Abstract
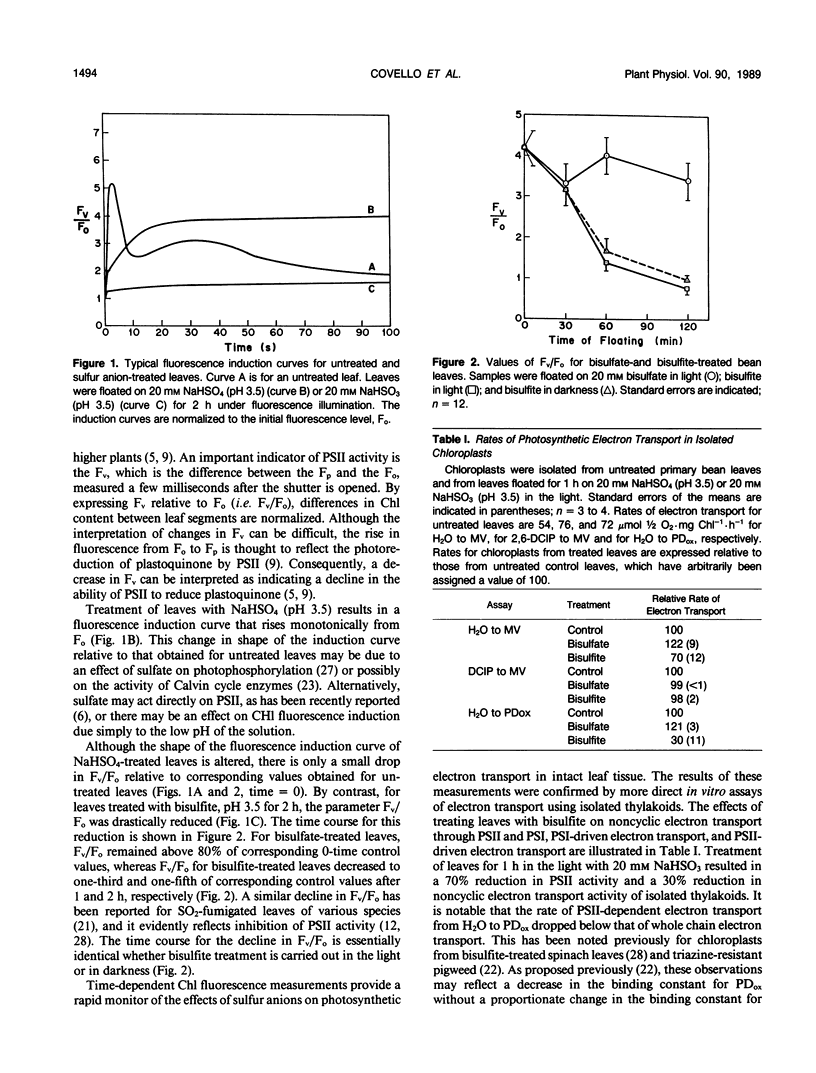
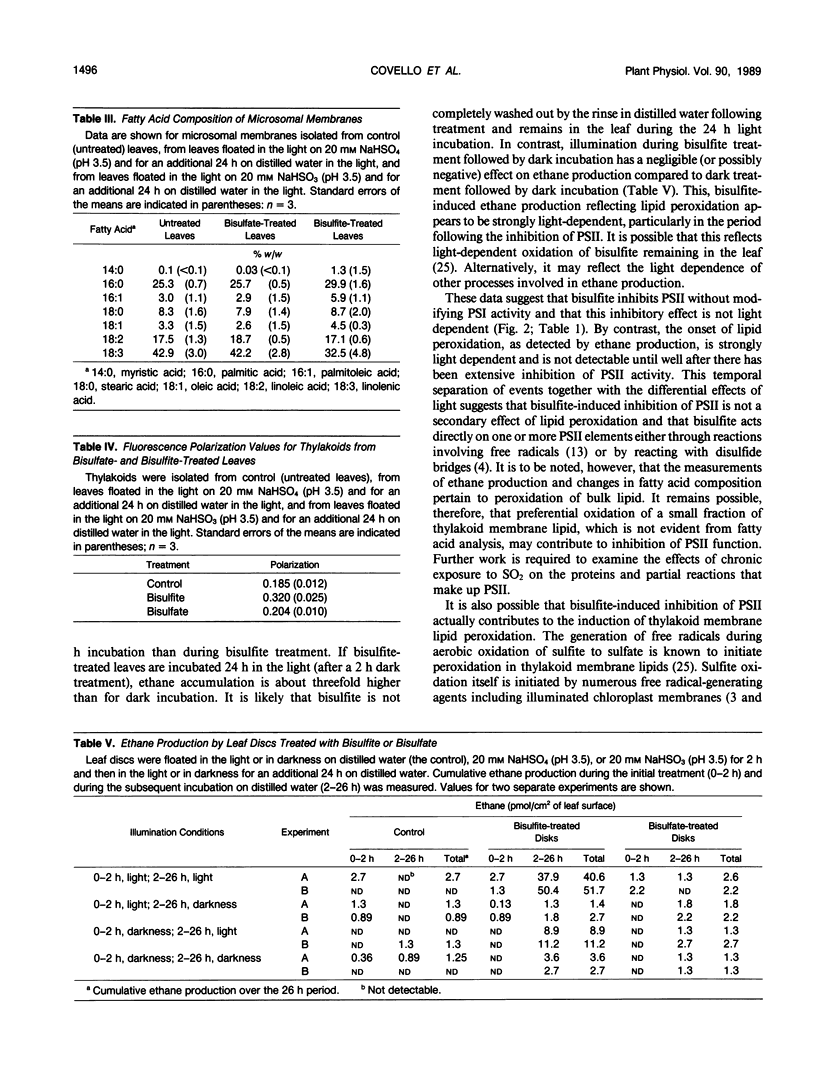
Exposure of leaves to SO2 or bisulfite is known to induce peroxidation of thylakoid lipids and to inhibit photosynthetic electron transport. In the present study, we have examined the temporal relationship between bisulfite-induced thylakoid lipid peroxidation and inhibition of electron transport in an attempt to clarify the primary mechanism of SO2 phytotoxicity. Primary leaves of bean (Phaseolus vulgaris L. cv Kinghorn) were floated on a solution of NaHSO3, and the effects of this treatment on photosynthetic electron transport were determined in vivo by measurements of chlorophyll a fluorescence induction and in vitro by biochemical measurements of the light reactions using isolated thylakoids. Lipid peroxidation in treated leaves was followed by monitoring ethane emission from leaf segments and by measuring changes in fatty acid composition and lipid fluidity in isolated thylakoids. A 1 hour treatment with bisulfite inhibited photosystem II (PSII) activity by 70% without modifying Photosystem I, and this inhibitory effect was not light-dependent. By contrast, lipid peroxidation was not detectable until after the inhibition of PSII and was strongly light dependent. This temporal separation of events together with the differential effect of light suggests that bisulfite-induced inhibition of PSII is not a secondary effect of lipid peroxidation and that bisulfite acts directly on one or more components of PSII.
Full text
PDF



Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Arnon D. I. COPPER ENZYMES IN ISOLATED CHLOROPLASTS. POLYPHENOLOXIDASE IN BETA VULGARIS. Plant Physiol. 1949 Jan;24(1):1–15. doi: 10.1104/pp.24.1.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Asada K., Kiso K. Initiation of aerobic oxidation of sulfite by illuminated spinach chloroplasts. Eur J Biochem. 1973 Mar 1;33(2):253–257. doi: 10.1111/j.1432-1033.1973.tb02677.x. [DOI] [PubMed] [Google Scholar]
- BAILEY J. L., COLE R. D. Studies on the reaction of sulfite with proteins. J Biol Chem. 1959 Jul;234(7):1733–1739. [PubMed] [Google Scholar]
