Abstract
Influenza vaccines could be improved by platforms inducing cross-reactive immunity. Immunodominance of the influenza hemagglutinin (HA) head in currently licensed vaccines impedes induction of cross-reactive neutralizing stem-directed antibodies. A vaccine without the variable HA head domain has the potential to focus the immune response on the conserved HA stem. This first-in-human dose-escalation open-label phase 1 clinical trial (NCT03814720) tested an HA stabilized stem ferritin nanoparticle vaccine (H1ssF) based on the H1 HA stem of A/New Caledonia/20/1999. Fifty-two healthy adults aged 18 to 70 years old enrolled to receive either 20 μg of H1ssF once (n = 5) or 60 μg of H1ssF twice (n = 47) with a prime-boost interval of 16 weeks. Thirty-five (74%) 60-μg dose participants received the boost, whereas 11 (23%) boost vaccinations were missed because of public health restrictions in the early stages of the COVID-19 pandemic. The primary objective of this trial was to evaluate the safety and tolerability of H1ssF, and the secondary objective was to evaluate antibody responses after vaccination. H1ssF was safe and well tolerated, with mild solicited local and systemic reactogenicity. The most common symptoms included pain or tenderness at the injection site (n = 10, 19%), headache (n = 10, 19%), and malaise (n = 6, 12%). We found that H1ssF elicited cross-reactive neutralizing antibodies against the conserved HA stem of group 1 influenza viruses, despite previous H1 subtype head-specific immunity. These responses were durable, with neutralizing antibodies observed more than 1 year after vaccination. Our results support this platform as a step forward in the development of a universal influenza vaccine.
INTRODUCTION
Influenza viruses cause seasonal epidemics with substantial morbidity and mortality, including 9 million to 41 million illnesses, 140,000 to 710,000 hospitalizations, and 12,000 to 52,000 deaths annually in the United States over the past decade (1). Currently, influenza vaccines require annual reformulation and immunization because of ongoing antigenic drift of circulating seasonal influenza strains (2, 3). The licensed vaccines are strain-specific, with immune responses biased toward the immunodominant and variable head region of hemagglutinin (HA) (4). This strain-specific response results in low vaccine efficacy during seasonal epidemics that ranges from 10 to 60% and hinders pandemic preparedness because strains and subtypes cannot be predicted in advance (5). Current manufacturing timelines are lengthy because of required steps including strain prediction and viral growth in either cell lines or embryonated eggs (6). These limitations highlight the need for a supraseasonal or universal influenza vaccine that can be efficiently manufactured and provides durable, cross-strain protection.
In contrast to the variable head domain of HA, the stem domain is highly conserved within the group 1 (including H1, H2, and H5) and group 2 (including H3 and H10) HA subtypes of influenza viruses. Although the HA head is immunodominant, subdominant stem responses are elicited after influenza vaccination or infection (7, 8). Because of the relative conservation of stem epitopes, these antibody responses are the primary recall response generated upon exposure to a novel influenza subtype within the same HA group (3, 9–12). Stem-specific antibodies with neutralizing activity against antigenically distinct influenza viruses have been described (9, 12, 13). Stem-binding antibodies can also mediate viral clearance through antibody-dependent cell cytotoxicity or phagocytosis and inhibit viral replication by blocking viral fusion machinery (3, 14, 15). Therefore, the HA stem is an advantageous target for universal or supraseasonal influenza vaccines.
To improve influenza vaccine platform technology, the Vaccine Research Center (VRC) at the National Institute of Allergy and Infectious Diseases (NIAID), National Institutes of Health (NIH) developed a Helicobacter pylori ferritin nanoparticle–based vaccine platform. H. pylori nonheme ferritin monomers spontaneously assemble into an extremely stable octahedral 24-mer complex (16), creating a nanoparticle. Proper folding of H. pylori ferritin nanoparticles has been demonstrated by cryo–electron microscopy (EM) and crystal structure imaging, despite the particles’ minimal iron incorporation capacity (17, 18). In addition, proper folding of influenza HA trimers on the nanoparticle surface has been shown to occur after genetic fusion of an HA ectodomain protomer to the H. pylori nonheme ferritin monomer (17). The ferritin nanoparticle vaccine platform previously exhibited safety and immunogenicity in a phase 1 trial (19). In that study, vaccination with ferritin nanoparticles displaying full-length H2 HA ectodomains elicited cross-reactive neutralizing stem-specific responses in H2-naïve participants. However, the immunodominant H2 head limited responses to the stem in individuals with previous exposure to H2 influenza strains.
To better focus immune responses on the HA stem, the VRC engineered a stabilized H1 (A/New Caledonia/20/1999) HA stem ferritin vaccine (H1ssF) as previously reported (18). The A/New Caledonia/20/1999 HA stem shares 93.2% amino acid conservation with the stem of a more recent HA, A/Michigan/45/2015. Because the H1ssF vaccine presents the HA stem to the immune system in the absence of the immunodominant head, we hypothesized that this vaccine would improve induction of stem-directed antibodies in adults regardless of previous influenza immunity (20, 21). In preclinical studies, the H1ssF vaccine elicited stem-directed neutralizing antibodies in multiple animal models and conferred protection against heterosubtypic H5N1 virus challenge in mice (18, 21). These results suggest that the H1ssF vaccine could elicit protective immunity across diverse group 1 influenza strains.
Given the strong preclinical data for H1ssF and a successful previous trial evaluating a full-length H2 HA ferritin nanoparticle vaccine (19, 21), we designed a first-in-human, phase 1 clinical trial to evaluate the safety, tolerability, and immunogenicity of H1ssF in adults between the ages of 18 and 70 years old. We assessed the vaccine at a single 20-μg dose and two 60-μg doses with a prime-boost interval of 16 weeks. The 60-μg dose group was stratified by age (18 to 40, 41 to 49, 50 to 59, and 60 to 70 years old), and participants were followed through 52 weeks after the last vaccination.
RESULTS
Clinical trial design and participants
Fifty-two healthy adults enrolled in the trial between 1 April 2019 and 9 March 2020 (Fig. 1). The 28 (54%) female and 24 (46%) male participants had a mean age of 46 years (range, 22 to 68; Table 1). Most participants (n = 29, 56%) received three or more seasonal influenza vaccinations in the 5 years before enrolling in the trial. Fifty-one (98%) participants completed the trial (Fig. 1), with a total of 87 H1ssF vaccinations administered. Five participants received a single 20-μg dose of H1ssF once in the dose-escalation portion of the trial. These participants provided safety data, but their immunogenicity results were not the focus of the immunological analyses and are reported in the supplement. Forty-seven participants received two 60-μg doses of H1ssF, with a prime-boost interval of 16 weeks. Twelve (23%) participants missed a scheduled second 60-μg dose vaccination, with the majority (n = 11, 92%) missed because of public health restrictions in the early stages of the coronavirus disease 2019 (COVID-19) pandemic (table S1). The other (2%) participant withdrew from the study because of relocation. Participants whose second vaccinations were interrupted because of the COVID-19 pandemic continued to be followed for safety and immunogenicity evaluations as allowable by Centers for Disease Control and Prevention and NIH guidance.
Fig. 1. Study CONSORT diagram.
