Highlights
-
•
This Clinical Practice Guideline provides recommendations for managing leptomeningeal metastases from solid tumours.
-
•
The guideline covers clinical, imaging and cytological diagnosis, staging and risk assessment, treatment and follow-up.
-
•
A treatment and management algorithm is provided.
-
•
The author panel encompasses a multidisciplinary group of experts from different institutions and countries in Europe.
-
•
Recommendations are based on available scientific data and the authors’ collective expert opinion.
Key words: central nervous system, cerebral, cerebrospinal fluid, clinical practice guideline, neurological, recommendations
Introduction
These joint European Association of Neuro-Oncology (EANO)–European Society for Medical Oncology (ESMO) recommendations for the diagnosis and treatment of leptomeningeal metastasis (LM) from solid tumours provide an update of the first joint EANO–ESMO guideline1 and complement the EANO–ESMO guideline on brain metastasis from solid tumours.2
LM is defined as the spread of tumour cells within the leptomeninges and the subarachnoid space. The present recommendations address LM from extra-central nervous system (CNS) solid tumours, but do not address LM from primary brain tumours, lymphoma or leukaemia. The recommendations cover diagnosis, treatment and follow-up, but do not cover the differential diagnosis, treatment-related adverse events (AEs) or supportive or palliative care in detail.
The authors propose diagnostic criteria and assign levels of certainty to the diagnosis of LM in order to provide guidance regarding when to treat versus when to intensify diagnostic efforts and which patients to include in clinical trials. The authors also provide a pragmatic treatment algorithm based on LM subtypes. Supporting evidence for this guideline focuses on LM-specific data with reference to the EANO–ESMO guideline on brain metastasis from solid tumours2 when LM-specific data are not available. Given the low level of evidence available, recommendations are often based on expert opinion and consensus rather than on evidence from informative clinical trials. Still, these EANO–ESMO multidisciplinary recommendations serve as a valuable source of information for physicians and other health care providers, as well as for patients and relatives.
Incidence and epidemiology
Details of incidence and epidemiology can be found in Section 1 of the Supplementary Material, available at https://doi.org/10.1016/j.esmoop.2023.101624.
Recommendation
-
•
LM should be considered, particularly in patients with breast or lung cancer or melanoma who present with neurological symptoms or signs [EANO: III, C; ESMO: IV, B].
Diagnosis, pathology and molecular biology
Clinical presentation
A detailed and standardised neurological examination is recommended (Table 1). Symptoms and signs depend on the specific CNS area of LM involvement. Multifocal clinical impairment is highly suggestive of LM, but patients may also present with isolated or subtle neurological symptoms or signs or may have a normal neurological evaluation. The typical clinical signs and symptoms include headache; nausea and vomiting; neurocognitive changes; gait difficulties; cranial nerve palsies, notably with diplopia or visual disturbance (cranial nerves II, III, IV, VI), facial palsy (cranial nerve VII) and hearing loss (cranial nerve VIII); radicular signs including weakness, voiding and cauda equina problems; and focal or radiating (radicular) neck and back pain.3, 4, 5, 6, 7, 8, 9, 10, 11 Neurological sequelae from previous or concomitant brain metastases or extra-CNS metastases, treatment, comorbidities or any other medical event, and also comedications, should be considered during the clinical assessment.
Table 1.
Recommended evaluation of suspected LM to establish the level of evidence for the diagnosis
| Recommended protocols of evaluation | Results | |
|---|---|---|
| Clinical evaluation | Thorough neurological examination focused on abnormalities typically seen in patients with LM | Presence of typical clinical signs of LMa Any other neurological abnormalityb Normal neurological evaluation |
| Neuroimaging | Field strength of 1.5 or preferably 3 T Gadolinium should be injected 10 min before data acquisition at a dose of 0.1 mmol/kg. The slice thickness should be ≤1 mm at the brain level and ≤3 mm at the spinal level Brain: 3D pre-contrast T1-weighted, 2D or 3D FLAIR, 2D diffusion-weighted imaging, 2D pre-contrast T2-weighted, post-gadolinium 3D T1-weighted. Post-gadolinium 3D FLAIR sequences should be considered Spinal axis: sagittal fat-suppression T2-weighted sequences, sagittal pre-contrast T1-weighted sequences, T1-weighted post-gadolinium sagittal fat-suppressed sequence |
Typical MRI findings of linear LM (type A)c Typical MRI findings of nodular LMD (type B) Both (type C) Hydrocephalus only (type D–hydrocephalus) Equivocal leptomeningeal findings or absence of leptomeningeal MRI findings (type D–normal) |
| CSF cytology | Fresh CSF samples should ideally be processed within 30 min after sampling CSF volume is ideally >10 ml but at least 5 ml After centrifugation, cytospins can be air-dried and subsequently May-Grünwald-Giemsa (MGG = Pappenheim) stained Alternatively, fresh CSF samples can be fixed with Ethanol-Carbowax (CSF–fixative ratio 1:1) to reduce time pressure, followed by Papanicolaou staining of the cytospins Upon indication and availability of material, additional immunocytochemical stainings for epithelial and melanocytic markers should be considered A second CSF sample should be analysed if the initial CSF sample is negative |
Positive: presence of tumour cells Equivocal: suspicious or atypical cells Negative: absence of tumour cells |
Adapted from Le Rhun et al.1
2D, two-dimensional; 3D, three-dimensional; CSF, cerebrospinal fluid; CNS, central nervous system; FLAIR, fluid-attenuated inversion recovery; LM, leptomeningeal metastasis; LMD, leptomeningeal disease; MRI, magnetic resonance imaging.
Typical clinical signs of LM include headache, nausea and vomiting, mental changes, gait difficulties, cranial nerve palsies with diplopia, visual disturbances, hearing loss, radicular signs including weakness, voiding and cauda equina problems and focal or radiating (radicular) neck and back pain.
Neurological sequelae from previous brain metastases or extra-CNS metastases, treatment, comorbidities or any other medical event, but also comedication should be considered during the clinical assessment.
See Table 2 and text.
A detailed and standardised clinical neurological scorecard for patients with LM would be welcome, notably in the context of clinical trials. However, the one proposed by the Response Assessment in Neuro-oncology (RANO) group LM committee has not been validated.12 The Neurological Assessment in Neuro-oncology (NANO) scale, which was developed for the evaluation of patients with brain tumours in general, does not cover all the clinical manifestations of LM.13
Neuroimaging
Cerebrospinal magnetic resonance imaging (MRI), without and with contrast enhancement, is the gold standard imaging method for the diagnosis and follow-up of patients with suspected or confirmed LM.1,12 The role of cerebrospinal MRI in addition to standard extracerebral staging for LM detection during the follow-up of patients at high risk of CNS metastases, e.g. patients with metastatic melanoma, lung or triple-negative breast cancer, has not been evaluated.2
The following technical aspects should be considered in order to acquire high-quality images (Table 1): a magnetic field strength of 1.5 or preferably 3 T, a slice thickness of 1 mm for brain sequences and 3 mm for spinal sequences and intravenous (i.v.) injection of gadolinium at 0.1 mmol/kg 10 min before T1-weighted post-contrast data acquisition. Brain MRI should include three-dimensional (3D) pre-contrast T1-weighted, two-dimensional (2D) or 3D fluid-attenuated inversion recovery (FLAIR), 2D diffusion-weighted imaging, 2D pre-contrast T2-weighted, post-gadolinium 3D T1-weighted and post-gadolinium 3D FLAIR sequences. Spinal MRI should include sagittal fat-suppression T2-weighted sequences, sagittal pre-contrast T1-weighted sequences and T1-weighted post-gadolinium sagittal fat-suppressed sequence. Additional sequences can be helpful, notably axial T1-weighted post-gadolinium images of regions of interest. Since lumbar punctures may induce dural enhancement, MRI should be carried out before a lumbar puncture whenever possible; the date(s) of the last cerebrospinal fluid (CSF) analysis should be documented when requesting an MRI.
MRI findings are not specific and should be interpreted in the specific clinical context. Various differential diagnoses must be considered. Typical MRI findings include contrast enhancement of cerebellar folia and sulci, basilar cisterns, cranial nerves, brain surface, surface of the lateral ventricles and lumbar nerve roots, notably the cauda equina. Leptomeningeal lesions can be linear or nodular. Cerebrospinal MRI can be normal even in patients with tumour cells in the CSF.6,14 In a retrospective review of 171 patients with LM from lung cancer, MRI at the time of LM presentation showed cranial meningeal-only involvement in 67 (40%) patients, spinal meningeal-only involvement in 17 (10%) patients, cranial and spinal meningeal involvement in 41 (24%) patients and a normal MRI in 46 (26%) patients.15 In a cohort study of 318 patients with LM from breast cancer, cranial meningeal-only involvement was reported in 135 (43%) patients, spinal meningeal-only involvement in 84 (26%) patients, cranial and spinal meningeal involvement in 83 (26%) patients and a normal MRI in 12 (4%) patients.16
The MRI presentation can be divided into five main subtypes: linear leptomeningeal disease (type A); nodular leptomeningeal disease (type B); both linear and nodular leptomeningeal disease, requiring a minimum of 20% of each pattern contributing to the disease burden (type C); hydrocephalus only (type D–hydrocephalus) or no neuroimaging evidence of LM (type D–normal). Due to the small volume and geometric complexity, a quantitative assessment of LM lesions is often not possible. Thus, it has been proposed to distinguish ‘measurable’ LM, defined by at least one measurable nodular lesion, from ‘non-measurable’ disease, which encompasses all other MRI abnormalities. A nodule is a contrast-enhancing lesion that is defined as leptomeningeal as opposed to parenchymal if there is direct contact (<2 mm) between the rim of the nodule and the leptomeninges on contrast-enhanced T1-weighted MRI. Nodules are considered as measurable if their size is ≥5 × 5 mm in orthogonal diameters in two planes.17
In a cohort of 254 patients with LM from various extra-CNS solid tumours, a linear presentation was noted in 117 (46%) patients, a combination of both linear and nodular enhancing disease in 55 (22%) patients and enhancing nodules only in 32 (13%) patients.17 Hydrocephalus has been reported in 11%-17% of patients with LM,18,19 but definitions of hydrocephalus taking both age and prior treatment into consideration are difficult to establish for patients with cancer.
A standardised and prospectively validated scorecard, such as the one proposed by the European Organisation for Research and Treatment of Cancer (EORTC) Brain Tumor Group (BTG) and the RANO group, should be used to assess LM disease burden, particularly within clinical trials.17 Concomitant brain metastases are frequently associated with LM. Indeed, concomitant brain metastases have been reported in 36%-66% of patients with LM from breast cancer, 66%-82% of patients with LM from lung cancer and 69%-87% of patients with LM from melanoma.6,7,10,16,19, 20, 21, 22 Spinal cord metastases and spinal epidural metastases may also be noted in patients with LM.