- BLIGH E. G., DYER W. J. A rapid method of total lipid extraction and purification. Can J Biochem Physiol. 1959 Aug;37(8):911–917. doi: 10.1139/o59-099. [DOI] [PubMed] [Google Scholar]
- Davies K. J. Protein damage and degradation by oxygen radicals. I. general aspects. J Biol Chem. 1987 Jul 15;262(20):9895–9901. [PubMed] [Google Scholar]
- McKersie B. D., Thompson J. E. Lipid crystallization in senescent membranes from cotyledons. Plant Physiol. 1977 May;59(5):803–807. doi: 10.1104/pp.59.5.803. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Omasa K., Shimazaki K., Aiga I., Larcher W., Onoe M. Image analysis of chlorophyll fluorescence transients for diagnosing the photosynthetic system of attached leaves. Plant Physiol. 1987 Jul;84(3):748–752. doi: 10.1104/pp.84.3.748. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ort D. R., Ahrens W. H., Martin B., Stoller E. W. Comparison of Photosynthetic Performance in Triazine-Resistant and Susceptible Biotypes of Amaranthus hybridus. Plant Physiol. 1983 Aug;72(4):925–930. doi: 10.1104/pp.72.4.925. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peiser G. D., Lizada M. C., Yang S. F. Sulfite-induced lipid peroxidation in chloroplasts as determined by ethane production. Plant Physiol. 1982 Oct;70(4):994–998. doi: 10.1104/pp.70.4.994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peiser G. D., Yang S. F. Ethylene and Ethane Production from Sulfur Dioxide-injured Plants. Plant Physiol. 1979 Jan;63(1):142–145. doi: 10.1104/pp.63.1.142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peiser G. D., Yang S. F. Ethylene and Ethane Production from Sulfur Dioxide-injured Plants. Plant Physiol. 1979 Jan;63(1):142–145. doi: 10.1104/pp.63.1.142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ryrie I. J., Jagendorf A. T. Inhibition of photophosphorylation in spinach chloroplasts by inorganic sulfate. J Biol Chem. 1971 Feb 10;246(3):582–588. [PubMed] [Google Scholar]
- Silvius J. E., Ingle M., Baer C. H. Sulfur dioxide inhibition of photosynthesis in isolated spinach chloroplasts. Plant Physiol. 1975 Sep;56(3):434–437. doi: 10.1104/pp.56.3.434. [DOI] [PMC free article] [PubMed] [Google Scholar]