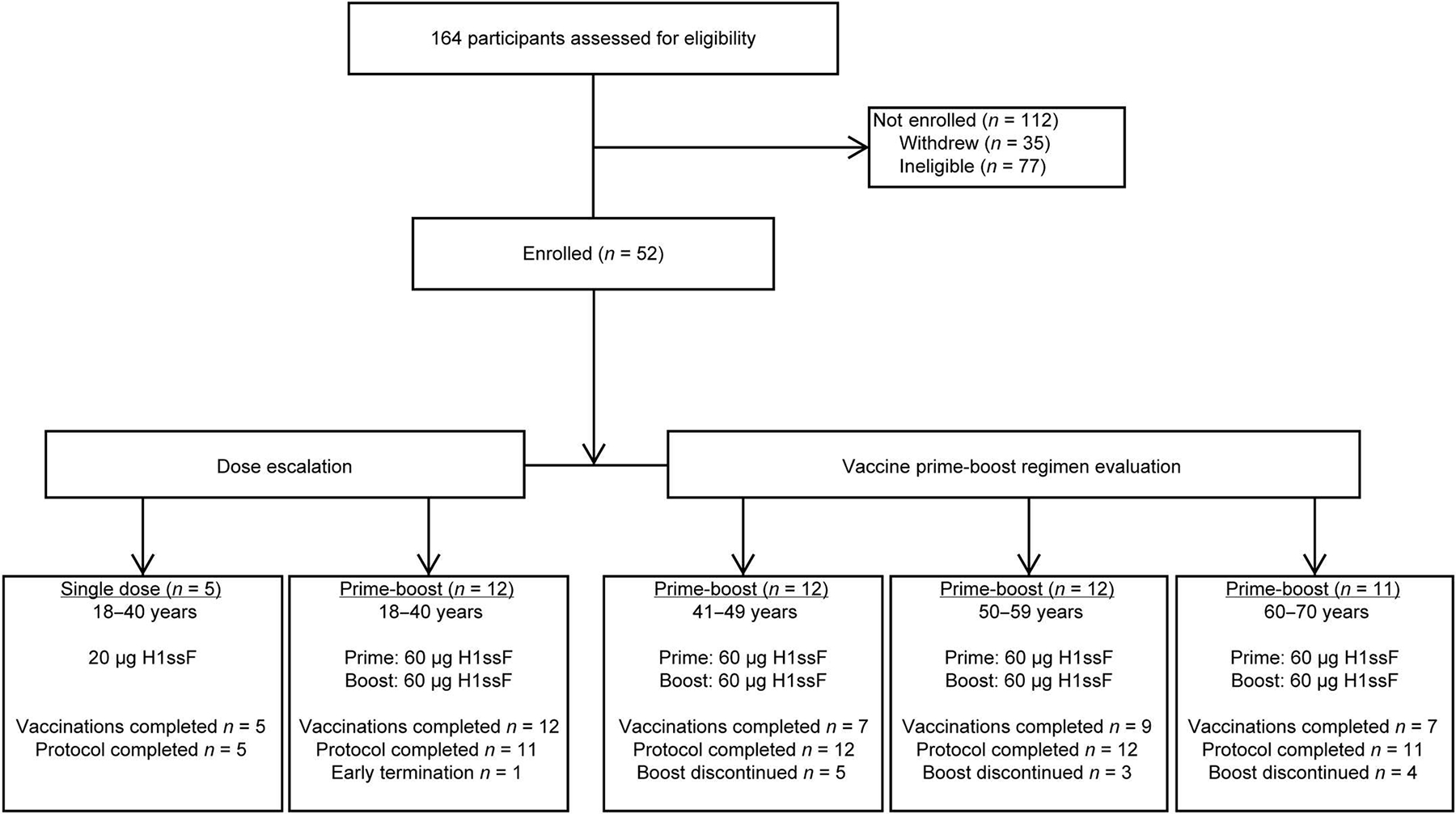
Fifty-two healthy adults aged 18 to 70 years old enrolled into the trial between 1 April 2019 and 9 March 2020 to receive either 20 μg of H1ssF once (n = 5) or 60 μg of H1ssF twice (n = 47) with a prime-boost interval of 16 weeks. The COVID-19 pandemic disrupted the administration of boost doses for 11 of 12 participants who missed the boost; the remaining participant withdrew because of relocation. Participants who had altered or discontinued vaccination schedules were monitored for safety and were included in the immunogenicity analysis until their vaccination schedules were changed.
Table 1. Demographic Information.
Data are provided as number (no.) and percentage (%) of participants unless indicated otherwise. GED, general educational development.
| 20 μg of H1ssF | 60 μg of H1ssF |
|||||
|---|---|---|---|---|---|---|
| 18–40 years (n = 5) | 18–40 years (n = 12) | 41–49 years (n = 12) | 50–59 years (n = 12) | 60–70 years (n = 11) | All 60 μg (n = 47) | |
|
| ||||||
| Sex, no. (%) | ||||||
|
| ||||||
| Male | 1 (20.0) | 8 (66.7) | 5 (41.7) | 5(41.7) | 5 (45.5) | 23 (48.9) |
|
| ||||||
| Female | 4 (80.0) | 4 (33.3) | 7 (58.3) | 7 (58.3) | 6 (54.5) | 24 (51.1) |
|
| ||||||
| Age, mean [range] | 34.0 [27, 40] | 29.2 [22, 38] | 45.3 [41,49] | 52.8 [50, 57] | 63.0 [60, 68] | 47.2 [22, 68] |
|
| ||||||
| Race, no. (%)* | ||||||
|
| ||||||
| Asian | 0 (0.0) | 2 (16.7) | 3 (25.0) | 0 (0.0) | 0 (0.0) | 5 (10.6) |
|
| ||||||
| Black or African American | 0 (0.0) | 0 (0.0) | 2 (16.7) | 2 (16.7) | 1 (9.1) | 5 (10.6) |
|
| ||||||
| White | 4 (80.0) | 8 (66.7) | 7 (58.3) | 10 (83.3) | 10 (90.9) | 35 (74.5) |
|
| ||||||
| Multiracial | 1 (20.0) | 2 (16.7) | 0 (0.0) | 0 (0.0) | 0 (0.0) | 2 (4.3) |
|
| ||||||
| Hispanic or Latino ethnic group, no. (%)* | ||||||
|
| ||||||
| Non-Hispanic/Latino | 4 (80.0) | 9 (75.0) | 12 (100.0) | 11 (91.7) | 11 (100.0) | 43 (91.5) |
|
| ||||||
| Hispanic/Latino | 1 (20.0) | 3 (25.0) | 0 (0.0) | 1 (8.3) | 0 (0.0) | 4 (8.5) |
|
| ||||||
| Body mass index, mean (SD)† | 22.8 (2.9) | 27.0 (3.5) | 27.0 (3.7) | 27.6 (3.0) | 26.3 (4.3) | 27.0 (3.6) |
|
| ||||||
| Educational level, no. (%) | ||||||
|
| ||||||
| High school graduate/GED | 1 (20.0) | 0 (0.0) | 0 (0.0) | 0 (0.0) | 0 (0.0) | 1 (1.9) |
|
| ||||||
| College/university | 1 (20.0) | 7 (58.3) | 3 (25.0) | 5(41.7) | 5 (45.5) | 20 (42.6) |
|
| ||||||
| Advanced degree | 3 (60.0) | 5 (41.7) | 9 (75.0) | 7 (58.3) | 6 (54.5) | 27 (57.4) |
Race and ethnic group were reported by the participants.
Body mass index is weight in kilograms divided by the square of height in meters.
This calculation was performed on the basis of weight and height measured at the time of enrollment.
Safety
The primary objective of this clinical trial was to examine the safety and tolerability of the H1ssF vaccine. The H1ssF vaccine was safe and well tolerated, with mild reported solicited local and systemic reactogenicity (Fig. 2). The only local reactogenicity reported was mild pain and tenderness (n = 10, 19%). Systemic reactogenicity most commonly included mild headache (n = 10, 19%) and malaise (n = 6, 12%). There were three adverse events (AEs) attributed to vaccination including mild lymphopenia, moderate neutropenia, and one report of abnormal dreams (table S2). All AEs self-resolved without sequelae. There were no severe AEs (SAEs) or study pauses in this trial. There were four cases of influenza-like illness in the 60-μg dose participants: one case in the 18- to 40-year cohort (laboratory-confirmed negative for influenza) and three in the 51- to 60-year cohort (two laboratory-confirmed positive for H1 influenza and one confirmed negative) (table S3).
Fig. 2. Mild solicited reactogenicity was reported after H1ssF vaccination.
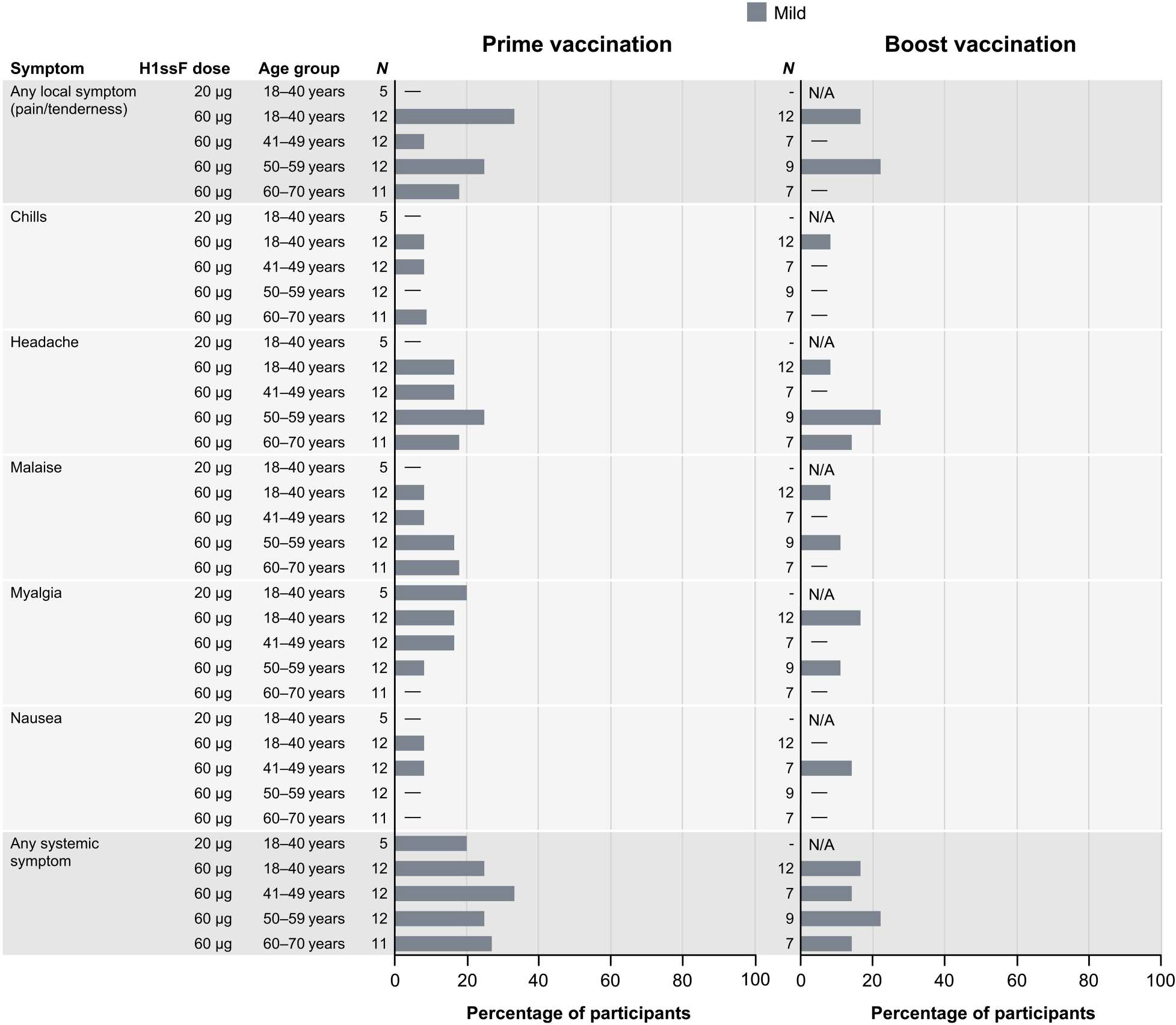
Percentage of participants (x axis) reporting solicited local or systemic symptoms (y axis) in the 7 days after each H1ssF vaccination. For symptoms persisting more than 1 day, a single count per person at the maximum severity of the symptom was used for the figure. Pain/tenderness was the only local symptom reported; no swelling or redness was noted. There were also no reports of fever or joint pain. All reactogenicity was mild. N/A indicates that no boost vaccination was administered to the dose group.