Computed tomography (CT) should be restricted to patients with contraindications to MRI and to emergency settings, e.g. to rule out CSF obstruction or cerebral haemorrhage in case of rapid neurological deterioration. [18F]2-fluoro-2-deoxy-D-glucose (FDG)–positron emission tomography (PET)–CT has no role in the diagnosis of LM due to technical limitations.23 Radionuclide studies using either 111indium-diethylenetriamine pentaacetic acid (In-DTPA) or 99technetium (Tc) macro-aggregated albumin can be carried out to establish CSF flow dynamics.24,25 However, these studies give no information regarding the actual dissemination of LM. Also, no recent data are available regarding the incidence of CSF flow abnormalities in LM. Radionuclide studies should therefore be considered in patients with suspected CSF flow blocks, e.g. in the presence of hydrocephalus, when intrathecal treatment is a therapeutic option, or in the presence of unexpected toxicity during the course of intrathecal treatment.12
CSF analysis
Lumbar punctures should be carried out after neuroimaging to avoid placing patients at risk from the procedure, e.g. from herniation because of major brain metastases or complications from local bulky disease. This sequence may also reduce possible challenges with interpreting dural versus leptomeningeal enhancement that may be seen after lumbar punctures. Unspecific abnormalities on routine CSF analysis are common in LM. These findings include an increased opening pressure (>200 mm H2O) in 21%-42% of patients,11,26,27 increased leukocyte counts (>4 per mm3) in 39%-77.5%, elevated protein (>500 mg/l) in 56%-91% and decreased glucose (<600 mg/l) in 22%-38% of patients.11,19,25,28
In contemporary large cohorts of patients with probable or confirmed LM, tumour cells have been detected at diagnosis in 60.5%-83%.1,14,15,27 CSF cytological analysis should be reported as positive, defined as the presence of malignant cells in the CSF; equivocal, corresponding to the detection of ‘suspicious’ or ‘atypical’ cells in the CSF; or negative, defined as the absence of malignant or potentially malignant (‘equivocal’) cells in the CSF (Table 1). The following simple measures may improve the sensitivity of CSF studies and should be followed in patients with suspected LM: obtaining sufficient volumes of CSF (ideally >10 ml but at least 5 ml), processing CSF within 30 min after sampling and avoiding haemorrhagic contamination12,29,30 (Table 1). Of note, several days, ideally 14 days, should be awaited between CNS surgery and a diagnostic CSF analysis when LM is suspected.
A higher sensitivity has been reported with thin-layer preparations (Thinprep) than with Cytospin-coupled Wright-Giemsa stains.31 If the first CSF analysis is negative, a second lumbar puncture should be carried out under optimised conditions as outlined above, which increases the sensitivity. The yield of further CSF assessments after a second negative assessment carried out according to contemporary recommendations remains doubtful. CSF sample storage tubes such as Transfix or CellSave™ preservative tubes may diminish the need for rapid sample processing and have been proposed for LM from haematological malignancies.32 Their value in clinical practice remains to be established in LM from solid tumours since the specificity of these assays remains controversial. Staining of neoplastic cells for specific alterations such as HER2 in breast cancer or BRAF V600E in melanoma by immunocytochemistry may be useful in selected equivocal cases. The assessment of tumour-specific markers or molecules thought to be involved in the metastatic process, such as vascular endothelial growth factor or matrix metalloproteinases, has a limited role in the diagnosis of LM in current clinical practice.
Novel techniques using epithelial cell adhesion molecule (Ep-CAM) antibodies or other tumour-specific antibody-covered magnetic nanoparticles to identify circulating tumour cells have shown promising results using various adaptations of the device initially designed for peripheral blood studies combined with flow cytometry or tumour marker immunofluorescence in situ hybridisation. A cut-off of 0.9-1 tumour cells per ml CSF has been proposed for the diagnosis of LM using such approaches.33,34 However, the value of this cut-off over standard cytopathology where one tumour cell defines LM remains to be explored. A reproducible quantification of circulating tumour cells in the CSF during follow-up could also potentially help to define response to treatment.35 Further validation in large prospective studies is required before the introduction of such technologies in routine clinical practice.
Genomic alterations can be detected in the CSF by DNA-based microarrays,36 digital or real-time PCR and targeted amplicon sequencing, whole exome sequencing or next-generation sequencing.37, 38, 39, 40 A higher sensitivity for the detection of genomic alterations, including EGFR mutation, ALK rearrangements and ROS1 rearrangements, has been reported for CSF compared with plasma samples in patients with LM from lung cancer.37,38 However, in clinical practice, there is still insufficient evidence to substitute a positive CSF cytology result with the detection of tumour-specific mutations at a DNA level in the CSF, e.g. BRAF V600E or EGFR T790M. It remains unclear whether tumour DNA detection in the CSF compartment always reflects the local presence of tumour cells or whether this DNA may be derived from concomitant brain metastases, tumour cells circulating in the blood or even from distant extracerebral metastases. Future studies need to address the question of what quantitative cut-off of tumour DNA in the CSF corresponds to clinically relevant LM. However, genomic analysis of the CSF can be considered in patients with LM from cancers where targeted therapies are available in order to define the molecular profile.37,38,41
Leptomeningeal biopsy
Leptomeningeal biopsies are rarely carried out to confirm the diagnosis of LM,42 but may be required to rule out differential diagnoses such as sarcoidosis or tuberculosis. It may be especially useful when the CSF cytology result is repeatedly negative, when there is no history of cancer or if there are doubts regarding the cause of the clinical and imaging features where therapeutic decision making is required. The site for leptomeningeal biopsies is guided by MRI findings.
Peripheral blood liquid biopsies
At present, there is no role for liquid biopsies from peripheral blood in the management of LM; the potential role for this still needs to be explored.
Recommendations
-
•
The clinical work-up in cases of suspected LM should include a detailed neurological evaluation, cerebrospinal MRI and CSF studies using optimised analysis conditions [EANO: III, B; ESMO: IV, B].
-
•
Typical clinical signs of LM, such as headache, nausea and vomiting, mental changes, gait difficulties, cranial nerve palsies with diplopia, visual disturbances, hearing loss, sensorimotor deficits of extremities and cauda equina syndrome, radicular, neck and back pain, especially in a patient with cancer, should alert clinicians to consider LM [EANO: III, B; ESMO: IV, B].
-
•
A detailed neurological examination, potentially using a standard evaluation form, such as the one proposed by the Leptomeningeal Assessment in Neuro-Oncology (LANO) group, should be carried out at diagnosis [EANO: IV, not applicable (NA); ESMO: V, NA].
-
•
Brain MRI should include axial T1-weighted, axial FLAIR, axial diffusion-weighted, axial T2-weighted, post-gadolinium 3D T1-weighted and post-gadolinium 3D FLAIR sequences. Spinal MRI should include post-gadolinium sagittal T1-weighted sequences. Spine sagittal T1-weighted sequences without contrast and sagittal fat-suppression T2-weighted sequences, combined with axial T1-weighted images with contrast of regions of interest, may also be considered [EANO: III, B; ESMO: III, B].
-
•
One repeat lumbar puncture with optimised analysis conditions should be carried out in patients with suspected LM and initial negative or equivocal cytological CSF studies [EANO: IV, NA; ESMO: V, NA].
Staging and risk assessment: EANO–ESMO diagnostic criteria for LM
The use of standardised diagnostic criteria is highly recommended in clinical trials and when reporting on retrospective cohorts. Interpretation of results from clinical trials and cohort studies should also take the applied inclusion and exclusion criteria into consideration. In the first EANO–ESMO Clinical Practice Guideline (CPG), a classification of LM from solid tumours based on clinical, MRI and CSF cytology presentation was developed to address the large spectrum of LM presentation and guide clinical decision making (Table 2).1 This classification has been confirmed as prognostic and is useful for guiding treatment decisions.42 The diagnosis of LM is confirmed in the presence of tumour cells in the CSF or by a positive leptomeningeal biopsy (type I). In the absence of tumour cells in the CSF or histological confirmation of meningeal metastases, the diagnosis of LM can be deemed as probable (type II) in patients with a history of histologically proven cancer with a reasonable risk of LM and after consideration of alternative diagnoses. The diagnosis is probable in the presence of typical clinical findings and typical MRI findings (clinical plus neuroradiological evidence) whereas the diagnosis is possible in the presence of typical MRI findings but without typical clinical findings or in the presence of typical clinical findings and an MRI demonstrating hydrocephalus only or a normal MRI (clinical or neuroradiological evidence only). In patients with a histologically proven cancer presenting with atypical clinical signs of LM only and with normal MRI or hydrocephalus only, a diagnosis of LM should not be made. The authors propose that such patients should be documented as having ‘lack of evidence’ for LM. The type of neuroimaging findings is also integrated into the EANO–ESMO criteria, and five imaging subtypes are now proposed (types A-D, as described above).
Table 2.
Diagnostic criteria and level of evidence for LM
| Cytology/biopsy | MRI | Confirmed | Probablea | Possiblea | Lack of evidenceb | ||
|---|---|---|---|---|---|---|---|
| Type I: positive CSF cytology or biopsy | IA | + | Linear | + | NA | NA | NA |
| IB | + | Nodular | + | NA | NA | NA | |
| IC | + | Linear + nodular | + | NA | NA | NA | |
| ID | + | Hydrocephalus | + | NA | NA | NA | |
| ID | + | Normal | + | NA | NA | NA | |
| Type II: clinical findings and neuroimaging only | IIA | − or equivocal | Linear | NA | With typical clinical signs | Without typical clinical signs | NA |
| IIB | − or equivocal | Nodular | NA | With typical clinical signs | Without typical clinical signs | NA | |
| IIC | − or equivocal | Linear + nodular | NA | With typical clinical signs | Without typical clinical signs | NA | |
| IID | − or equivocal | Hydrocephalus | NA | NA | With typical clinical signs | Without typical clinical signs | |
| IID | − or equivocal | Normal | NA | NA | With typical clinical signs | Without typical clinical signs |
Adapted from Le Rhun et al.1
Type A: LM with typical linear MRI abnormalities; type B: LM with nodular disease; type C: LM with both linear and nodular disease; type D: LM without MRI abnormalities (except hydrocephalus).
CSF, cerebrospinal fluid; LM, leptomeningeal metastasis; MRI, magnetic resonance imaging; NA, not applicable.
Requires a history of cancer with a reasonable risk of LM and consideration of alternative diagnoses.
Including in patients with a history of cancer.
Recommendations
-
•
Clinical, imaging and CSF cytology assessments are all mandatory to classify LM [EANO: IV, NA; ESMO: IV, B].
-
•
The use of standardised diagnostic criteria to define cohorts of patients with LM is highly recommended [EANO: III, C; ESMO: IV, A].
-
•
Only patients with confirmed or probable LM after a complete clinical, cerebrospinal MRI and CSF analysis should be enrolled into LM-specific clinical trials [EANO: IV, NA; ESMO: IV, B].
Management of advanced and metastatic disease
Given the poor prognosis of patients with LM, the goal of treatment is to prolong survival with an acceptable quality of life and to prevent or delay neurological deterioration. Tumour-specific approaches are usually used in isolation or as part of combination therapy with the specific aim of controlling LM, but preferably to also control CNS parenchymal and systemic disease. Only a limited number of prospective clinical trials specifically evaluating LM have been completed and reported, and interpretation of these data is difficult due to the heterogeneity of inclusion criteria, incomplete diagnostic work-up, pooled cohorts of different tumour entities and the use of poorly defined response criteria. Trials enrolling patients with LM should be dedicated to these patients only or have a pre-planned arm or subgroup analysis for patients with LM with adequate assessment tools and statistical power. Given the paucity of clinical trials dedicated to LM, most of which addressed intrathecal pharmacotherapy, the authors also considered results for LM patient subgroups enrolled into trials of pharmacotherapy focused primarily on brain metastases, most of which were non-randomised and require further confirmation and validation of the results. Trials focussing on brain metastases have been analysed elsewhere.2 No clinical trial data are available to assess the efficacy of radiotherapy (RT). Thus, the recommendations below are largely based on expert opinion.
