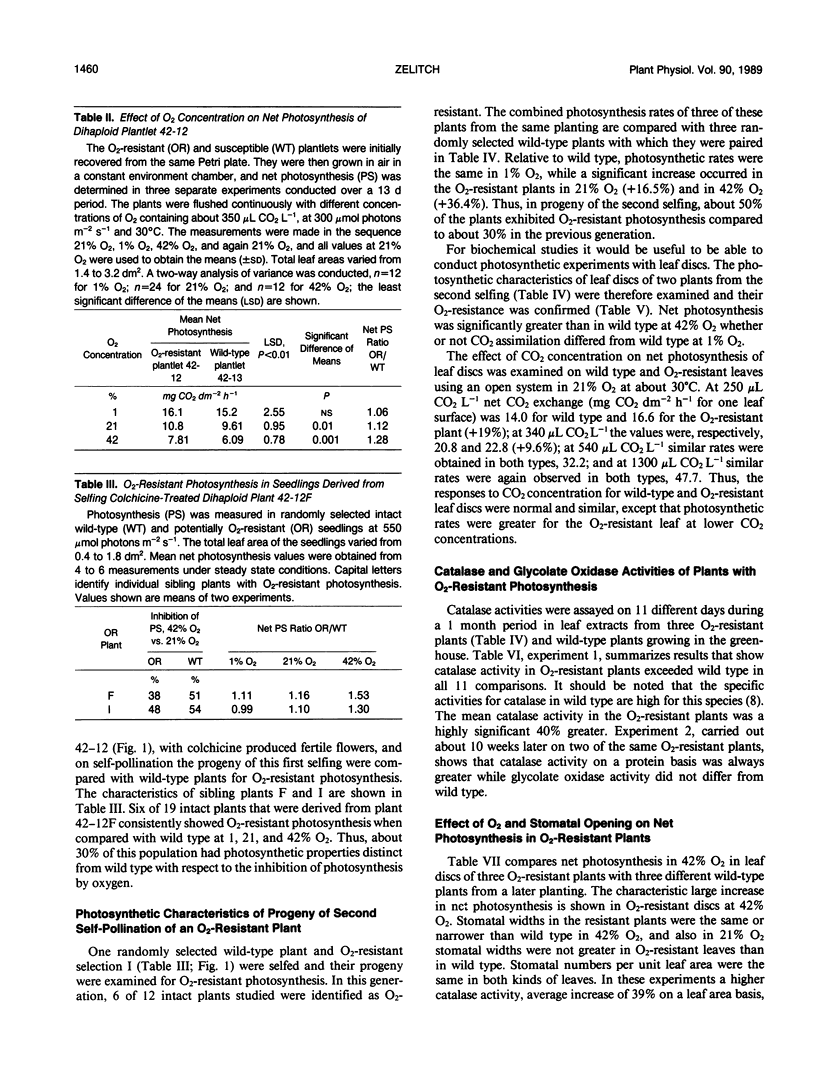
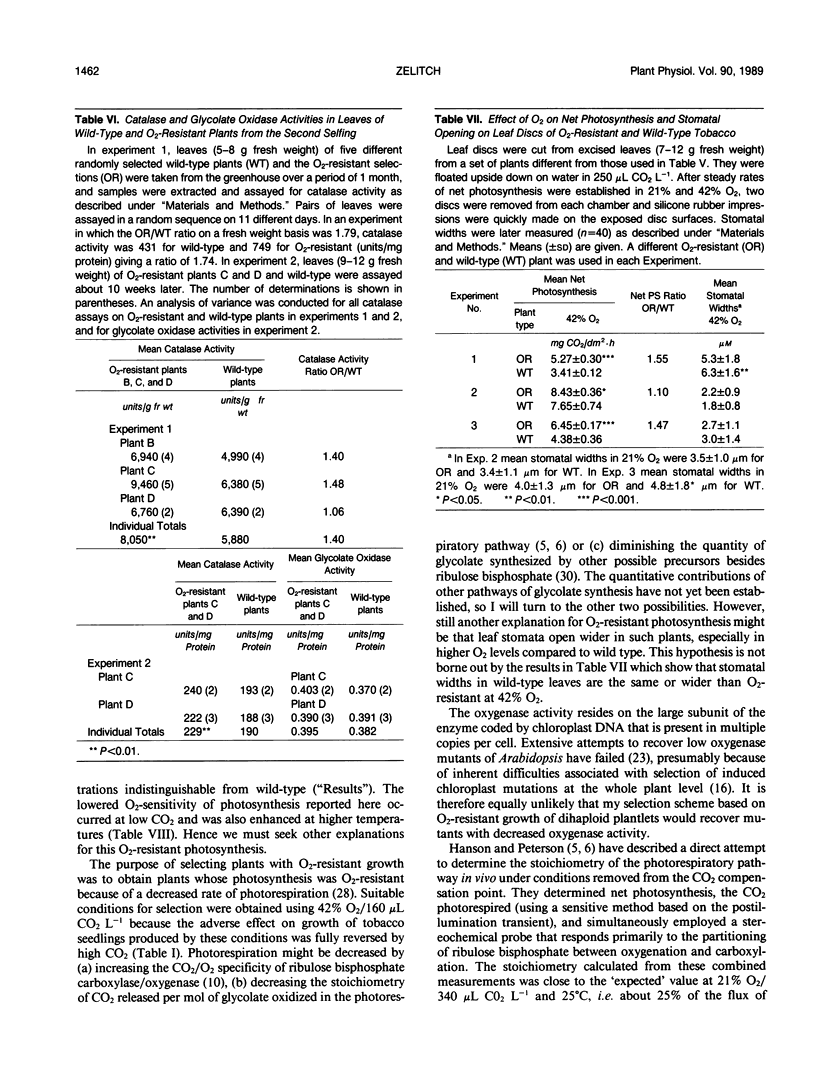
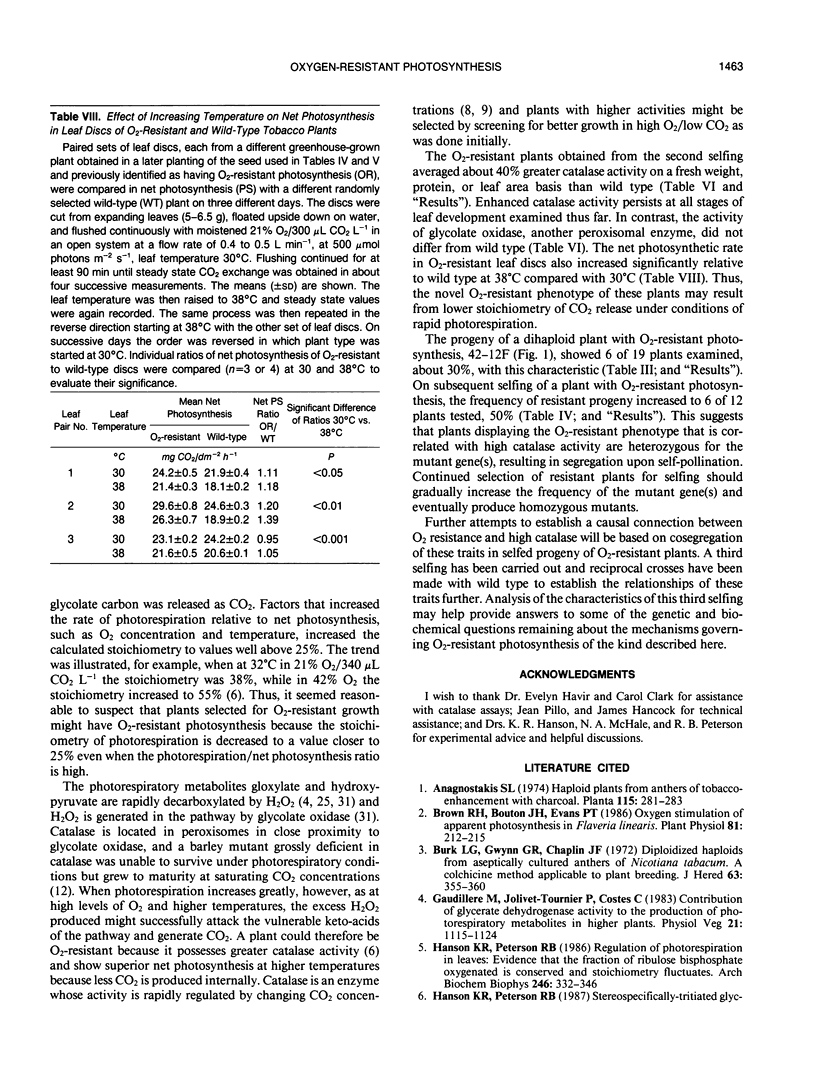
Plants were obtained with novel O2-resistant photosynthetic characteristics. At low CO2 (250-350 μL CO2 L−1) and 30°C when O2 was increased from 1% to 21% to 42%, the ratio of net CO2 uptake in O2-resistant whole plants or leaf discs compared to wild type increased progressively, and this was not related to stomatal opening. Dihaploid plantlets regenerated from anther culture were initially screened and selected for O2-resistant growth in 42% O2/160 μL CO2 L−1 and 0.18% of the plantlets showed O2-resistant photosynthesis. About 30% of the progeny (6 of 19 plants) of the first selfing of a fertile plant derived from a resistant dihaploid plant had O2-resistant photosynthesis, and after a second selfing this increased to 50% (6 of 12 plants). In 21% O2 and low CO2, net photosynthesis of the resistant plants was about 15% greater on a leaf area basis than wild type. Net photosynthesis was compared in leaf discs at 30 and 38°C in 21% O2, and at the higher temperature O2-resistant plants showed still greater photosynthesis than wild type. The results suggest that the O2-resistant photosynthesis described here is associated with a decreased stoichiometry of CO2 release under conditions of rapid photorespiration. This view was supported by the finding that leaves of O2-resistant plants averaged 40% greater catalase activity than wild type.