To address the theoretical possibility of the H. pylori ferritin nanoparticle generating an immune response against human ferritin proteins, antibody responses to the human ferritin heavy and light chain, as well as responses to H. pylori ferritin, were assessed by enzyme-linked immunoassay (ELISA) in an exploratory objective. As in our previous trial, we observed immune responses to H. pylori ferritin (fig. S1A) (19). However, no antibody responses were seen against either human ferritin antigen after vaccination that increased over the range of vaccine-naïve controls (fig. S1, B and C).
H1ssF resulted in a response against autologous H1 HA
The H1ssF vaccine elicited binding antibodies against the stem domain of the H1 HA protein irrespective of dose or age. In an exploratory analysis, we examined the binding antibody response after vaccination by electrochemiluminescence immunoassay (ECLIA). Similar antibody kinetics were observed for all age cohorts that received two 60-μg doses (fig. S2, A and B). In light of these comparable responses and the COVID-19 impact on sample collections (table S4), the 60-μg cohorts were combined for antibody analyses (Fig. 3). H1 stem–binding antibody responses peaked 2 weeks after the 60-μg prime (Fig. 3A) at a value sixfold higher than baseline (Fig. 3B). The week 16 response remained more than fourfold higher than baseline. The second dose at week 16 boosted the binding antibodies to a peak sevenfold higher than baseline by week 18. Although the response waned after the boost, the degree of binding antibodies remained more than twofold higher than baseline at week 40. The 20-μg dose group followed a similar pattern after a single vaccination (fig. S2A).
Fig. 3. H1ssF elicits broad antibody responses to group 1 influenza HA antigens.
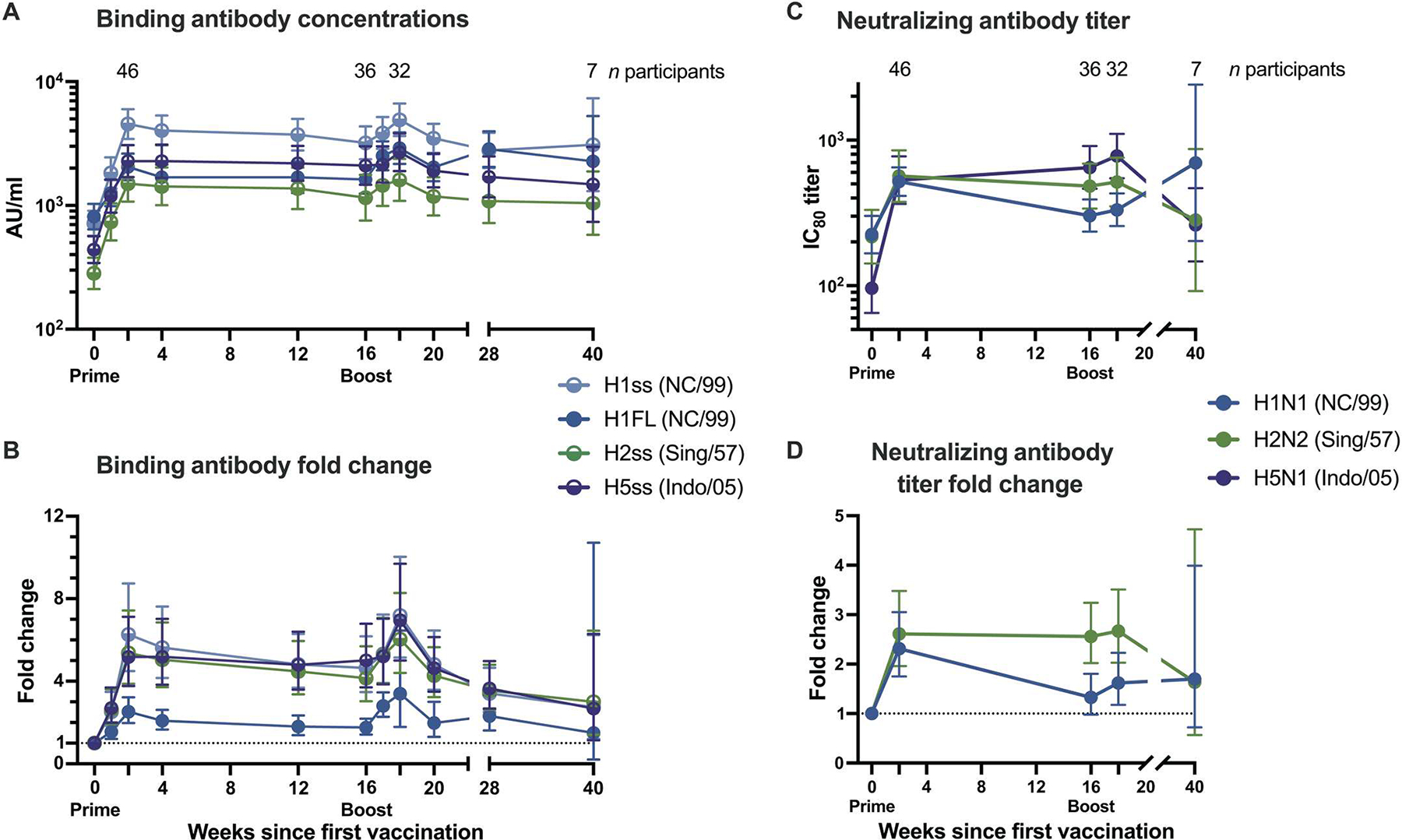
(A) Geometric means and 95% confidence intervals (CIs) are shown for all 60-μg dose recipients’ binding antibody concentrations. (B) Fold change of binding antibody concentrations from baseline for group 1 influenza virus antigens. Binding was assessed by ECLIA. (C) Neutralizing IC80 antibody titers for three viruses and (D) fold change from baseline for two viruses, as assessed by reporter-based microneutralization assay. Half circles in (A) and (B) denote stabilized stem (ss) antigens. Dotted lines in (B) and (D) indicate baseline. In (D), H5N1 fold change calculations could not be performed because of low baseline titers. Exact numbers of participant samples analyzed at each time point are listed in table S4. AU, arbitrary units; NC/99, A/New Caledonia/20/1999; Sing/57, A/Singapore/1/1957; Indo/05, A/Indonesia/5/2005.
An increase in binding antibody responses was also observed against the full-length H1 HA (H1FL) after H1ssF vaccination (Fig. 3A and fig. S3, A and B). The H1FL-binding response peaked 2.5-fold over baseline at 2 weeks after prime (Fig. 3B) and was boosted 2.8-fold over baseline by week 18. The binding antibody response remained significantly higher than baseline through week 28 (P < 0.001). Neutralizing antibody titers followed a similar trend, whether assessed by an exploratory microneutralization assay (Fig. 3, C and D, and fig. S4A) or the secondary endpoint pseudotyped lentiviral neutralization assay (fig. S4B).
To investigate the durability of the vaccine-induced response, the neutralizing antibody titers were assessed in an exploratory analysis. Neutralization titers remained significantly (P= 0.037) elevated over baseline at 68 weeks after the first dose of H1ssF vaccine as assessed by the pseudotyped lentiviral neutralization assay (fig. S4B). This may be partly attributable to the second dose of H1ssF, which effectively increased the magnitude of the stem-binding antibody response (fig. S5). Assessment of the impact of the boost on the longevity of the immune response was hindered by the missed sample collections in the post-boost period. In addition, most participants received a seasonal quadrivalent influenza vaccine (QIV) at some point during the trial (table S5), but we did not detect differences in binding antibody titers between participants who did and did not receive a QIV (fig. S6). Among three 60-μg H1ssF recipients with no known influenza vaccinations or infections during the trial, pseudotyped lentiviral neutralization titers remained elevated at week 68, with a geometric mean 80% inhibitory concentration (IC80) titer of 1926.8 over a baseline of 740.8. These data indicate that H1ssF elicited a durable neutralizing antibody response to the H1 stem that persisted for more than a year after vaccination.