Pharmacotherapy
General considerations
LM often occurs in the setting of progressive brain metastases and extra-CNS disease, and so the systemic treatment should target the CNS and the extra-CNS compartment, if feasible. Systemic pharmacotherapy includes cytotoxic chemotherapy (ChT), targeted therapy and immunotherapy. The choice of pharmacotherapy depends on its efficacy in the primary cancer and its molecular type, ability to penetrate the CNS compartment, number and type of previous lines of treatment and patient characteristics. The rationale for choosing an intrathecal route of administration is to achieve higher CSF drug concentrations while minimising systemic toxicity. Only a few dedicated LM trials are available. In the absence of data from such trials, therapeutic options are often selected based on trials in patients with parenchymal brain metastases or those with metastatic disease. Available data on CSF penetration are provided below but these data should be interpreted with caution due to the low number of cases, potential variability according to the systemic dose administered, lack of established pharmacological tests and potential interpatient variability.
Intrathecal pharmacotherapy of LM
Although leptomeningeal contrast enhancement and increased levels of protein are observed in the vast majority of patients, the penetration rate of many drugs into the CSF is limited, with reported CSF:blood ratios usually below 5%.43,44 The intrathecal route may be more efficient for targeting floating tumour cells in the CSF42 and in the absence of blood–CSF barrier dysfunction, e.g. in case of diffuse leptomeningeal or ependymal spread. The penetration of drugs after intrathecal administration into nodules or brain parenchyma is probably limited to a few millimeters;45 however, no contemporary data for novel pharmacotherapy are available. Intrathecal administration is not appropriate in the presence of CSF flow blocks.
When intra-CSF ChT is used, administration may be via repeated lumbar punctures or preferably via a subgaleal reservoir linked to an intraventricular catheter. The best surgical procedure for reservoir implantation must be defined by the neurosurgeon in charge of the patient. The conceptual advantages of the ventricular route include the certainty that the drug is delivered into the CSF compartment and not the epidural or subdural space, a more uniform distribution of the agent, less invasiveness, improved patient comfort and a faster procedure, all of which improve compliance and safety of drug administration, particularly in patients requiring anticoagulation. No survival benefit of the ventricular versus the lumbar route has been demonstrated. However, in a subanalysis of a randomised trial using the ventricular route, a longer progression-free survival (PFS) was observed for methotrexate (MTX) whereas no significant difference was seen for liposomal cytarabine, presumably due to the different half-lives of these agents.25 The safety of ventricular devices has been shown in several cohort studies using different technologies and devices,46, 47, 48 but careful handling is required to ensure strictly aseptic puncture and drug application to minimise the risk of infection. Lumbar catheters have not been systematically studied in LM and thus remain experimental. Alternative approaches such as ventriculolumbar perfusion are interesting but require further study.49,50
In shunts with programmable valves (which are the most frequently used), the opening pressure can be temporarily raised to maximum so that the shunt does not drain CSF and drugs can be injected through the reservoir into the ventricles. Otherwise, shunts with a valve that can be switched on and off by pressing a button are available. Shunts can be blocked by either method for 12-24 h to allow the drug to be distributed within the CSF without drainage into the peritoneal space. A test occlusion without drug delivery for at least 12 h is recommended to check whether shunt-dependent patients will tolerate this procedure.51 Successful drug delivery via VP shunt devices to the ventriculothecal space with minimal relative peritoneal drug uptake was demonstrated using In-DTPA scintigraphy.52
An equivalent or larger volume of CSF should be removed before each intra-CSF injection. After lumbar injection, patients are often placed in a flat position for 1 h.53 Standard operating procedures are proposed in Table 3.
Table 3.
Proposed technical standard operating procedure for administration of intra-CSF anticancer pharmacotherapy
| Administration via the ventricular route (ventricular device in place) | Administration via the lumbar route | |
|---|---|---|
| Specific contraindications |
|
|
| Medial technical procedure |
|
|
| Comments |
No pressure should be necessary for the withdrawal of CSF or the injection of intrathecal pharmacotherapy. If no CSF can be withdrawn, stop the intervention and explore with the neurosurgeon on call whether neuroimaging is required. |
|
| Main risks |
|
|
Patients must only receive intrathecal pharmacotherapy in designated areas where staff are routinely involved in the administration of drugs by the intrathecal route. The nurse should first install the patient comfortably (seated position, leaning well forward, arched back or in left lateral decubitus). The whole procedure must respect rigorous aseptic conditions. The patient should remain lying in decubitus position for 1 h (strict decubitus after lumbar administration, as feasible). The absence of possible immediate side-effects should be checked before discharge.
CSF, cerebrospinal fluid; SOP, standard operating procedure.
A three-way connector can also be used, depending on the physician’s preferences.
The choice of intrathecal therapy should be guided by local approval as well as available safety and efficacy data for the considered tumour entity. Three agents are commonly used for the intrathecal treatment of LM: MTX, cytarabine, including liposomal cytarabine (not available at present), and thiotepa. Thus, the compounds routinely used for intra-CSF treatment do not have a key role as single agents for the systemic treatment of most common cancers causing LM. Different schedules for these agents have been proposed (Table 4), although there is currently no consensus regarding the optimal dose, frequency of administration or duration of treatment.
Table 4.
Characteristics and proposed schedules of administration of intra-CSF therapy
| Agent | Description | Half-life in the CSF | Recommended schedules of administration | Prophylaxis of AEs |
|---|---|---|---|---|
| MTX | Folate antimetabolite, cell cycle specific | 4.5-8 h | 10-15 mg twice weekly (total 4 weeks), then 10-15 mg once weekly (total 4 weeks), then 10-15 mg once monthly | Folinic acid rescue 25 mg every 6 h orally for 24 h starting 6 h after administration |
| Cytarabine | Pyrimidine nucleoside analogue, cell cycle specific | <1 h | 10 mg twice weekly (total 4 weeks), then 10 mg once weekly (total 4 weeks), then 10 mg once monthly | None |
| Liposomal cytarabinea | Pyrimidine nucleoside analogue, cell cycle specific | 14-21 days | 50 mg every 2 weeks (total 8 weeks), then 50 mg once monthly | Oral steroids, e.g. 6 mg/day dexamethasone or equivalent (day 1-4) |
| Thiotepa | Alkylating ethyleneimine compound, cell cycle non-specific | 3-4 h | 10 mg twice weekly (total 4 weeks), then 10 mg once weekly (total 4 weeks), then 10 mg once monthly | None |
AE, adverse event; CSF, cerebrospinal fluid; MTX, methotrexate.
This medicine is currently not available.
A critical review of the first six randomised trials of patients with LM from solid tumours (Supplementary Table S1, available at https://doi.org/10.1016/j.esmoop.2023.101624) revealed a lack of standardisation for evaluating response as well as methodological limitations regarding the tumour type (haematological versus solid tumours), baseline evaluation, evaluation of response to treatment and safety, and all trials experienced long accrual times.54 Moreover, all trials were open label and most compared different intrathecal agents, but no agent showed a significant survival advantage over another upon intrathecal administration.55,56 Longer time to neurological progression was reported for liposomal cytarabine compared with MTX.56 Combination intra-CSF ChT did not demonstrate superiority over single intra-CSF agents.57 Only two trials evaluated the addition of intrathecal treatment to systemic treatment versus systemic treatment alone, both in patients with breast cancer. The first trial attempted to explore the value of adding intra-CSF MTX to systemic therapy and involved-field RT, but it was closed prematurely.58 In this trial, 35 patients were evaluated based on clinical findings alone. There were no differences in clinical response or overall survival (OS; primary endpoint), but intra-CSF ChT was associated with more treatment-related neurotoxicity (scored according to a local scale) compared with the control arm (47% versus 6%). The complication rate in the intra-CSF ChT arm was also high (18% of reservoir revisions) compared with other cohorts (<7.3% of reservoir revisions).46, 47, 48 The second trial enrolled 73 patients and showed a significantly longer LM PFS (primary endpoint) with intrathecal liposomal cytarabine plus systemic therapy versus systemic therapy alone {3.8 months [95% confidence interval (CI) 2.3-6.8 months] versus 2.2 months [95% CI 1.3-3.1 months]};59 the median OS was numerically superior with intrathecal therapy: 7.3 months (95% CI 3.9-9.6 months) in the experimental group versus 4.0 months (95% CI 2.2-6.3 months) in the control group.
Toxicities of the various intrathecal agents differ. More neurological complications were observed with MTX than with thiotepa.55 For MTX and liposomal cytarabine, Cancer and Leukemia Group B (CALGB)-expanded Common Toxicity Criteria (CTC) treatment-related grade ≥3 toxicity was similar.56 The combination of MTX and whole brain RT (WBRT) is not recommended, especially MTX after WBRT, based on older literature from the haematological cancer field.
No significant difference in incidence rates of severe AEs was noted between systemic therapy alone versus systemic therapy plus intrathecal liposomal cytarabine, except for more systemic infections in the experimental group; the Quality-adjusted Time Without Symptoms of disease and Toxicity (Q-TWiST) difference was also not significant.59
Intrathecal topotecan was explored in a phase II trial in 62 patients with LM from various primaries.60 However, further data are needed to assess its role in LM. New therapeutic intrathecal agents are currently being explored. A meta-analysis of 58 patients with HER2-positive breast cancer and LM treated with intrathecal trastuzumab alone (n = 20) or in combination with systemic pharmacotherapy (n = 37) reported a median OS of 13.2 months.61 Two phase I/II studies have shown a good tolerance of intrathecal trastuzumab in human epidermal growth factor receptor 2 (HER2)-positive breast cancer. In the first trial (NCT01373710), intrathecal trastuzumab was administered alone or in combination with systemic pharmacotherapy once weekly. The recommended phase 2 dose (RP2D) was 150 mg.62 In the other trial (NCT01325207), intrathecal trastuzumab was administered twice per week for 4 weeks, then weekly for 4 weeks and then every 2 weeks; the RP2D was 80 mg.63 In both trials, the final dose selected was not associated with significant toxicity but was the predefined highest dose. A trial evaluating any type of CNS RT followed by intrathecal trastuzumab and pertuzumab in patients with LM from HER2-positive breast cancer is ongoing (NCT04588545).64
Intrathecal pemetrexed was explored in a phase I/II trial of 30 patients with LM from epidermal growth factor receptor (EGFR)-mutant non-small-cell lung cancer (NSCLC). In this trial, 50 mg pemetrexed was combined with vitamin B12 and folic acid supplementation.65 Treatment was well tolerated and a clinical response rate of 85% was reported, the significance of which remains uncertain.
Intrathecal nivolumab has been explored in a phase I/II trial in patients with LM from melanoma (NCT03025256). At doses of 20 and 50 mg every 14 days, nivolumab was well tolerated in combination with systemic nivolumab.66 However, the introduction of such strategies into routine clinical practice should be based on data from randomised studies.
The optimal duration of intra-CSF treatment has also not been adequately explored. Most patients are treated until progression or for 1 year, if tolerated. In the absence of evidence from appropriate clinical trials, clinical symptoms, MRI and CSF findings and tolerance of treatment should guide individual decisions on the duration of treatment. Notably, the role of persisting positive CSF cytology alone for decision making regarding the continuation of treatment remains controversial.12
Systemic pharmacotherapy of LM
Breast cancer
Very few data are available on systemic treatment of LM from breast cancer. Most data come from sub-cohorts or pilot studies with <30 patients and most of them had limitations in the inclusion criteria. More details are provided in Section 2.1 of the Supplementary Material, available at https://doi.org/10.1016/j.esmoop.2023.101624.