Full text
PDF



Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Brown R. H., Bouton J. H., Evans P. T. Oxygen Stimulation of Apparent Photosynthesis in Flaveria linearis. Plant Physiol. 1986 May;81(1):212–215. doi: 10.1104/pp.81.1.212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hanson K. R., Peterson R. B. Regulation of photorespiration in leaves: evidence that the fraction of ribulose bisphosphate oxygenated is conserved and stoichiometry fluctuates. Arch Biochem Biophys. 1986 Apr;246(1):332–346. doi: 10.1016/0003-9861(86)90478-9. [DOI] [PubMed] [Google Scholar]
- Harris G. C., Cheesbrough J. K., Walker D. A. Effects of mannose on photosynthetic gas exchange in spinach leaf discs. Plant Physiol. 1983 Jan;71(1):108–111. doi: 10.1104/pp.71.1.108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Havir E. A., McHale N. A. Biochemical and developmental characterization of multiple forms of catalase in tobacco leaves. Plant Physiol. 1987 Jun;84(2):450–455. doi: 10.1104/pp.84.2.450. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Havir E. A., McHale N. A. Regulation of Catalase Activity in Leaves of Nicotiana sylvestris by High CO(2). Plant Physiol. 1989 Mar;89(3):952–957. doi: 10.1104/pp.89.3.952. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McHale N. A. Conditions for strict autotrophic culture of tobacco callus. Plant Physiol. 1985 Jan;77(1):240–242. doi: 10.1104/pp.77.1.240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morgan J. A., Brown R. H. Photosynthesis in Grass Species Differing in Carbon Dioxide Fixation Pathways: III. OXYGEN RESPONSE AND ENZYME ACTIVITIES OF SPECIES IN THE LAXA GROUP OF PANICUM. Plant Physiol. 1980 Jan;65(1):156–159. doi: 10.1104/pp.65.1.156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peterson R. B. Estimation of Photorespiration Based on the Initial Rate of Postillumination CO(2) Release: I. A Nonsteady State Model for Measurement of CO(2) Exchange Transients. Plant Physiol. 1983 Dec;73(4):978–982. doi: 10.1104/pp.73.4.978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peterson R. B., Zelitch I. Relationship between Net CO(2) Assimilation and Dry Weight Accumulation in Field-Grown Tobacco. Plant Physiol. 1982 Sep;70(3):677–685. doi: 10.1104/pp.70.3.677. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sharkey T. D. O(2)-insensitive photosynthesis in c(3) plants : its occurrence and a possible explanation. Plant Physiol. 1985 May;78(1):71–75. doi: 10.1104/pp.78.1.71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vollmer R. R., Boccagno J. A., Steinbacher T. E., Horovitz Z. P., Murthy V. S. Antihypertensive activity of captopril (SQ 14,225), an orally active inhibitor of angiotensin converting enzyme in conscious two-kidney perinephritic hypertensive dogs. J Pharmacol Exp Ther. 1981 Feb;216(2):225–231. [PubMed] [Google Scholar]
- Walker D. A., Zelitch I. Some Effects of Metabolic Inhibitors, Temperature, & Anaerobic Conditions on Stomatal Movement. Plant Physiol. 1963 Jul;38(4):390–396. doi: 10.1104/pp.38.4.390. [DOI] [PMC free article] [PubMed] [Google Scholar]
- ZELITCH I., OCHOA S. Oxidation and reduction of glycolic and glyoxylic acids in plants. I. Glycolic and oxidase. J Biol Chem. 1953 Apr;201(2):707–718. [PubMed] [Google Scholar]
- Zelitch I. Improving the efficiency of photosynthesis. Science. 1975 May 9;188(4188):626–633. doi: 10.1126/science.188.4188.626. [DOI] [PubMed] [Google Scholar]
- Zelitch I. Synthesis of Glycolate from Pyruvate via Isocitrate Lyase by Tobacco Leaves in Light. Plant Physiol. 1988 Feb;86(2):463–468. doi: 10.1104/pp.86.2.463. [DOI] [PMC free article] [PubMed] [Google Scholar]