H1ssF elicited a cross-reactive antibody response against group 1 HAs
The H1ssF vaccine also elicited antibodies cross-reactive with heterologous group 1 HA stems by an exploratory ECLIA (Fig. 3A and figs. S7, A and B, and S8). For H5, the stem-binding antibody response after the H1ssF prime increased fivefold at 2 weeks (Fig. 3B) and was sustained through week 16. The boost increased the response further to eightfold over baseline by week 18, and this response remained elevated out to week 40. The neutralizing antibody titers against the matching H5N1 virus also increased after both prime and boost (Fig. 3C and fig. S9A). These responses remained significantly (P = 0.034) greater than baseline values out to week 40 (Fig. 3C).
The binding and neutralizing antibody responses against H2 followed similar trends to the H5 responses (Fig. 3, A and C, and figs. S8 and S9B). However, in contrast to the comparable results observed between age groups for H1 stem, full-length H1, and H5 stem responses (figs. S2B, S3B, and S7B), we observed a significantly greater quantity of both H2 stem–binding (Fig. 4A) and H2N2-neutralizing (Fig. 4B) antibodies among the 60 to 70 year olds compared with the other 60-μg dose participants from baseline through at least week 16 (P ≤ 0.03). We previously demonstrated that individuals born before 1968 have greater H2 antibody concentrations at baseline because of exposure to the H2 subtype during childhood (19). Therefore, we stratified the 60-μg dose participants by likelihood of previous H2 exposure, categorizing those born after 1968 as H2 naïve and those born in or before 1968 as H2 exposed. H1ssF significantly increased binding (P < 0.001) and neutralizing (P ≤ 0.004) antibodies for both H2-naïve and H2-exposed participants 2 weeks after vaccination compared with baseline (Fig. 4, C and D). As expected, H2-exposed participants had significantly greater H2 stem–binding antibody responses at both baseline (P < 0.001) and 1 week after prime (P = 0.017) compared with H2-naïve participants. However, the H1ssF vaccine induced a greater fold change in the H2 stem–binding responses of H2-naïve participants than H2-exposed participants 2 weeks after both prime and boost (Fig. 4C) such that from weeks 16 to 28 there was no difference between the H2-naïve and H2-exposed groups. Similarly, H2-exposed participants had higher H2N2-neutralizing antibody titers only at baseline (P = 0.002) and 2 weeks after prime (P = 0.014) (Fig. 4D). Together, these responses to heterologous HA antigens indicate that H1ssF can induce cross-reactive group 1 binding and neutralizing antibodies in both H2-exposed and H2-naïve individuals.
Fig. 4. H1ssF vaccination decreases the baseline difference in H2 binding and neutralizing antibodies between H2-exposed and H2-naïve individuals.
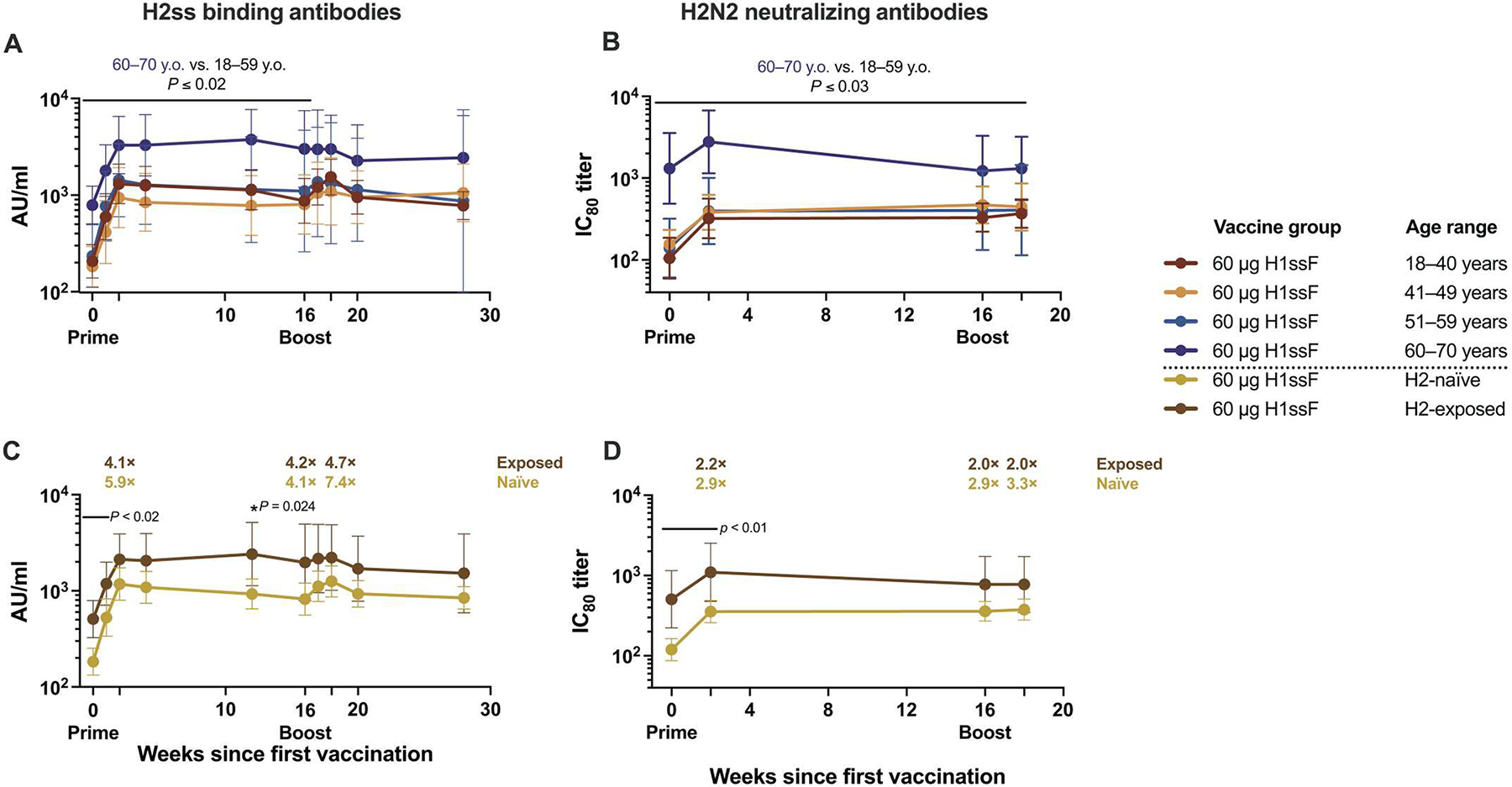
(A and B) Binding antibody concentrations to H2ss assessed by ECLIA (A) and neutralizing IC80 antibody titers against H2N2 assessed by reporter-based microneutralization assay (B) stratified by age group; y.o., years old. (C and D) Binding antibody concentrations to H2ss (C) and neutralizing IC80 antibody titers (D) stratified by expected exposure to H2 influenza; participants born in or after 1969 are considered “H2-naïve,” and those born in or before 1968 are considered “H2-exposed.” Geometric means (GMs) and 95% CI are shown. Fold changes over baseline are indicated at weeks 2, 16, and 18 for (C) and (D). Results of two-sample t tests are shown. In (A) and (B), comparisons were made at each time point for the GMs of the 60- to 70-year-old individuals to the GM of the 18- to 59-year-old individuals combined. In (C) and (D), the GMs of the H2-naïve and H2-exposed individuals were compared. Significance noted applies to each time point under the corresponding black lines. Exact numbers of participant samples analyzed at each time point are listed in table S4.
Because stem-binding antibodies are thought to mediate protection through Fc-mediated functions, the capacity of H1ssF to elicit antibody-dependent cellular cytotoxicity (ADCC) activity in participants’ sera was assessed after vaccination. ADCC activity assayed with an H6 head/H1 stem construct increased 7.3-fold on average by 2 weeks after vaccination (fig. S10) and was maintained at a value 7.5-fold greater than baseline at week 18. There was no difference between the age groups in their ADCC response to the vaccine. These results demonstrate that the H1ssF vaccine induces stem-specific antibodies with Fc-mediated activity.
Next, we compared the 60-μg dose participants’ geometric mean binding and neutralizing antibody responses against all the tested influenza antigens over time in a post hoc analysis. As expected, we found that the H1ssF vaccine elicited the greatest binding antibody responses against the homologous H1 stem and H1FL antigens (Fig. 3A), followed by responses against heterologous group 1 H5 and H2 stem antigens (P < 0.001 for both H5 and H2 stem antigen fold changes compared with H1 stem). In contrast, we observed little to no increase in binding antibody responses against the group 2 H3 and H10 stem antigens compared with baseline (fig. S11, A and B). Neutralizing antibody titers comparably increased against all tested group 1 antigens (Fig. 3C), but no increase in microneutralization titers was observed for group 2 viruses at week 2 after prime (table S6). Because no change in neutralization titers was observed for the group 2 viruses after the prime, these responses were not assayed at later time points. These data indicate that the H1ssF vaccine-induced response primarily targets group 1 influenza viruses.