NSCLC
Only preliminary data are available from cohorts with <45 patients and without complete work-up for the assessment of the LM diagnosis. Oncogene addiction offers the prospect of new generations of CNS-penetrant and active targeted therapies in LM. Promising results have been reported in patients with EGFR-mutated NSCLC and LM using osimertinib, the agent with the most available data. A phase II trial was also carried out exploring ceritinib in 18 patients with confirmed or probable LM from ALK-positive NSCLC. Very few patients with LM from NSCLC treated with an immune checkpoint inhibitor at the time of LM diagnosis have been evaluated. More details are provided in Section 2.2 of the Supplementary Material, available at https://doi.org/10.1016/j.esmoop.2023.101624.
Melanoma
No dedicated trials have been published for patients with LM from melanoma. Four patients with LM from melanoma were treated with systemic nivolumab in a phase II trial within a cohort of 16 patients with brain metastases progressing after local therapy, with neurological symptoms or with LM. The median OS for this cohort was 5.1 months.67 Small retrospective cohorts have also been reported, mostly with heterogeneous interventions.10,14,68
LM from other cancers
No trial evaluating systemic pharmacotherapy in patients with LM from other cancers has been published. Therapeutic options should be discussed considering the recommendations and results of trials in brain metastases or advanced metastatic cancer.
High-dose systemic pharmacotherapy of LM
Cytotoxic CSF concentrations of MTX or thiotepa may be achieved using high-dose systemic administration, and these agents have induced responses in patients with LM from various solid tumours.69, 70, 71 The major limitations of these approaches are haematological toxicity and their incompatibility with other systemic regimens potentially needed for the control of systemic disease. Accordingly, these regimens are not used in current clinical practice.
RT
No randomised clinical trial to assess the efficacy and tolerance of RT has been conducted in patients with LM. RT can result in rapid symptom improvement but has not been shown to improve OS. Focal RT, given as involved-field hypofractionated stereotactic RT or stereotactic radiosurgery, is the consensual preferred option to treat nodular disease and symptomatic cerebral or spinal sites, notably in patients with favourable prognostic factors. In exceptional cases, focal RT can be carried out for cauda equina syndrome or cranial nerve palsies after exclusion of other causes, even in the absence of corresponding MRI findings at the symptomatic level and positive CSF cytology. For patients with cauda equina syndrome, target volumes usually include the lumbosacral vertebrae. Typical target volumes for cranial neuropathies include the skull base, including the cribriform plate and the whole pituitary fossa, the entire length of the optic nerves, the basilar cistern and the spinal canal to the plane up to the second cervical vertebra. A margin of 5 mm below the cribriform plate and at least 10 mm for the rest of the skull base is recommended in order to cover cranial nerve meningeal reflections. In the presence of CSF flow obstruction, restoration of CSF flow can be obtained by focal RT in 30% of patients with spinal blocks and in 50% of patients with intracranial blocks.72 RT in this setting has been proposed to reduce the toxicity from, and enhance the efficacy of, intra-CSF therapy.
No association between WBRT and OS has been reported in retrospective studies of contemporary LM patients.7,9,14,20,27,28,49,73 Accordingly, WBRT should not be considered standard of care for patients with newly diagnosed LM, mainly because of the absence of a survival benefit but also because of toxicity, notably in patients receiving concomitant systemic treatment. WBRT is usually considered for patients with extensive nodular or symptomatic linear LM or co-existing brain metastases as an entirely palliative intervention. WBRT at doses of 30 Gy in 10 fractions of 3 Gy is usually administered, although an abbreviated course of 20 Gy in 5 fractions of 4 Gy may be considered in selected patients with a poor prognosis.7,74
Cerebrospinal RT is rarely an option for adults with LM from solid cancers because of the risk of bone marrow toxicity, enteritis and mucositis, as well as the usual co-existence of systemic disease. Common Terminology Criteria for Adverse Events (CTCAE) grade 3-4 myelosuppression was observed in up to 37% of patients. A survival time of 3.4-4.8 months was reported in small cohorts of patients selected for this approach, and up to half of the patients did not finish the planned course of RT.75,76 A randomised phase II trial comparing proton craniospinal irradiation and photon involved-field RT showed a benefit for CNS PFS and OS in patients treated with protons.77 This trial compared two different techniques of RT and different volumes of irradiation. More data are required to define how proton craniospinal therapy should be integrated with other therapeutic options. Concomitant craniospinal RT and systemic or intra-CSF treatment should be avoided outside of clinical trials to prevent severe toxicity, notably myelosuppression for systemic treatment and neurotoxicity for intra-CSF treatment. Intra-CSF administration of radioisotopes or radiolabelled monoclonal antibodies is currently being explored in the context of clinical trials.
Integrated therapeutic approaches
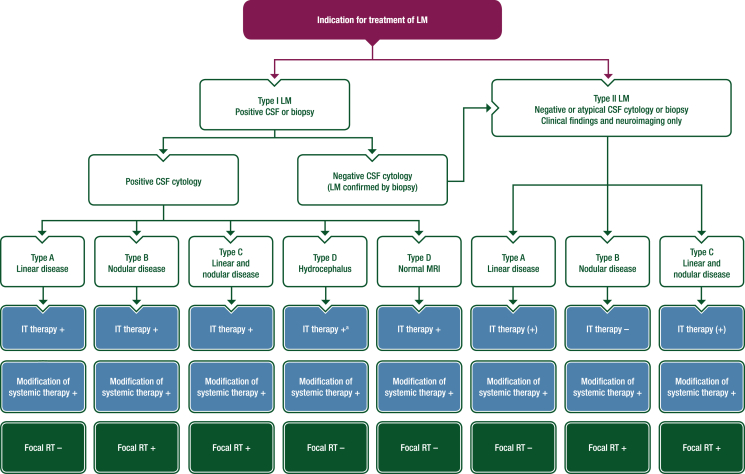
The management of patients with LM should follow multidisciplinary tumour board recommendations throughout the disease course. The therapeutic strategy should consider the general health of the patient, histological and molecular subtype of the primary cancer, medical history and oncological history with previous lines of treatment, extent and available therapeutic options for extra-CNS disease, presence of concomitant brain metastases, clinical neurological status, imaging presentation of LM and the CSF cytology. The therapeutic recommendations summarised in Figure 1 and Table 5 are largely based on small prospective cohort data, retrospective cohort data or expert agreements and must be considered as low level of evidence. Randomised clinical trials with standardised and complete criteria for diagnosis and response, including clinical status, neuroimaging and CSF analysis, in well-defined patient populations with appropriate endpoints are urgently needed to better define the role of systemic and intra-CSF treatments. This EANO–ESMO expert group strongly recommends prioritising randomised studies whenever possible and recommends against considering any approach as standard of care based on preliminary results, however promising, obtained from non-randomised small prospective studies or retrospective cohorts. The limited evidence should be made transparent when discussing treatment options with patients when no data from clinical trials are available.
Figure 1.
Treatment algorithm for the management of patients with LM. Intrathecal therapy: Intrathecal therapy should be considered in the presence of tumour cells in the CSF or in the presence of linear LM. Systemic pharmacotherapy: A modification of systemic therapy should always be discussed in case of progressive brain metastases or progressive extra-CNS disease. RT: Progressive brain metastases or progressive extra-CNS disease should be considered when selecting the choice of RT technique (in case of progressive brain metastases amenable to SRT, SRT should be preferred; in case of progressive brain metastases not amenable to SRT or in case of progressive extra-CNS disease without effective options for systemic treatment, WBRT can be considered). +, recommended; (+), can be considered; –, not recommended. CNS, central nervous system; CSF, cerebrospinal fluid; IT, intrathecal; LM, leptomeningeal metastasis; MRI, magnetic resonance imaging; RT, radiotherapy; SRT, stereotactic radiotherapy; VP, ventriculoperitoneal; WBRT, whole brain radiotherapy.aIf no CSF obstruction and no indication for a VP shunt without ON/OFF valve.
Table 5.
Overall EANO–ESMO response assessment and guidance for LM treatment
| Clinical | Cerebrospinal imaging | CSF cytology | Response determination | Action |
|---|---|---|---|---|
| Improved or stable | Improved | Improved or stable | Response | Continue treatment |
| Stable | Stable | Stable | Stable | Continue treatment |
| Worse | Improved or stable | Improved or stable | Suspicion of progression | Consider alternative neurological diagnoses or other reasons for clinical deterioration, change treatment only if there is no other explanation and if there is significant worsening of clinical signs for >2 weeks |
| Improved or stable | Improved or stable | Worse | Suspicion of progressiona or progression in case of de novo appearance of tumour cells in the CSFb |
aContinue treatment with close follow-up (e.g. for 4 weeks) bChange treatment for de novo appearance of tumour cells from the same CSF site (lumbar or ventricular) |
| Worse | Improved or stable | Worse | Suspicion of progressiona or progression in case of de novo appearance of tumour cells in the CSFb |
aConsider alternative neurological diagnoses; continue treatment with close follow-up (e.g. for 4 weeks) bChange treatment if there is worsening of clinical signs for >2 weeks or if there is appearance of tumour cells from the same CSF site (lumbar or ventricular) |
| Improved or stable | Worse | Improved or stable | Progression | Change treatment |
| Improved or stable | Worse | Worse | Progression | Change treatment |
| Worse | Worse | Improved or stable or worse | Progression | Change treatment |
Adapted from Le Rhun et al.1
In case of suspicion of clinical deterioration or uncertain imaging assessment, the response should be considered as stable. In these situations, a new assessment should be planned within a reasonably short time interval.
CSF, cerebrospinal fluid; EANO, European Association of Neuro-Oncology; ESMO, European Society for Medical Oncology; LM, leptomeningeal metastasis.
Recommendations
-
•
Trials enrolling patients with LM should be dedicated to patients with LM, ideally newly diagnosed LM, or have a pre-planned arm or subgroup analysis for patients with LM with adequate assessment tools and statistical power [EANO: IV, NA; ESMO: IV, C].
-
•
Intra-CSF pharmacotherapy should be considered for patients with type IA/C LM [EANO: III, B; ESMO: III, B].
-
•
Intra-CSF ChT should be administered via the ventricular rather than lumbar route whenever feasible [EANO: IV, NA; ESMO: V, NA].
-
•
Systemic pharmacotherapy based on the primary tumour and previous treatment should be considered for most patients with type B/C LM [EANO: IV, NA; ESMO: V, NA].
-
•
Focal RT should be considered for circumscribed, notably symptomatic lesions [EANO: IV, NA; ESMO: V, NA].
-
•
WBRT can be considered for extensive nodular or symptomatic linear LM [EANO: IV, NA; ESMO: V, NA].
-
•
Randomised studies should be conducted whenever possible [EANO: IV, NA; ESMO: V, NA].
-
•
Any approach based on preliminary results, even if promising, obtained from non-randomised small prospective studies or retrospective cohorts should not be considered as standard of care [EANO: IV, NA; ESMO: V, NA].
Follow-up, long-term implications and survivorship
Monitoring and follow-up
No robust data are available and monitoring recommendations for LM are based on consensus and expert opinion. Response should be evaluated based on a complete neurological assessment, cerebrospinal neuroimaging evaluation and standard CSF cytology (Table 5). Clinical, imaging and CSF evaluations should be carried out at baseline and at defined timepoints thereafter to assess response. Evaluations should be planned every 2 months for the first 6 months in order to modify the treatment early, if needed, before clinical deterioration and every 3 months thereafter in stable patients. However, evaluations should be carried out earlier whenever there is suspicion of progression based on clinical assessment. Ideally, assessment of CNS disease should be synchronised with general disease assessment.