DISCUSSION
This first-in-human phase 1 clinical trial in healthy adults demonstrated that the H1ssF vaccine is safe, well tolerated, and immunogenic without the use of adjuvant. This influenza ss ferritin-based nanoparticle vaccine generated cross-reactive antibodies with neutralizing and Fc-mediated activity, specific for the conserved group 1 influenza HA stem in trial participants, regardless of age. This was demonstrated by increases in binding and neutralizing antibodies against multiple group 1 HA stems, as well as increased H1 stem–directed ADCC activity, after a single vaccination. Neither prime nor boost elicited more than mild reactogenicity. Our results, along with the associated B cell analysis (22), support this platform as a cross-reactive group 1 influenza vaccine candidate.
A major challenge in influenza vaccine development is eliciting immune responses directed at an epitope of interest in the face of nearly universal preexisting influenza exposure (23, 24). Any epitopes shared between an antigenically distinct strain of influenza and those seen previously will recall memory responses that inhibit the development of immunity against novel epitopes [reviewed in (25) and (26)]. Furthermore, immunodominance of the HA head is disadvantageous for stem-specific responses. First encounter with an antigenically distinct HA can recall responses against conserved stem epitopes, but these are subdominant to the head-specific responses upon further exposure to the same full-length HA [(27) and reviewed in (28)]. This phenomenon was evident in our previous trial evaluating a full-length H2 ferritin nanoparticle vaccine: stem-specific responses were only elicited by the full-length H2 antigen in the absence of preexisting immunity to the H2 head (19). However, by removing the immunodominant HA head, the H1ssF vaccine was effective at eliciting binding antibodies against the conserved group 1 HA stem in adults with preexisting head-specific immunity.
We also observed increased neutralizing antibody titers against diverse group 1 influenza viruses after vaccination with H1ssF. Because we previously demonstrated that the ferritin nanoparticle platform can generate stem-specific neutralizing antibody responses (19), and a stem-only immunogen was used in this study, it is expected that stem-specific antibodies engendered the increased neutralization. The H1ssF vaccine at least doubled neutralization titers post-prime against H2N2 viruses regardless of the participants’ previous H2 exposure. When viewed alongside our previous results with a full-length HA vaccine (19), these data provide further evidence that the immunodominance of the head domain is a barrier to priming and boosting stem responses and that the H1ssF vaccine can overcome this barrier.
In interpreting our results, it is important to note that humans are not a naïve population for influenza virus infection. Antibody responses were evaluated by full-length HAs or conserved HA stem antigens, which are expected to be cross-reactive to previously encountered influenza strains. Thus, we expect that the “prime” vaccination of our trial boosted preexisting immune responses, resulting in the multiple-fold increases shown 2 weeks after the first vaccination. The “boost” vaccination of our trial is then a repeated exposure 16 weeks later and is not expected to result in the same magnitude of response. It is possible that the 16-week interval is not optimal for this vaccine platform.
Similar to preclinical findings (18, 21), in this trial, the H1ssF vaccine elicited broad influenza group 1 antibody responses but was limited in eliciting responses against group 2 influenza viruses. No neutralization titer increases were noted for H3 or H10 viruses, and only very modest increases in binding antibodies were observed. This result is expected given the structural and antigenic distance between group 1 and group 2 HA stems (29). However, a bivalent group 1 and group 2 stem vaccine would be expected to have increased breadth. In ongoing clinical trials, the VRC is currently evaluating a group 2 ss ferritin nanoparticle vaccine (H10ssF, NCT04579250), and Emergent BioSolutions is currently evaluating a combined influenza A group 1 (H1) and group 2 (H10) ss ferritin nanoparticle vaccine (UFluA) using nanoparticle technology developed by and licensed from the VRC (NCT05155319).
Concerns exist about the potential for HA stem–specific antibodies to cause antibody-mediated disease enhancement (ADE) [reviewed in (28) and (30)]. However, it is clear from this study and others that stem-specific antibodies occur naturally in humans (7, 31). No signs of ADE were observed in preclinical studies in mice or ferrets (17, 18, 32). Furthermore, the two confirmed influenza A infections that occurred during the trial presented as typical influenza infections, resolving within 7 days without sequelae. Overall, we did not observe any safety signals associated with ADE; later-stage trials will be needed to assess safety with greater power.
Ferritin is a ubiquitous iron storage protein, but the H. pylori ferritin used in the H1ssF vaccine has minimal iron incorporation activity and therefore is unlikely to affect iron homeostasis in vivo (17). Furthermore, single-chain H. pylori ferritin is highly divergent genetically and antigenically from both heterodimers of mammalian ferritin (17). In an abundance of caution, we monitored for antibody responses specific for the human ferritin proteins. As expected, antibodies specific for H. pylori ferritin increased, especially after two 60-μg doses of H1ssF. However, antibody titers against the human ferritins did not increase over the range of vaccine-naïve controls, indicating that there were no cross-reactive antibody responses. We did not assess antibody responses against the dimeric human ferritin/apoferritin. It is highly improbable that a single-chain H. pylori ferritin would elicit an antibody response only to quaternary epitopes at the junction of human heavy and light chain proteins but not to the rest of the human ferritin protein. Furthermore, we did not observe clinical signs of an anti-human ferritin antibody response: AEs for anemia evaluated as related to study product or product-related trends in participants’ iron and ferritin concentrations. These data support the safety of the H. pylori ferritin nanoparticle for use in human vaccines.
Our study has limitations. As with all phase 1 trials, the small number of participants limit the strength of the conclusions that can be drawn, including statements regarding the vaccine’s efficacy. The heterogeneity of preexisting influenza virus immunity in the human population is a major confounder for the investigation of vaccine-induced immune responses. In addition, this clinical trial was especially hampered by the COVID-19 pandemic, which caused 23% of participants to miss their boost and resulted in 131 missed sample collections. However, the H1ssF vaccine increased group 1 influenza binding antibodies post-prime in all age groups, during a time with few missed samples. Furthermore, unlike currently licensed influenza vaccines that are less effective in elderly adults (33, 34), the influenza ferritin vaccine platform displayed no difference in immune responses based on age (19). An additional limitation of this trial is that the H1ss vaccine antigen was derived from a pre-2009 pandemic H1 and differs in sequence from more recent H1 stems by 6.8%. However, we expect to observe similar responses against current H1 stem antigens, because we observed broad heterosubtypic responses against stems that diverge more than 20% in sequence from H1, including H5 and H2. In summary, the purpose of this clinical trial was as a safety evaluation and proof of principle that a ferritin nanoparticle presenting a stem-only HA antigen could elicit broad immunity. Future studies will be needed to determine the optimal stem antigens for a vaccine moving forward and will continue monitoring for safety signals related to the ferritin nanoparticle.
The results of this first-in-human phase 1 clinical trial demonstrate the tremendous promise for this vaccine platform. In contrast to most commercially available influenza virus vaccines, the H1ssF vaccine can be produced without reliance on embryonated eggs. Furthermore, ferritin-based nanoparticle vaccines can be more rapidly designed and manufactured and are therefore more adaptable in the face of changing epidemic and pandemic trends. This technology is amenable to mRNA-based delivery (5). Ongoing and planned studies of this technology include phase 1 trials evaluating mRNA-based delivery of H1ssF and delivery of a group 2 stem antigen by ferritin nanoparticles (NCT04579250) as well as computationally designed nanoparticle-based platforms (NCT04896086). The ferritin-based nanoparticle technology is being evaluated in ongoing clinical trials for severe acute respiratory syndrome coronavirus 2 (NCT04784767) and Epstein-Barr virus (NCT04645147) and has been nonexclusively licensed for future clinical development. The results of these studies will further contribute to the safety assessment and inform development of HA stem nanoparticle influenza vaccines capable of eliciting broad group 1 influenza immunity potentially effective over multiple influenza seasons.
MATERIALS AND METHODS
Study design
This study was a phase 1, open-label, dose-escalation clinical trial (Clinicaltrials.gov, NCT03814720) to examine the H1ssF vaccine at one 20-μg or two 60-μg doses in healthy adults aged 18 to 70 years. The primary outcomes of the study were safety and tolerability as determined by the occurrence of solicited local and systemic reactogenicity symptoms, AEs, SAEs, and new chronic medical conditions. The secondary outcome was immunogenicity as measured by the elicitation of vaccine-induced HA-specific antibodies.
The H1ssF (VRC-FLUNPF099-00-VP) vaccine was produced under Good Manufacturing Practice at the VRC Pilot Plant (VPP) operated under contract by the Vaccine Clinical Materials Program, Leidos Biomedical Research. H1ssF is a ferritin-based nanoparticle displaying eight stabilized headless stems of the H1 A/New Caledonia/20/1999 virus HA protein. The H1 stem antigen was designed by replacing the immunodominant head domain of the HA protomer with a short glycine-rich linker and mutating residues at the protomer interfaces to improve stability (17). The C terminus of each protomer was genetically fused to H. pylori nonheme ferritin as previously described (18). The vaccine was provided at 0.7 ± 0.1 ml in 1-ml vials at 180 mg/ml in a sodium phosphate solution.
H1ssF was administered intramuscularly through needle and syringe in the deltoid muscle. Participants were observed for a minimum of 30 min after each vaccination. A single dose escalation occurred after three participants received a 20-μg dose and were monitored for safety for 2 weeks. After safety review, the trial opened to administration of two 60-μg doses as a prime-boost regimen with an interval of about 16 weeks. The 60-μg dose arm of the trial was divided into age cohorts as follows: 18 to 40, 41 to 50, 51 to 60, and 61 to 70 years of age. Interim safety reviews occurred before enrollment opened for subsequent dose and age cohorts. Safety evaluations included participant-reported solicited reactogenicity collected for 7 days after each vaccination. Clinical and laboratory assessments occurred at baseline and at protocol-specified visit intervals. Unsolicited AEs were recorded for 28 days after each vaccination and were graded according to the modified U.S. Food and Drug Administration (FDA) Toxicity Grading Scale for Healthy Adult and Adolescent Volunteers Enrolled in Preventative Vaccine Clinical Trials (35). Influenza-like illnesses, SAEs, and new chronic medical conditions were recorded for the duration of the trial. A nasopharyngeal swab was obtained upon the report of influenza-like illnesses for polymerase chain reaction–based influenza testing. Participants were followed for 52 weeks after the last study product administration, including through an influenza season.
To address the primary outcome of safety and reactogenicity, sample size was determined by the power to identify SAEs. For the 20-μg dose participants (n = 5), there was a 90% chance to observe at least one SAE if the true rate was at least 0.369 and a more than 90% chance to observe no SAE if the true rate was less than 0.021. For the 60-μg dose participants, within each age cohort (n = 12), there was a greater than 90% chance to observe at least one SAE if the true rate was at least 0.175 and a more than 90% chance to observe no SAE if the true rate was no more than 0.009. When combining all four age cohorts from the 60-μg dose participants (n = 48), there was a greater than 90% chance to observe at least one SAE if the true rate was at least 0.047 and a greater than 90% chance to observe no SAE if the true rate was no more than 0.002. Enrollment for the 60- to 70-year-old age group was halted prematurely because of COVID-19 pandemic restrictions, so the final n for that group is 11, and the final n of all 60-μg dose participants is 47. Participants who had altered or discontinued vaccination schedules were monitored for safety and were included in the immunogenicity analysis until their vaccination schedules were changed. Immunogenicity data from all 60-μg dose participants were grouped.
The trial was conducted at the NIH Clinical Center by the VRC Clinical Trials Program, NIAID, NIH in Bethesda, MD. The study followed the guidelines for conducting clinical research with human subjects in accordance with 45 CFR Part 46 from the U.S. Department of Health and Human Services, and U.S. FDA regulations for investigational products, and principles expressed in the Declaration of Helsinki. The clinical trial protocol was reviewed and approved by the NIAID Institutional Review Board (IRB). Participants were recruited from the greater Washington, DC area by IRB-approved written and electronic media. Written informed consent was obtained from all participants before enrollment. Inclusion criteria included general good health determined by laboratory tests, medical history, and physical exam, with receipt of at least one licensed influenza vaccine since 2014. Exclusion criteria included the previous receipt of a licensed influenza vaccine within 6 weeks and previous investigational H1 influenza or ferritin-based vaccines. A complete list of inclusion and exclusion criteria can be found in the trial protocol (www.clinicaltrials.gov/ProvidedDocs/20/NCT03814720/Prot_SAP_ICF_000.pdf). The CONSORT checklist is provided in data file S1. All raw, individual-level data for experiments including groups of n < 20 are presented in data file S2.
Electrochemiluminescence assay
H1FL- and HA ss–binding antibodies in participant sera were assayed by Meso Scale Discovery (MSD) ECLIA as previously described (19). Briefly, blocked MSD 384-well streptavidin plates were coated with H1FL (1 μg/ml) or ss proteins specifically biotinylated at an AviTag site located proximal to the C terminus from the trimer foldon. The proteins included H2ss from A/Singapore/1/1957, H5ss from A/Indonesia/5/2005, H1ss from A/New Caledonia/20/1999, H1FL from A/New Caledonia/20/1999, H3ss from A/Finland/486/2004, and H10ss from A/Jiangxi-Donghu/346/2013. Blocked plates were washed, and serially diluted test samples, reference standards, and controls were added to the assay plates for 1 hour with shaking. A group 1 and group 2 binding antibody, 315-53-1F12 (36), was used as a reference standard in each assay. Binding of 315-53-1F12 (1 μg/ml) to H1ss was assigned a concentration of 100 arbitrary units per milliliter (AU/ml). AU/ml values for 315-53-1F12 binding to the heterologous HAs were assigned relative to H1ss binding. After sample incubation, plates were washed, and SULFO-TAG–labeled (0.5 μg/ml; MSD, catalog no. R91AO-1) anti-human immunoglobulin G (IgG), IgM, and IgA (H+L) secondary antibody (Thermo Fisher Scientific, catalog no. 31128, RRID AB_228255, lot #0031128) was added for 1 hour with shaking. Plates were read using MSD Sector Imager S600. All samples were tested in duplicate. Samples with a replicate coefficient of variation (CV) > 30% were retested. Serial dilutions of sample within the dynamic range of the standard curve were interpolated to assign a sample concentration in AU/ml. Results were plotted and analyzed using Prism version 8 or newer (GraphPad).
Anti-ferritin ELISA
Anti-ferritin antibodies in participant sera were measured by ELISA as previously described, with the exception that biotinylated rabbit anti-human IgG + IgM + IgA (H+L) (Jackson ImmunoResearch, catalog no. 309-065-064, RRID:AB_2339676) was used as the detection reagent (19). The human ferritin heavy chain and H. pylori ferritin antigens were produced by the VPP. Correct folding was verified by size exclusion chromatography, Western blot/SDS–polyacrylamide gel electrophoresis (PAGE) (human heavy chain), and transmission electron microscopy (H. pylori ferritin). The human light chain antigen was purchased commercially (LifeSpan BioSciences Inc., #109413) and verified by SDS-PAGE. All samples were tested in duplicate. Samples with a fold change greater than 1.15 from baseline or CV >15% underwent confirmatory testing in triplicate.
Microneutralization assay
The reporter-based microneutralization assay used in these analyses has been demonstrated to produce comparable results to the World Health Organization–recommended gold standard microneutralization assay using authentic live viruses (36). The reporter assay has several advantages over the gold standard assay, including safety, a better signal-to-noise ratio, a larger dynamic range, and no signal amplification steps that exaggerate errors, making it more reliable and reproducible. For this assay, replication-restricted reporter (R3) viruses were used where the viral genomic RNA encoding either the HA (R3ΔHA) (H2N2: A/Singapore/1/1957, H5N1: A/Indonesia/5/2005) or PB1 (R3ΔPB1) (H1N1: A/New Caledonia/20/1999) was replaced with the fluorescent gene TdKatushka2 per published methods (36, 37). Virus rescue, titration, and the neutralization assay were performed as previously described (19). The MDCK-SIAT1 PB1 clone A4 cell line was used in all microneutralization assays. The canine-origin parental line, MDCK-SIAT, was obtained from Sigma-Millipore (catalog no. 05071502). All participant samples were measured in quadruplicate by microneutralization assay.
Pseudotyped lentiviral neutralization assay
The pseudotyped lentiviral neutralization assay against A/New Caledonia/20/1999 was conducted as previously described (38–42). A human-origin 293T/17 cell line (American Type Culture Collection, catalog no. ACS-4500) was used to produce pseudotyped lentiviruses. The human-origin 293A cell line (Thermo Fisher Scientific, catalog no. R70507) was used as the reporter cell in the pseudotyped lentivirus neutralization. Each participant sample was assayed as true replicates serially diluted for eight dilution titers with duplicate wells per titer point. The analysis of neutralization at 80% (IC80) was interpolated off five-parameter nonlinear regression analysis of the eight dilution titers (points) where the signal of duplicate wells was averaged for curve fitting. CV values were calculated for each duplicate pair, and any dilution titer points between 50 and 100% neutralization with a CV greater than 30% were excluded from analysis. No sample was found to have a CV greater than 30% between the 50 and 100% neutralization range.
ADCC assay
The ADCC assays were conducted using a reporter assay (Promega) as previously reported (19). The chimeric virus used for evaluation displayed a chimeric HA with the head of an H6 subtype (A/mallard/Sweden/81/2002), the stalk from an H1 subtype (A/California/04/2009), and a neuraminidase from an H5 strain (A/mallard/Sweden/86/2003) on an A/Puerto Rico/8/1934 backbone. Serum samples were analyzed by a single five-point 1:3 dilution curve per sample starting at a 1:50 dilution. The positive value for each plate was determined as the average of all positive control wells. The negative value for each plate was determined as the average of all negative control wells plus three times the standard deviation of the negative control wells. A nonlinear regression line was fit to the data in GraphPad Prism 8.0 using the [Agonist] vs. response - Variable slope (four parameters) formula. Area under the curve (AUC) was analyzed using the negative value calculated for each plate as the baseline.