Compartmental response assessment criteria
Clinical baseline and follow-up assessments should be carried out by the same physician wherever possible. Standardised scorecards should be developed, validated and used for clinical,12,13 imaging17 and cytological assessment1,12 of LM in clinical trials, but can also be used in routine practice.
Neurological symptoms or signs that develop during follow-up and are related or suspected to be related to comorbidities or any other medical event should be considered as non-evaluable and appropriate tests should be carried out to exclude a different concomitant condition or treatment-related toxicity. If these symptoms and signs are retrospectively considered as LM-related (and not related to any comorbidity), the date of appearance of the symptoms or signs should be considered to assess the response. Comedications such as steroids, pain killers and anti-emetics should be considered when evaluating the clinical response. A general overall assessment (improvement, stability, deterioration) should be carried out at the end of the clinical evaluation. This rating should be compared with the baseline neurological evaluation or the best clinical evaluation after enrolment to determine the best clinical response.
A cerebrospinal MRI is recommended to evaluate the imaging response in clinical trials and is also preferable in routine practice. The value and diagnostic yield of spinal sequences in patients asymptomatic for spinal disease remain controversial. However, as 34%-52% of patients15,16 may present with spinal LMs and neurological deficits rarely improve, the addition of spinal sequences to brain sequences, ideally during the same procedure, is recommended to optimise the clinical management of patients with LM. Ideally, brain and spinal MRI should be carried out on the same day or within an interval of not more than 7 days. During follow-up, MRI scans should be carried out preferably on the same scanner or at least a device of identical field strength and the standardised imaging protocol should be used at all timepoints during follow-up (Table 1). Validated criteria should be used for response assessment. The response assessment should consider changes in size but not changes in intensity of contrast enhancement (Supplementary Table S2, available at https://doi.org/10.1016/j.esmoop.2023.101624).17
In trials using immunotherapy, iRANO criteria should be adapted for the imaging response assessment.78 If a patient is clinically stable or if clinical decline can be attributed to causes other than LM, then deterioration on MRI should not result in premature termination of treatment. In these cases, imaging may be repeated at least 4 weeks later. If progression is confirmed, the date of progression within clinical trials should be backdated to the initial MRI with criteria of progression. In clinical trials, concomitant brain metastases or extradural spinal metastases are evaluated separately for response. Other imaging modalities, such as magnetic resonance (MR) spectroscopy, MR perfusion or PET, currently have no role in the assessment of LM during follow-up.
CSF studies should be carried out at the time of each injection in patients undergoing intrathecal pharmacotherapy, and every 2-3 months in patients not undergoing intra-CSF pharmacotherapy. Criteria for CSF response assessment are proposed in Supplementary Table S3, available at https://doi.org/10.1016/j.esmoop.2023.101624. A complete CSF cytological response requires a conversion of a previously positive to a negative CSF response maintained for at least 4 weeks. If only lumbar CSF was positive and the patient is treated via a ventricular reservoir, the CSF response cannot be evaluated unless further lumbar CSF samples are obtained. An unequivocal de novo appearance of malignant cells in the CSF after repeated negative CSF cytology carried out under optimised conditions should be considered as progression and does not require a confirmatory analysis. In contrast, a change from negative to equivocal is not considered relevant for clinical decision making. CSF cytology may remain positive in patients with stable or improved clinical or imaging features. CSF cell counts could, in principle, be obtained specifically for tumour, as opposed to non-neoplastic, cells but this has remained challenging and requires more sophisticated techniques than those commonly available at most centres. The levels of CSF protein, glucose or lactate, or novel biomarkers or new methodologies for the identification of tumour cells in the CSF or circulating tumour DNA load have not been integrated into routine response determination.
LM overall response assessment
The overall response should consider the three levels of assessment (Table 5). Alternative neurological diagnoses, decline related to comorbid events or concomitant medication or other reasons for clinical deterioration should be considered in case of clinical deterioration with improved or stable cerebrospinal imaging and standard CSF cytology. The treatment should be changed only if there is no other explanation and if there is significant worsening of clinical signs for >2 weeks. If the progression is confirmed at the next evaluation, the date of progression within clinical trials should be backdated to initial MRI with criteria of progression. When reporting on treatment efficacy, response to treatment and the prognosis of LM, mixed populations including both patients with newly diagnosed and recurrent LM should be avoided.
Response to treatment and prognosis
Most neurological deficits in patients with LM are irreversible. The best anticipated clinical response is usually stable disease. Almost no prospective studies report on the best response to treatment in patients with newly diagnosed LM and standardising response assessment has remained challenging.1,79 Moreover, few clinical trials have used standardised scorecards for the response assessment. The best responses are usually clinical stabilisation, imaging stabilisation or deterioration.59,62,80,81 CSF cytology responses are more frequently reported.59
Median OS in large contemporary cohorts of >90 patients with newly diagnosed LM varies: 3.8-5.4 months in breast cancer,6,27,82 4.2-8.1 months in lung cancer,15,28 4.8 months in melanoma14 and 3 months in cohorts including various tumours.83 Long-term survivors may be observed, notably in breast cancer, with 63 out of 318 patients (20%) surviving >1 year in one study,16 thereby underlying the necessity to consider potential treatment-related toxicity in patients with LM.
Favourable prognostic factors reported in at least two large contemporary patient cohorts with confirmed or probable LM include younger age at LM diagnosis,49,82,84 good performance status at LM diagnosis,14,26-28,49,84 low tumour burden with absence of tumour cells in the CSF at LM diagnosis,15,42 absence of CSF flow interruptions,24,25,85 administration of systemic treatment6,14,27,28,42,84 and administration of intrathecal treatment.14,26,42,49,84 In most reports, WBRT has not been associated with improved OS,6,7,14,27,28,49 although rare reports of a link with improved OS do exist.26,84 In breast cancer, LM from triple-negative tumours was associated with a worse prognosis compared with other subtypes of breast cancers.6,82 In LM from NSCLC, the absence of a druggable oncogenic target was associated with a poor prognosis in one cohort.15 However, in LM from melanoma, BRAF status was not prognostic.14
Recommendations
-
•
The use of standardised scorecards for the assessment of clinic status, as well as imaging and CSF cytology data, are recommended for patient follow-up [EANO: IV, NA; ESMO: V, NA].
-
•
A detailed neurological examination using a standard evaluation form should be carried out every 2 months for the first 6 months and every 3 months thereafter in stable patients or at radiological progression or when new neurological symptoms or signs are reported [EANO: IV, NA; ESMO: V, NA].
-
•
Cerebrospinal MRI should be carried out every 6-12 weeks and at any timepoint where clinical progression is suspected [EANO: IV, NA; ESMO: V, NA].
-
•
CSF studies should be carried out every 6-12 weeks in patients undergoing intra-CSF pharmacotherapy [EANO: IV, NA; ESMO: V, NA].
Supportive care
Although the aim of this guideline is not to comprehensively describe palliative and supportive care for patients with LM, a few points deserve consideration. The role of steroids has not been specifically studied in patients with LM, notwithstanding their role for associated brain metastases, chemical meningitis or other systemic complications of cancer. When required clinically, the lowest dose of steroids should be used for the shortest time possible. Seizures should be managed using drugs that do not interact with systemic treatments. Primary prophylaxis is not recommended.86,87 Symptoms and signs related to increased intracranial pressure related to CSF circulation disturbances may be rapidly alleviated by CSF drainage. VP shunting may provide durable relief from symptomatic hydrocephalus88 and does not carry a relevant risk of peritoneal seeding in patients with LM from solid cancers. Shunt failure during the lifetime of patients with LM is not of major concern.88 In a cohort of 190 patients with LM who underwent a CSF shunt procedure, 83% of the patients experienced clinical improvement and 56% underwent further oncological treatment. One hundred and fifty patients (79%) had no complications; infections were observed in 9 patients (5%), subdural hygroma or haematoma in 25 patients [13%; which was symptomatic in 12 patients (6.3%)] and shunt malfunction in 9 patients (5%). An externalisation, removal or revision was indicated in 15 patients (8%).88 The median protein level was 0.68 g/l and high protein levels were not associated with shunt complications. National and institutional supportive care guidelines may provide further guidance.87,89
Outlook
Guidelines reflect knowledge and consensus at a given timepoint. Updates on these recommendations will be announced on the websites of EANO (www.eano.org) and ESMO (www.esmo.org).
Randomised trials based on well-defined diagnostic and inclusion criteria, in appropriately selected subgroups of LM patients, enriched for molecular genetic signatures, where feasible, and with adequate criteria for evaluation, are required to improve outcomes for patients with LM in a primary cancer-specific manner. Important questions to address include the role of liquid biopsies for the diagnosis and the management of LM, the role of intrathecal pharmacotherapy and novel systemic therapies, notably targeted agents and immunotherapy.
Methodology
This CPG was developed in accordance with the ESMO standard operating procedures for CPG development (http://www.esmo.org/Guidelines/ESMO-Guidelines-Methodology). The relevant literature has been selected by the expert authors. References were identified through searches of PubMed with the search terms ‘carcinomatous meningitis’, ‘cerebrospinal fluid’, ‘CNS’, ‘intrathecal’, ‘leptomeningeal’, ‘metastasis’, ‘neoplastic meningitis’, ‘trial’, ‘clinical’, ‘surgery’, ‘radiotherapy’, ‘chemotherapy’, ‘targeted therapy’, ‘immunotherapy’, ‘imaging’, ‘MRI’ and ‘PET’ in various combinations from 01 March 1993 to 31 April 2022. Articles were also identified through searches of the authors’ own files. Only papers in English were reviewed. The final reference list was generated by consensus of the authors and based on originality and relevance to the broad scope of this guideline. Levels of evidence and grades of recommendation were applied using the European Federation of Neurological Societies criteria, as recommended by EANO (Supplementary Table S4, available at https://doi.org/10.1016/j.esmoop.2023.101624),90 as well as an adapted version of the Infectious Disease Society of America-United States Public Health Service Grading System, as recommended by ESMO (Supplementary Table S5, available at https://doi.org/10.1016/j.esmoop.2023.101624).91 Statements without grading were considered justified standard clinical practice by the experts.
Acknowledgements
Manuscript editing support was provided by Jennifer Lamarre and Claire Bramely (ESMO Guidelines staff) and Martina Habeck and Angela Corstorphine of Kstorfin Medical Communications Ltd (KMC); this support was funded by ESMO.
Funding
No external funding has been received for the preparation of these guidelines. Production costs have been covered by ESMO from central funds.