Statistical analysis
To address the secondary outcome of immunogenicity, the age cohorts in the 60-μg dose portion were compared pairwise using two-sample t tests but are exploratory because of small group sizes. Comparisons of binding antibody concentrations or neutralization titers between weeks within the 60-μg dose group or subgroups were made using paired t tests. Fold changes were calculated using raw binding antibody concentrations or neutralization titers. For the microneutralization assay, values at the limit of detection were imputed to half the limit of detection to calculate group averages. This affected baseline titers for 2 participants for H1N1, 1 participant for H2N2, and 11 participants for H5N1. The substantial percentage of participants (24%, 11 of 46) with undetectable baseline H5 neutralizing antibody titers meant that fold change calculations could not be performed. Comparisons of binding antibody concentrations or neutralization titers between antigens were made using generalized estimating equations to account for individual-level clustering over time. Comparisons between pre-boost and post-boost binding antibody concentrations were made as follows: Pre-boost concentrations were defined as the average on the raw scale of weeks 12 and 16 concentrations, and post-boost concentrations were the average on the raw scale of weeks 18 and 20 concentrations. Paired t tests were then used to compare log10 transforms of the averages. Unless otherwise noted, neutralizing antibody titers were log10-transformed before analysis. All tests were two sided, and an alpha level of 0.05 was used for significance. Per protocol, no adjustments were made for multiple comparisons for either safety or immunogenicity outcomes. Statistical analysis was performed using Statistical Analysis System (SAS), R version 4.1.1, or S-Plus statistical software.
Supplementary Material
Acknowledgments:
We thank the participants of the VRC 321 clinical trial for furthering influenza vaccine research.
Funding:
This work was supported by the Intramural Research Program of the VRC, NIAID, NIH. This work was partially funded by the NIAID-funded Centers of Excellence for Influenza Research and Response (75N93021C00014, to A.W.F., R.N., and P.P.), by NIAID grant P01 AI097092 (to A.W.F., R.N., and P.P.), by NIAID grant R01 AI145870 (to A.W.F., R.N., and P.P.), and by Collaborative Vaccine Innovation Centers contract 75N93019C00051 (to A.W.F., R.N., and P.P.).
The VRC 321 study team
In addition to VRC-affiliated authors, the following VRC 321 study team members have contributed to conceptualization of the study, development of the methodology, clinical trial investigation, and conducting of the analysis:
Allison Beck1, Joseph Casazza1, Christopher L. Case1, John Misasi1, Abidemi O. Ola1, Karen Parker1, Richard Wu1, Pamela Costner1, Jamie Saunders1, Laura Novik1, William Whalen1, Xiaolin Wang1, Aba Mensima Eshun1, Jennifer Cunningham1, Anita Arthur1, Morgan Anderson1, Justine Jones1, Brenda Larkin1, Thuy Nguyen1, Sandra Sitar1, Lam Le1, Iris Pittman1, Olga Vasilenko1, Galina Yamshchikov1, Ro Shauna Rothwell1, Eugenia Burch1, Olga Trofymenko1, Sarah Plummer1, Catina Evans1, Cora Trelles Cartagena1, Renunda Hicks1, LaShawn Requilman1, Pernell Williams1, Carmencita Graves1, and Shinyi Telscher1.
We would also like to thank the VRC Vaccine Production Program (VPP) members: Gabriela Albright1, Jessica Bahorich1, Sashikanth Banappagari1, Michael Bender1, Alegria T. Caringal1, Juliane Carvalho1, Rajoshi Chaudhuri1, Mythili Chintamani1, Jonathan Cooper1, Jacob Demirji1, Tracey Dinh1, Gelu Dobrescu1, Alvenne Goh1, Deepika Gollapudi1, Raju Gottumukkala1, Daniel Gowetski1, Janel Holland-Linn1, Jin Sung Hong1, Joe Horwitz1, Vera Ivleva1, Lisa Kueltzo1, Nadji Lambert1, Alaina LaPanse1, Heather Lawlor1, Kristin Leach1, James Lee1, Paula Lei1, Yile Li1, Jie (Amy) Liu1, Slobodanka Manceva1, Aakash Patel1, Rahul Ragunathan1, Lori Romaine1, Erwin Rosales1, Nikki Schneck1, William Shadrick1, Andrew Shaddeau1, Sudesh Upadhyay1, Karen Vickery1, Xiangchun (Eric) Wang1, Xin Wang1, Jack Yang1, Rong (Sylvie) Yang1, Yanhong Yang1, Yansong Yi1, Weidong Zhao1, and Zhong Zhao1.
Footnotes
Competing interests: M.K., B.S.G., and J.R.M. are named inventors of U.S. patents 9,441,019, titled “Influenza hemagglutinin protein-based vaccines”; 10,137,190, titled “Nucleic acid molecules encoding ferritin-hemagglutinin fusion proteins”; and 10,363,301, titled “Stabilized influenza hemagglutinin stem region trimers and uses thereof” filed by the Department of Health and Human Services (NIH). R.N. and A.W.F. are employees and shareholders of Moderna Inc. The other authors declare no competing interests.
Supplementary Materials
This PDF file includes:
Other Supplementary Material for this manuscript includes the following:
Data files S1 and S2
Data and materials availability:
All data associated with this study are present in the paper or the Supplementary Materials. Data generated in this study are available as deidentified data at ClinicalTrials.gov, NCT03814720. The study protocol, statistical analysis plan, and informed consent form are available on ClinicalTrials.gov (www.clinicaltrials.gov/ProvidedDocs/20/NCT03814720/Prot_SAP_ICF_000.pdf). Individual participant data that underlie the results reported in this article are available, after de-identification, in the Supplementary Materials. Additional data may be made available upon reasonable request to the corresponding author and jstein@mail.nih.gov for MTA requests, for investigators whose proposed use of the data has been approved by the NIAID IRB. All unique materials used in this study are available from the authors upon reasonable request.
References
- 1.Centers for Disease Control and Prevention, Disease Burden of Flu (Centers for Disease Control and Prevention, 2022); www.cdc.gov/flu/about/burden/index.html. [Google Scholar]
- 2.Wei C-J, Crank MC, Shiver J, Graham BS, Mascola JR, Nabel GJ, Next-generation influenza vaccines: Opportunities and challenges. Nat. Rev. Drug Discov. 19, 239–252 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Taubenberger JK, Kash JC, Morens DM, The 1918 influenza pandemic: 100 years of questions answered and unanswered. Sci. Transl. Med. 11, eaau5485 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Wiley DC, Skehel JJ, The structure and function of the hemagglutinin membrane glycoprotein of influenza virus. Annu. Rev. Biochem. 56, 365–394 (1987). [DOI] [PubMed] [Google Scholar]
- 5.Kanekiyo M, Graham BS, Next-generation influenza vaccines. Cold Spring Harb. Perspec. Med. 11, a038448 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Lambert LC, Fauci AS, Influenza vaccines for the future. N. Engl. J. Med. 363, 2036–2044 (2010). [DOI] [PubMed] [Google Scholar]
- 7.Corti D, Suguitan AL Jr., Pinna D, Silacci C, Fernandez-Rodriguez BM, Vanzetta F, Santos C, Luke CJ, Torres-Velez FJ, Temperton NJ, Weiss RA, Sallusto F, Subbarao K, Lanzavecchia A, Heterosubtypic neutralizing antibodies are produced by individuals immunized with a seasonal influenza vaccine. J. Clin. Invest. 120, 1663–1673 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Margine I, Hai R, Albrecht RA, Obermoser G, Harrod AC, Banchereau J, Palucka K, Garcia-Sastre A, Palese P, Treanor JJ, Krammer F, H3N2 influenza virus infection induces broadly reactive hemagglutinin stalk antibodies in humans and mice. J. Virol. 87, 4728–4737 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Thomson CA, Wang Y, Jackson LM, Olson M, Wang W, Liavonchanka A, Keleta L,Silva V, Diederich S, Jones RB, Gubbay J, Pasick J, Petric M, Jean F, Allen VG, Brown EG, Rini JM, Schrader JW, Pandemic H1N1 influenza infection and vaccination in humans induces cross-protective antibodies that target the hemagglutinin stem. Front. Immunol. 3, 87 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Ellebedy AH, Krammer F, Li GM, Miller MS, Chiu C, Wrammert J, Chang CY, Davis CW, McCausland M, Elbein R, Edupuganti S, Spearman P, Andrews SF, Wilson PC, Garcia-Sastre A, Mulligan MJ, Mehta AK, Palese P, Ahmed R, Induction of broadly cross-reactive antibody responses to the influenza HA stem region following H5N1 vaccination in humans. Proc. Natl. Acad. Sci. U.S.A. 111, 13133–13138 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Andrews SF, Joyce MG, Chambers MJ, Gillespie RA, Kanekiyo M, Leung K, Yang ES, Tsybovsky Y, Wheatley AK, Crank MC, Boyington JC, Prabhakaran MS, Narpala SR, Chen X, Bailer RT, Chen G, Coates E, Kwong PD, Koup RA, Mascola JR, Graham BS, Ledgerwood JE, McDermott AB, Preferential induction of cross-group influenza A hemagglutinin stem-specific memory B cells after H7N9 immunization in humans. Sci. Immunol. 2, eaan2676 (2017). [DOI] [PubMed] [Google Scholar]