Disclosure
ELR reports personal financial interests as an advisory board member for Bayer, Janssen, Leo Pharma, Pierre Fabre and Seattle Genetics; institutional funding from Bristol Myers Squibb (BMS). MW reports personal financial interests as an advisory board member for Bayer, Curevac, Merck (EMD), Novartis, Novocure and Philogen; personal financial interests as an independent data monitoring committee member for Orbus; institutional research grants from Quercis and Versameb. He also reports non-financial interests from a leadership role for the European Organisation for Research and Treatment of Cancer (EORTC). MvdB reports personal financial interests as an advisory board member from Agios, AstraZeneca, Boehringer, Carthera, Chimerix, Genenta, Nerviano, Roche and Sumitomo. He also reports non-financial interests as Chair of the EANO Guidelines Committee and for an advisory role as member of the steering committee (EUDRACT: 2022-000627-20) for Fore Biotherapeutics. DB reports institutional financial interests as a local principal investigator for BMS and invited speaker for AstraZeneca and Medtalks. JF reports personal financial interests as an invited speaker for Novartis and SeaGen. RR reports personal financial interests for advisory board membership for GENENTA, expert testimony for Novocure and as an invited speaker for Bayer and UCB. DS reports personal financial interests as an advisory board member for 4SC, Array Biopharma, BMS, Immunocore, Merck Serono, Merck Sharp & Dohme (MSD), NEKTAR, Neracare, Novartis, Pfizer, Philogen, Pierre Fabre, Roche, Sandoz and Sanofi/Regeneron; personal financial interests as an invited speaker for BMS, Merck, Merck Serono, MSD, Novartis, Roche and Sanofi; institutional financial interests as steering committee member for 4SC, BMS, MSD, Nektar and Novartis; institutional financial interests as coordinating principal investigator for 4SC, BMS, MSD, Nektar, Novartis and Pierre Fabre; institutional financial interests as local principal investigator for Philogen, Roche and Sanofi; institutional research grants from Array/Pfizer, BMS, MSD and Novartis. He also reports non-financial interests as a member of the board of directors of the EORTC Melanoma Group. JS is co-founder of Mosaic Biomedicals and reports honoraria for providing consultancy and attending advisory boards from Mestaq Biomedicals, Merck Serono, Northern Biologics, Mosaic Biomedicals, Omniscope, Sanofi and Sumitomo; receipt of research support from Roche Glycart AG, Hoffmann-La Roche Ltd, AstraZeneca, Isarna Therapeutics, Mosaic Biomedicals SL, Northern Biologics Inc. and Ridgeline Therapeutic. JCT reports personal financial interests as royalties from Springer and receipt of a research grant from Novocure to fund a study nurse and institutional funding from Munich Surgical Instruments. He also reports non-financial interests as Chairman of the neuro-oncology section for the World Federation of Neurological Societies. WW reports institutional funding for clinical trials from Apogenix, Pfizer and Roche; institutional financial interests related to coordinating principal investigator (PI) roles for Enterome and Vaximm. He reports non-financial interests for leadership roles at Deutscher Wissenschaftsrat, EANO, Neuroonkologische Arbeitsgemeinschaft and as a member of Leopoldina/Deutsche Gesellschaft der Wissenschaften. GM reports personal financial interests as an invited speaker for BrainLab and treasurer for the EORTC; and institutional funding from AstraZeneca. SP reports personal fees for an editorial role as an Associate Editor for Annals of Oncology; fees paid to her institution as an invited speaker from AstraZeneca, BMS, Boehringer Ingelheim, e-cancer, Eli Lilly, Fishawack, Illumina, Imedex, Medscape, Mirati, MSD, Novartis, OncologyEducation, PER, Pfizer, PRIME, RMEI, Roche/Genentech, RTP, Sanofi and Takeda; fees paid to her institution for advisory board membership from AbbVie, Amgen, Arcus, AstraZeneca, Bayer, BeiGene, Bio Invent, Biocartis, Blueprint Medicines, BMS, Boehringer Ingelheim, Daiichi Sankyo, Debiopharm, Eli Lilly, F-Star, Foundation Medicine, Genzyme, Gilead, GSK, Illumina, Incyte, IQVIA, iTeos, Janssen, Merck Serono, Mirati, MSD, Novartis, Novocure, Pfizer, Pharma Mar, Phosplatin Therapeutics, Regeneron, Roche/Genentech, Sanofi, Seattle Genetics, Takeda and Vaccibody; institutional funding as a steering committee member from AstraZeneca, BeiGene, BMS, iTeos, Mirati, MSD, Pharma Mar, Phosplatin Therapeutics and Roche/Genentech; institutional funding as a coordinating PI from AstraZeneca; institutional funding as a trial chair from GSK and Roche/Genentech; non-remunerated role as President and Council Member for the Ballet Béjart Lausanne Foundation; non-remunerated leadership roles as President of ESMO (2020-2022), Vice-President of Swiss Academy of Multidisciplinary Oncology (SAMO), Vice-President of Lung Group for SAKK (Swiss Group for Clinical Cancer Research); non-remunerated role as PI involved in academic trials for the European Thoracic Oncology Platform (ETOP)/EORTC/SAKK; non-remunerated role as Council Member and Scientific Committee Chair for ETOP/International Breast Cancer Study Group (IBCSG) Partners; member of the American Association for Cancer Research (AACR), American Society of Clinical Oncology (ASCO), ASMAC/VSAO (Association of Swiss Interns and Residents), FMH (Association of Swiss Physicians) and the International Association for the Study of Lung Cancer (IASLC). GC reports personal fees for advisory board membership from AstraZeneca, BMS, Celcuity, Daiichi Sankyo, Ellipsis, Exact Sciences, Eli Lilly, Merck, Pfizer, Roche and Veracyte; personal fees as an invited speaker from AstraZeneca, Daiichi Sankyo, Novartis, Pfizer and Roche; personal fees for a writing engagement from Pfizer; an institutional research grant from Merck for an investigator-initiated trial; institutional funding for phase I studies from Astellas, AstraZeneca, Blueprint Medicine, BMS, Daiichi Sankyo, Kymab, Novartis, Philogen, Relay Therapeutics (coordinating PI), Roche and Sanofi; non-remunerated roles as Advisor for the Ministry of Health at the Italian National Health Council; as a member of the scientific council at Europa Donna, of the advisory council for European Society of Breast Cancer Specialists (EUSOMA) and of the Board of Directors at Lega Italiana Lotta ai Tumori; and a non-remunerated advisory role at Fondazione Beretta. MP reports personal financial interests for advisory board membership for Abbvie, Adastra, AstraZeneca, Bayer, BMJ Journals, BMS, CMC Contrast, Daiichi Sankyo, Gan & Lee Pharmaceuticals, Gerson Lehrman, GSK, Eli Lilly, Medahead, MedMedia, MSD, Mundipharma, Novartis, Roche, Sanofi and Tocagen; institutional funding as coordinating PI for PharmaMar and institutional research grants from Abbvie, Boehringer-Ingelheim, BMS, Daiichi Sankyo, GSK, MSD, Novocure and Roche. He reports non-financial interests as Past President of EANO, Brain Tumor Group Chair for the EORTC and as a member of the Multi-Site Guideline Advisory Group for ASCO. PW has declared no conflicts of interest.
Supplementary data
References
- 1.Le Rhun E., Weller M., Brandsma D., et al. EANO-ESMO Clinical Practice Guidelines for diagnosis, treatment and follow-up of patients with leptomeningeal metastasis from solid tumours. Ann Oncol. 2017;28(suppl 4):iv84–iv99. doi: 10.1093/annonc/mdx221. [DOI] [PubMed] [Google Scholar]
- 2.Le Rhun E., Guckenberger M., Smits M., et al. EANO-ESMO Clinical Practice Guidelines for diagnosis, treatment and follow-up of patients with brain metastasis from solid tumours. Ann Oncol. 2021;32(11):1332–1347. doi: 10.1016/j.annonc.2021.07.016. [DOI] [PubMed] [Google Scholar]
- 3.de Azevedo C.R.A.S., Cruz M.R.S., Chinen L.T.D., et al. Meningeal carcinomatosis in breast cancer: prognostic factors and outcome. J Neurooncol. 2011;104(2):565–572. doi: 10.1007/s11060-010-0524-y. [DOI] [PubMed] [Google Scholar]
- 4.Lara-Medina F., Crismatt A., Villarreal-Garza C., et al. Clinical features and prognostic factors in patients with carcinomatous meningitis secondary to breast cancer. Breast J. 2012;18(3):233–241. doi: 10.1111/j.1524-4741.2012.01228.x. [DOI] [PubMed] [Google Scholar]
- 5.Meattini I., Livi L., Saieva C., et al. Prognostic factors and clinical features in patients with leptominengeal metastases from breast cancer: a single center experience. J Chemother. 2012;24(5):279–284. doi: 10.1179/1973947812Y.0000000034. [DOI] [PubMed] [Google Scholar]
- 6.Le Rhun E., Taillibert S., Zairi F., et al. A retrospective case series of 103 consecutive patients with leptomeningeal metastasis and breast cancer. J Neurooncol. 2013;113(1):83–92. doi: 10.1007/s11060-013-1092-8. [DOI] [PubMed] [Google Scholar]
- 7.Morris P.G., Reiner A.S., Szenberg O.R., et al. Leptomeningeal metastasis from non-small cell lung cancer: survival and the impact of whole brain radiotherapy. J Thorac Oncol. 2012;7(2):382–385. doi: 10.1097/JTO.0b013e3182398e4f. [DOI] [PubMed] [Google Scholar]
- 8.Gwak H.S., Joo J., Shin S.H., et al. Ventriculolumbar perfusion chemotherapy with methotrexate for treating leptomeningeal carcinomatosis: a phase II study. Oncologist. 2014;19(10):1044–1045. doi: 10.1634/theoncologist.2014-0199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Kuiper J.L., Hendriks L.E., van der Wekken A.J., et al. Treatment and survival of patients with EGFR-mutated non-small cell lung cancer and leptomeningeal metastasis: a retrospective cohort analysis. Lung Cancer. 2015;89(3):255–261. doi: 10.1016/j.lungcan.2015.05.023. [DOI] [PubMed] [Google Scholar]
- 10.Geukes Foppen M.H., Brandsma D., Blank C.U., et al. Targeted treatment and immunotherapy in leptomeningeal metastases from melanoma. Ann Oncol. 2016;27(6):1138–1142. doi: 10.1093/annonc/mdw134. [DOI] [PubMed] [Google Scholar]
- 11.Kwon J., Chie E.K., Kim K., et al. Impact of multimodality approach for patients with leptomeningeal metastases from solid tumors. J Korean Med Sci. 2014;29(8):1094–1101. doi: 10.3346/jkms.2014.29.8.1094. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Chamberlain M., Junck L., Brandsma D., et al. Leptomeningeal metastases: a RANO proposal for response criteria. Neuro Oncol. 2017;19(4):484–492. doi: 10.1093/neuonc/now183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Nayak L., DeAngelis L.M., Brandes A.A., et al. The Neurologic Assessment in Neuro-Oncology (NANO) scale: a tool to assess neurologic function for integration into the Response Assessment in Neuro-Oncology (RANO) criteria. Neuro Oncol. 2017;19(5):625–635. doi: 10.1093/neuonc/nox029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Ferguson S.D., Bindal S., Bassett R.L., et al. Predictors of survival in metastatic melanoma patients with leptomeningeal disease (LMD) J Neurooncol. 2019;142(3):499–509. doi: 10.1007/s11060-019-03121-2. [DOI] [PubMed] [Google Scholar]
- 15.Nevel K.S., DiStefano N., Lin X., et al. A retrospective, quantitative assessment of disease burden in patients with leptomeningeal metastases from non-small-cell lung cancer. Neuro Oncol. 2020;22(5):675–683. doi: 10.1093/neuonc/noz208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Morikawa A., Jordan L., Rozner R., et al. Characteristics and outcomes of patients with breast cancer with leptomeningeal metastasis. Clin Breast Cancer. 2017;17(1):23–28. doi: 10.1016/j.clbc.2016.07.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Le Rhun E., Devos P., Winklhofer S., et al. Prospective validation of a new imaging scorecard to assess leptomeningeal metastasis: a joint EORTC BTG and RANO effort. Neuro Oncol. 2022;24(10):1726–1735. doi: 10.1093/neuonc/noac043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Niwińska A., Rudnicka H., Murawska M. Breast cancer leptomeningeal metastasis: propensity of breast cancer subtypes for leptomeninges and the analysis of factors influencing survival. Med Oncol. 2013;30(1):408. doi: 10.1007/s12032-012-0408-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Yust-Katz S., Garciarena P., Liu D., et al. Breast cancer and leptomeningeal disease (LMD): hormone receptor status influences time to development of LMD and survival from LMD diagnosis. J Neurooncol. 2013;114(2):229–235. doi: 10.1007/s11060-013-1175-6. [DOI] [PubMed] [Google Scholar]
- 20.Park J.H., Kim Y.J., Lee J.O., et al. Clinical outcomes of leptomeningeal metastasis in patients with non-small cell lung cancer in the modern chemotherapy era. Lung Cancer. 2012;76(3):387–392. doi: 10.1016/j.lungcan.2011.11.022. [DOI] [PubMed] [Google Scholar]
- 21.Debnam J.M., Mayer R.R., Chi T.L., et al. Most common sites on MRI of intracranial neoplastic leptomeningeal disease. J Clin Neurosci. 2017;45:252–256. doi: 10.1016/j.jocn.2017.07.020. [DOI] [PubMed] [Google Scholar]
- 22.Umemura S., Tsubouchi K., Yoshioka H., et al. Clinical outcome in patients with leptomeningeal metastasis from non-small cell lung cancer: Okayama Lung Cancer Study Group. Lung Cancer. 2012;77(1):134–139. doi: 10.1016/j.lungcan.2012.03.002. [DOI] [PubMed] [Google Scholar]
- 23.Lombardi G., Zustovich F., Farina P., et al. Neoplastic meningitis from solid tumors: new diagnostic and therapeutic approaches. Oncologist. 2011;16(8):1175–1188. doi: 10.1634/theoncologist.2011-0101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Grossman S.A., Trump D.L., Chen D.C., et al. Cerebrospinal fluid flow abnormalities in patients with neoplastic meningitis. An evaluation using 111indium-DTPA ventriculography. Am J Med. 1982;73(5):641–647. doi: 10.1016/0002-9343(82)90404-1. [DOI] [PubMed] [Google Scholar]
- 25.Glantz M.J., Hall W.A., Cole B.F., et al. Diagnosis, management, and survival of patients with leptomeningeal cancer based on cerebrospinal fluid-flow status. Cancer. 1995;75(12):2919–2931. doi: 10.1002/1097-0142(19950615)75:12<2919::aid-cncr2820751220>3.0.co;2-9. [DOI] [PubMed] [Google Scholar]
- 26.Lee S.J., Lee J.I., Nam D.H., et al. Leptomeningeal carcinomatosis in non-small-cell lung cancer patients: impact on survival and correlated prognostic factors. J Thorac Oncol. 2013;8(2):185–191. doi: 10.1097/JTO.0b013e3182773f21. [DOI] [PubMed] [Google Scholar]
- 27.Griguolo G., Pouderoux S., Dieci M.V., et al. Clinicopathological and treatment-associated prognostic factors in patients with breast cancer leptomeningeal metastases in relation to tumor biology. Oncologist. 2018;23(11):1289–1299. doi: 10.1634/theoncologist.2018-0200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Lee J., Choi Y.L., Han J., et al. Osimertinib improves overall survival in patients with EGFR-mutated NSCLC with leptomeningeal metastases regardless of T790M mutational status. J Thorac Oncol. 2020;15(11):1758–1766. doi: 10.1016/j.jtho.2020.06.018. [DOI] [PubMed] [Google Scholar]
- 29.Glantz M.J., Cole B.F., Glantz L.K., et al. Cerebrospinal fluid cytology in patients with cancer: minimizing false-negative results. Cancer. 1998;82(4):733–739. doi: 10.1002/(sici)1097-0142(19980215)82:4<733::aid-cncr17>3.0.co;2-z. [DOI] [PubMed] [Google Scholar]
- 30.Dux R., Kindler-Röhrborn A., Annas M., et al. A standardized protocol for flow cytometric analysis of cells isolated from cerebrospinal fluid. J Neurol Sci. 1994;121(1):74–78. doi: 10.1016/0022-510x(94)90159-7. [DOI] [PubMed] [Google Scholar]
- 31.Pan Z., Yang G., Wang Y., et al. Thinprep plus Papanicolaou stain method is more sensitive than cytospin-coupled Wright Giems stain method in cerebrospinal fluid cytology for diagnosis of leptomeningeal metastasis from solid tumors. PLoS One. 2015;10(4) doi: 10.1371/journal.pone.0122016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.de Jongste A.H., Kraan J., van den Broek P.D., et al. Use of TransFix™ cerebrospinal fluid storage tubes prevents cellular loss and enhances flow cytometric detection of malignant hematological cells after 18 hours of storage. Cytometry B Clin Cytom. 2014;86(4):272–279. doi: 10.1002/cyto.b.21097. [DOI] [PubMed] [Google Scholar]
- 33.van Bussel MTJ, Pluim D., Milojkovic Kerklaan B., et al. Circulating epithelial tumor cell analysis in CSF in patients with leptomeningeal metastases. Neurology. 2020;94(5):e521–e528. doi: 10.1212/WNL.0000000000008751. [DOI] [PubMed] [Google Scholar]
- 34.Torre M., Lee E.Q., Chukwueke U.N., et al. Integration of rare cell capture technology into cytologic evaluation of cerebrospinal fluid specimens from patients with solid tumors and suspected leptomeningeal metastasis. J Am Soc Cytopathol. 2020;9(1):45–54. doi: 10.1016/j.jasc.2019.09.001. [DOI] [PubMed] [Google Scholar]
- 35.Malani R., Fleisher M., Kumthekar P., et al. Cerebrospinal fluid circulating tumor cells as a quantifiable measurement of leptomeningeal metastases in patients with HER2 positive cancer. J Neurooncol. 2020;148(3):599–606. doi: 10.1007/s11060-020-03555-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Magbanua M.J.M., Roy R., Sosa E.V., et al. Genome-wide copy number analysis of cerebrospinal fluid tumor cells and their corresponding archival primary tumors. Genom Data. 2014;2:60–62. doi: 10.1016/j.gdata.2014.04.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Ying S., Ke H., Ding Y., et al. Unique genomic profiles obtained from cerebrospinal fluid cell-free DNA of non-small cell lung cancer patients with leptomeningeal metastases. Cancer Biol Ther. 2019;20(4):562–570. doi: 10.1080/15384047.2018.1538614. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Zheng M.M., Li Y.S., Jiang B.Y., et al. Clinical utility of cerebrospinal fluid cell-free DNA as liquid biopsy for leptomeningeal metastases in ALK-rearranged NSCLC. J Thorac Oncol. 2019;14(5):924–932. doi: 10.1016/j.jtho.2019.01.007. [DOI] [PubMed] [Google Scholar]
- 39.Fitzpatrick A., Iravani M., Mills A., et al. Assessing CSF ctDNA to improve diagnostic accuracy and therapeutic monitoring in breast cancer leptomeningeal metastasis. Clin Cancer Res. 2022;28(6):1180–1191. doi: 10.1158/1078-0432.CCR-21-3017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.White M.D., Klein R.H., Shaw B., et al. Detection of leptomeningeal disease using cell-free DNA from cerebrospinal fluid. JAMA Netw Open. 2021;4(8) doi: 10.1001/jamanetworkopen.2021.20040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.De Mattos-Arruda L., Mayor R., Ng C.K.Y., et al. Cerebrospinal fluid-derived circulating tumour DNA better represents the genomic alterations of brain tumours than plasma. Nat Commun. 2015;6:8839. doi: 10.1038/ncomms9839. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Le Rhun E., Devos P., Weller J., et al. Prognostic validation and clinical implications of the EANO ESMO classification of leptomeningeal metastasis from solid tumors. Neuro Oncol. 2021;23(7):1100–1112. doi: 10.1093/neuonc/noaa298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Stemmler H.J., Schmitt M., Willems A., et al. Ratio of trastuzumab levels in serum and cerebrospinal fluid is altered in HER2-positive breast cancer patients with brain metastases and impairment of blood-brain barrier. Anticancer Drugs. 2007;18(1):23–28. doi: 10.1097/01.cad.0000236313.50833.ee. [DOI] [PubMed] [Google Scholar]
- 44.Song Y., Liu P., Huang Y., et al. Osimertinib quantitative and gene variation analyses in cerebrospinal fluid and plasma of a non-small cell lung cancer patient with leptomeningeal metastases. Curr Cancer Drug Targets. 2019;19(8):666–673. doi: 10.2174/1568009618666181017114111. [DOI] [PubMed] [Google Scholar]
- 45.Muldoon L.L., Soussain C., Jahnke K., et al. Chemotherapy delivery issues in central nervous system malignancy: a reality check. J Clin Oncol. 2007;25(16):2295–2305. doi: 10.1200/JCO.2006.09.9861. [DOI] [PubMed] [Google Scholar]
- 46.Zairi F., Le Rhun E., Bertrand N., et al. Complications related to the use of an intraventricular access device for the treatment of leptomeningeal metastases from solid tumor: a single centre experience in 112 patients. J Neurooncol. 2015;124(2):317–323. doi: 10.1007/s11060-015-1842-x. [DOI] [PubMed] [Google Scholar]
- 47.Kennedy B.C., Brown L.T., Komotar R.J., et al. Stereotactic catheter placement for Ommaya reservoirs. J Clin Neurosci. 2016;27:44–47. doi: 10.1016/j.jocn.2015.11.005. [DOI] [PubMed] [Google Scholar]
- 48.Morgenstern P.F., Connors S., Reiner A.S., et al. Image guidance for placement of Ommaya reservoirs: comparison of fluoroscopy and frameless stereotactic navigation in 145 patients. World Neurosurg. 2016;93:154–158. doi: 10.1016/j.wneu.2016.04.090. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Gwak H.S., Joo J., Kim S., et al. Analysis of treatment outcomes of intraventricular chemotherapy in 105 patients for leptomeningeal carcinomatosis from non-small-cell lung cancer. J Thorac Oncol. 2013;8(5):599–605. doi: 10.1097/JTO.0b013e318287c943. [DOI] [PubMed] [Google Scholar]
- 50.Choi Y.H., Gwak H.S., Joo J., et al. Efficacy of slow rate ventriculolumbar perfusion chemotherapy for leptomeningeal carcinomatosis: interim result of a phase II study. Brain Tumor Res Treat. 2019;7(2):85–91. doi: 10.14791/btrt.2019.7.e33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Burger M.C., Wagner M., Franz K., et al. Ventriculoperitoneal shunts equipped with on-off valves for intraventricular therapies in patients with communicating hydrocephalus due to leptomeningeal metastases. J Clin Med. 2018;7(8):216. doi: 10.3390/jcm7080216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.McThenia S.S., Pandit-Taskar N., Grkovski M., et al. Quantifying intraventricular drug delivery utilizing programmable ventriculoperitoneal shunts as the intraventricular access device. J Neurooncol. 2022;157(3):457–463. doi: 10.1007/s11060-022-03989-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Blaney S.M., Poplack D.G., Godwin K., et al. Effect of body position on ventricular CSF methotrexate concentration following intralumbar administration. J Clin Oncol. 1995;13(1):177–179. doi: 10.1200/JCO.1995.13.1.177. [DOI] [PubMed] [Google Scholar]
- 54.Chamberlain M., Soffietti R., Raizer J., et al. Leptomeningeal metastasis: a Response Assessment in Neuro-Oncology critical review of endpoints and response criteria of published randomized clinical trials. Neuro Oncol. 2014;16(9):1176–1185. doi: 10.1093/neuonc/nou089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Grossman S.A., Finkelstein D.M., Ruckdeschel J.C., et al. Randomized prospective comparison of intraventricular methotrexate and thiotepa in patients with previously untreated neoplastic meningitis. Eastern Cooperative Oncology Group. J Clin Oncol. 1993;11(3):561–569. doi: 10.1200/JCO.1993.11.3.561. [DOI] [PubMed] [Google Scholar]
- 56.Glantz M.J., Jaeckle K.A., Chamberlain M.C., et al. A randomized controlled trial comparing intrathecal sustained-release cytarabine (DepoCyt) to intrathecal methotrexate in patients with neoplastic meningitis from solid tumors. Clin Cancer Res. 1999;5(11):3394–3402. [PubMed] [Google Scholar]
- 57.Hitchins R.N., Bell D.R., Woods R.L., et al. A prospective randomized trial of single-agent versus combination chemotherapy in meningeal carcinomatosis. J Clin Oncol. 1987;5(10):1655–1662. doi: 10.1200/JCO.1987.5.10.1655. [DOI] [PubMed] [Google Scholar]
- 58.Boogerd W., van den Bent M.J., Koehler P.J., et al. The relevance of intraventricular chemotherapy for leptomeningeal metastasis in breast cancer: a randomised study. Eur J Cancer. 2004;40(18):2726–2733. doi: 10.1016/j.ejca.2004.08.012. [DOI] [PubMed] [Google Scholar]
- 59.Le Rhun E., Wallet J., Mailliez A., et al. Intrathecal liposomal cytarabine plus systemic therapy versus systemic chemotherapy alone for newly diagnosed leptomeningeal metastasis from breast cancer. Neuro Oncol. 2020;22(4):524–538. doi: 10.1093/neuonc/noz201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Groves M.D., Glantz M.J., Chamberlain M.C., et al. A multicenter phase II trial of intrathecal topotecan in patients with meningeal malignancies. Neuro Oncol. 2008;10(2):208–215. doi: 10.1215/15228517-2007-059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Zagouri F., Sergentanis T.N., Bartsch R., et al. Intrathecal administration of trastuzumab for the treatment of meningeal carcinomatosis in HER2-positive metastatic breast cancer: a systematic review and pooled analysis. Breast Cancer Res Treat. 2013;139(1):13–22. doi: 10.1007/s10549-013-2525-y. [DOI] [PubMed] [Google Scholar]
- 62.Bonneau C., Paintaud G., Trédan O., et al. Phase I feasibility study for intrathecal administration of trastuzumab in patients with HER2 positive breast carcinomatous meningitis. Eur J Cancer. 2018;95:75–84. doi: 10.1016/j.ejca.2018.02.032. [DOI] [PubMed] [Google Scholar]
- 63.Kumthekar P.U., Avram M.J., Lassman A.B., et al. A phase I/II study of intrathecal trastuzumab in human epidermal growth factor receptor 2-positive (HER2-positive) cancer with leptomeningeal metastases: safety, efficacy, and cerebrospinal fluid pharmacokinetics. Neuro Oncol. 2023;25(3):557–565. doi: 10.1093/neuonc/noac195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Ahmed K.A., Kim Y., DeJesus M., et al. Trial in progress: phase I/II study of radiation therapy followed by intrathecal trastuzumab/pertuzumab in the management of HER2+ breast leptomeningeal disease. J Clin Oncol. 2021;39(suppl 15):TPS1099. [Google Scholar]
- 65.Fan C., Zhao Q., Li L., et al. Efficacy and safety of intrathecal pemetrexed combined with dexamethasone for treating tyrosine kinase inhibitor-failed leptomeningeal metastases from EGFR-mutant NSCLC-a prospective, open-label, single-arm phase 1/2 clinical trial (unique identifier: ChiCTR1800016615) J Thorac Oncol. 2021;16(8):1359–1368. doi: 10.1016/j.jtho.2021.04.018. [DOI] [PubMed] [Google Scholar]
- 66.Glitza Oliva I.C., Ferguson S.D., Bassett R., et al. Concurrent intrathecal and intravenous nivolumab in leptomeningeal disease: phase 1 trial interim results. Nat Med. 2023;29(4):898–905. doi: 10.1038/s41591-022-02170-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Long G.V., Atkinson V., Lo S., et al. Combination nivolumab and ipilimumab or nivolumab alone in melanoma brain metastases: a multicentre randomised phase 2 study. Lancet Oncol. 2018;19(5):672–681. doi: 10.1016/S1470-2045(18)30139-6. [DOI] [PubMed] [Google Scholar]
- 68.Chorti E., Kebir S., Ahmed M.S., et al. Leptomeningeal disease from melanoma-poor prognosis despite new therapeutic modalities. Eur J Cancer. 2021;148:395–404. doi: 10.1016/j.ejca.2021.02.016. [DOI] [PubMed] [Google Scholar]
- 69.Glantz M.J., Cole B.F., Recht L., et al. High-dose intravenous methotrexate for patients with nonleukemic leptomeningeal cancer: is intrathecal chemotherapy necessary? J Clin Oncol. 1998;16(4):1561–1567. doi: 10.1200/JCO.1998.16.4.1561. [DOI] [PubMed] [Google Scholar]
- 70.Lassman A.B., Abrey L.E., Shah G.D., et al. Systemic high-dose intravenous methotrexate for central nervous system metastases. J Neurooncol. 2006;78(3):255–260. doi: 10.1007/s11060-005-9044-6. [DOI] [PubMed] [Google Scholar]
- 71.Chahal J., Stopeck A., Clarke K., et al. Intravenous thiotepa for treatment of breast cancer-related leptomeningeal carcinomatosis: case series. Neurol Sci. 2015;36(9):1691–1693. doi: 10.1007/s10072-015-2259-1. [DOI] [PubMed] [Google Scholar]
- 72.Chamberlain M.C. Radioisotope CSF flow studies in leptomeningeal metastases. J Neurooncol. 1998;38(2-3):135–140. doi: 10.1023/a:1005982826121. [DOI] [PubMed] [Google Scholar]
- 73.Abouharb S., Ensor J., Loghin M.E., et al. Leptomeningeal disease and breast cancer: the importance of tumor subtype. Breast Cancer Res Treat. 2014;146(3):477–486. doi: 10.1007/s10549-014-3054-z. [DOI] [PubMed] [Google Scholar]
- 74.Gani C., Müller A.C., Eckert F., et al. Outcome after whole brain radiotherapy alone in intracranial leptomeningeal carcinomatosis from solid tumors. Strahlenther Onkol. 2012;188(2):148–153. doi: 10.1007/s00066-011-0025-8. [DOI] [PubMed] [Google Scholar]
- 75.El Shafie R.A., Böhm K., Weber D., et al. Palliative radiotherapy for leptomeningeal carcinomatosis-analysis of outcome, prognostic factors, and symptom response. Front Oncol. 2018;8:641. doi: 10.3389/fonc.2018.00641. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Devecka M., Duma M.N., Wilkens J.J., et al. Craniospinal irradiation(CSI) in patients with leptomeningeal metastases: risk-benefit-profile and development of a prognostic score for decision making in the palliative setting. BMC Cancer. 2020;20(1):501. doi: 10.1186/s12885-020-06984-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Yang J.T., Wijetunga N.A., Pentsova E., et al. Randomized phase II trial of proton craniospinal irradiation versus photon involved-field radiotherapy for patients with solid tumor leptomeningeal metastasis. J Clin Oncol. 2022;40(33):3858–3867. doi: 10.1200/JCO.22.01148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Okada H., Weller M., Huang R., et al. Immunotherapy response assessment in neuro-oncology: a report of the RANO working group. Lancet Oncol. 2015;16(15):e534–e542. doi: 10.1016/S1470-2045(15)00088-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Le Rhun E., Devos P., Boulanger T., et al. The RANO Leptomeningeal Metastasis Group proposal to assess response to treatment: lack of feasibility and clinical utility and a revised proposal. Neuro Oncol. 2019;21(5):648–658. doi: 10.1093/neuonc/noz024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Oberkampf F., Gutierrez M., Trabelsi Grati O., et al. Phase II study of intrathecal administration of trastuzumab in patients with HER2-positive breast cancer with leptomeningeal metastasis. Neuro Oncol. 2023;25(2):365–374. doi: 10.1093/neuonc/noac180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Brastianos P.K., Strickland M.R., Lee E.Q., et al. Phase II study of ipilimumab and nivolumab in leptomeningeal carcinomatosis. Nat Commun. 2021;12(1):5954. doi: 10.1038/s41467-021-25859-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Kingston B., Kayhanian H., Brooks C., et al. Treatment and prognosis of leptomeningeal disease secondary to metastatic breast cancer: a single-centre experience. Breast. 2017;36:54–59. doi: 10.1016/j.breast.2017.07.015. [DOI] [PubMed] [Google Scholar]
- 83.Hyun J.W., Jeong I.H., Joung A., et al. Leptomeningeal metastasis: clinical experience of 519 cases. Eur J Cancer. 2016;56:107–114. doi: 10.1016/j.ejca.2015.12.021. [DOI] [PubMed] [Google Scholar]
- 84.Niwińska A., Pogoda K., Michalski W., et al. Determinants of prolonged survival for breast cancer patient groups with leptomeningeal metastasis (LM) J Neurooncol. 2018;138(1):191–198. doi: 10.1007/s11060-018-2790-z. [DOI] [PubMed] [Google Scholar]
- 85.Chamberlain M.C., Kormanik P.A. Prognostic significance of 111indium-DTPA CSF flow studies in leptomeningeal metastases. Neurology. 1996;46(6):1674–1677. doi: 10.1212/wnl.46.6.1674. [DOI] [PubMed] [Google Scholar]
- 86.Roth P., Pace A., Le Rhun E., et al. Neurological and vascular complications of primary and secondary brain tumours: EANO-ESMO Clinical Practice Guidelines for prophylaxis, diagnosis, treatment and follow-up. Ann Oncol. 2021;32(2):171–182. doi: 10.1016/j.annonc.2020.11.003. [DOI] [PubMed] [Google Scholar]
- 87.Walbert T., Harrison R.A., Schiff D., et al. SNO and EANO practice guideline update: anticonvulsant prophylaxis in patients with newly diagnosed brain tumors. Neuro Oncol. 2021;23(11):1835–1844. doi: 10.1093/neuonc/noab152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Bander E.D., Yuan M., Reiner A.S., et al. Cerebrospinal fluid diversion for leptomeningeal metastasis: palliative, procedural and oncologic outcomes. J Neurooncol. 2021;154(3):301–313. doi: 10.1007/s11060-021-03827-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Pace A., Dirven L., Koekkoek J.A.F., et al. European Association for Neuro-Oncology (EANO) guidelines for palliative care in adults with glioma. Lancet Oncol. 2017;18(6):e330–e340. doi: 10.1016/S1470-2045(17)30345-5. [DOI] [PubMed] [Google Scholar]
- 90.Brainin M., Barnes M., Baron J.C., et al. Guidance for the preparation of neurological management guidelines by EFNS scientific task forces--revised recommendations 2004. Eur J Neurol. 2004;11(9):577–581. doi: 10.1111/j.1468-1331.2004.00867.x. [DOI] [PubMed] [Google Scholar]
- 91.Dykewicz C.A., Centers for Disease Control and Prevention (U.S.) Infectious Diseases Society of America, American Society of Blood and Marrow Transplantation. Summary of the Guidelines for Preventing Opportunistic Infections among Hematopoietic Stem Cell Transplant Recipients. Clin Infect Dis. 2001;33(2):139–144. doi: 10.1086/321805. [adapted from Gross PA, Barrett TL, Dellinger EP, et al. Clin Infect Dis. 1994;18(3):421] [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.