- 12.Li G-M, Chiu C, Wrammert J, McCausland M, Andrews SF, Zheng N-Y, Lee J-H, Huang M, Qu X, Edupuganti S, Mulligan M, Das SR, Yewdell JW, Mehta AK, Wilson PC, Ahmed R, Pandemic H1N1 influenza vaccine induces a recall response in humans that favors broadly cross-reactive memory B cells. Proc. Natl. Acad. Sci. U.S.A. 109, 9047–9052 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Ekiert DC, Bhabha G, Elsliger MA, Friesen RH, Jongeneelen M, Throsby M, Goudsmit J, Wilson IA, Antibody recognition of a highly conserved influenza virus epitope. Science 324, 246–251 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Brandenburg B, Koudstaal W, Goudsmit J, Klaren V, Tang C, Bujny MV, Korse HJWM, Kwaks T, Otterstrom JJ, Juraszek J, van Oijen AM, Vogels R, Friesen RHE, Mechanisms of hemagglutinin targeted influenza virus neutralization. PLOS ONE 8, e80034 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Nachbagauer R, Miller MS, Hai R, Ryder AB, Rose JK, Palese P, Garcia-Sastre A, Krammer F, Albrecht RA, Hemagglutinin stalk immunity reduces influenza virus replication and transmission in ferrets. J. Virol. 90, 3268–3273 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Yamashita I, Iwahori K, Kumagai S, Ferritin in the field of nanodevices. Biochim. Biophys. Acta 1800, 846–857 (2010). [DOI] [PubMed] [Google Scholar]
- 17.Kanekiyo M, Wei C-J, Yassine HM, McTamney PM, Boyington JC, Whittle JRR, Rao SS, Kong W-P, Wang L, Nabel GJ, Self-assembling influenza nanoparticle vaccines elicit broadly neutralizing H1N1 antibodies. Nature 499, 102–106 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Yassine HM, Boyington JC, McTamney PM, Wei C-J, Kanekiyo M, Kong W-P, Gallagher JR, Wang L, Zhang Y, Joyce MG, Lingwood D, Moin SM, Andersen H, Okuno Y, Rao SS, Harris AK, Kwong PD, Mascola JR, Nabel GJ, Graham BS, Hemagglutinin-stem nanoparticles generate heterosubtypic influenza protection. Nat. Med. 21, 1065–1070 (2015). [DOI] [PubMed] [Google Scholar]
- 19.Houser KV, Chen GL, Carter C, Crank MC, Nguyen TA, Florez MCB, Berkowitz NM, Mendoza F, Hendel CS, Gordon IJ, Coates EE, Vazquez S, Stein J, Case CL, Lawlor H, Carlton K, Gaudinski MR, Strom L, Hofstetter AR, Liang CJ, Narpala S, Hatcher C, Gillespie RA, Creanga A, Kanekiyo M, Raab JE, Andrews SF, Zhang Y, Yang ES, Wang L, Leung K, Kong WP, Freyn AW, Nachbagauer R, Palese P, Bailer RT, McDermott AB, Koup RA, Gall JG, Arnold F, Mascola JR, Graham BS, Ledgerwood JE; the VRC 316 Study Team, Safety and immunogenicity of a ferritin nanoparticle H2 influenza vaccine in healthy adults: A phase 1 trial. Nat. Med. 28, 383–391 (2022). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Joyce MG, Wheatley AK, Thomas PV, Chuang GY, Soto C, Bailer RT, Druz A, Georgiev IS, Gillespie RA, Kanekiyo M, Kong WP, Leung K, Narpala SN, Prabhakaran MS, Yang ES, Zhang B, Zhang Y, Asokan M, Boyington JC, Bylund T, Darko S, Lees CR, Ransier A, Shen CH, Wang L, Whittle JR, Wu X, Yassine HM, Santos C, Matsuoka Y, Tsybovsky Y, Baxa U; Program 6 NISC Comparative Sequencing, Mullikin JC, Subbarao K, Douek DC, Graham BS, Koup RA, Ledgerwood JE, Roederer M, Shapiro L, Kwong PD, Mascola JR, B A. McDermott, Vaccine-induced antibodies that neutralize group 1 and group 2 influenza A viruses. Cell 166, 609–623 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Darricarrere N, Qiu Y, Kanekiyo M, Creanga A, Gillespie RA, Moin SM, Saleh J, Sancho J, Chou TH, Zhou Y, Zhang R, Dai S, Moody A, Saunders KO, Crank MC, Mascola JR, Graham BS, Wei C-J, Nabel GJ, Broad neutralization of H1 and H3 viruses by adjuvanted influenza HA stem vaccines in nonhuman primates. Sci. Transl. Med. 13, eabe5449 (2021). [DOI] [PubMed] [Google Scholar]
- 22.Andrews SF, Cominsky LY, Shimberg GD, Gillespie RA, Gorman J, Raab JE, Brand J, Creanga A, Gajjala SR, Narpala S, Cheung CSF, Harris DR, Zhou T, Gordon IJ, Holman L, Mendoza F, Houser KV, Chen GL, Mascola JR, Graham BS, Kwong PD, Widge A, Dropulic LK, Ledgerwood JE, Kanekiyo M, McDermott AB, An influenza H1 hemagglutinin stem-only immunogen elicits a broadly cross-reactive B cell response in humans. Sci. Transl. Med. 15, eade4976 (2023). [DOI] [PubMed] [Google Scholar]
- 23.Bodewes R, de Mutsert G, van der Klis FR, Ventresca M, Wilks S, Smith DJ, Koopmans M, Fouchier RA, Osterhaus AD, Rimmelzwaan GF, Prevalence of antibodies against seasonal influenza A and B viruses in children in Netherlands. Clin. Vaccine Immunol. 18, 469–476 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Sauerbrei A, Langenhan T, Brandstädt A, Schmidt-Ott R, Krumbholz A, Girschick H, Huppertz H, Kaiser P, Liese J, Streng A, Niehues T, Peters J, Sauerbrey A, Schroten H, Tenenbaum T, Wirth S, Wutzler P, Prevalence of antibodies against influenza A and B viruses in children in Germany, 2008 to 2010. Euro Surveill. 19, 20687 (2014). [DOI] [PubMed] [Google Scholar]
- 25.Zhang A, Stacey HD, Mullarkey CE, Miller MS, Original antigenic sin: How first exposure shapes lifelong anti–influenza virus immune responses. J. Immunol. 202, 335–340 (2019). [DOI] [PubMed] [Google Scholar]
- 26.Knight M, Changrob S, Li L, Wilson PC, Imprinting, immunodominance, and other impediments to generating broad influenza immunity. Immunol. Rev. 296, 191–204 (2020). [DOI] [PubMed] [Google Scholar]
- 27.Ellebedy AH, Nachbagauer R, Jackson KJL, Dai YN, Han J, Alsoussi WB, Davis CW, Stadlbauer D, Rouphael N, Chromikova V, McCausland M, Chang CY, Cortese M, Bower M, Chennareddy C, Schmitz AJ, Zarnitsyna VI, Lai L, Rajabhathor A, Kazemian C, Antia R, Mulligan MJ, Ward AB, Fremont DH, Boyd SD, Pulendran B, Krammer F, Ahmed R, Adjuvanted H5N1 influenza vaccine enhances both cross-reactive memory B cell and strain-specific naive B cell responses in humans. Proc. Natl. Acad. Sci. U.S.A. 117, 17957–17964 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Crowe JE Jr., Is it possible to develop a “universal” influenza virus vaccine? Cold Spring Harb. Perspect. Biol. 10, a029496 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Russell RJ, Gamblin SJ, Haire LF, Stevens DJ, Xiao B, Ha Y, Skehel JJ, H1 and H7 influenza haemagglutinin structures extend a structural classification of haemagglutinin subtypes. Virology 325, 287–296 (2004). [DOI] [PubMed] [Google Scholar]
- 30.Jang YH, Seong BL, The quest for a truly universal influenza vaccine. Front. Cell. Infect. Microbiol. 9, 344 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Aydillo T, Escalera A, Strohmeier S, Aslam S, Sanchez-Cespedes J, Ayllon J, RocaOporto C, Perez-Romero P, Montejo M, Gavalda J, Munoz P, Lopez-Medrano F, Carratala J, Krammer F, García-Sastre A, Cordero E, Pre-existing hemagglutinin stalk antibodies correlate with protection of lower respiratory symptoms in flu-infected transplant patients. Cell Rep. Med. 1, 100130 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Boyoglu-Barnum S, Hutchinson GB, Boyington JC, Moin SM, Gillespie RA, Tsybovsky Y, Stephens T, Vaile JR, Lederhofer J, Corbett KS, Fisher BE, Yassine HM, Andrews SF, Crank MC, McDermott AB, Mascola JR, Graham BS, Kanekiyo M, Glycan repositioning of influenza hemagglutinin stem facilitates the elicitation of protective cross-group antibody responses. Nat. Commun. 11, 791 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Rondy M, El Omeiri N, Thompson MG, Levêque A, Moren A, Sullivan SG, Effectiveness of influenza vaccines in preventing severe influenza illness among adults: A systematic review and meta-analysis of test-negative design case-control studies. J. Infect. 75, 381–394 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.U.S. Food and Drug Administration, Flucelvax Quadrivalent Package Insert (U.S. Food and Drug Administration, 2021); www.fda.gov/media/115862/download. [Google Scholar]
- 35.U.S. Food and Drug Administration, Guidance for Industry: Toxicity Grading Scale for Healthy Adult and Adolescent Volunteers Enrolled in Preventive Vaccine Clinical Trials (U.S. Food and Drug Administration, 2007); www.fda.gov/regulatory-information/search-fda-guidance-documents/toxicity-grading-scale-healthy-adult-and-adolescent-volunteers-enrolled-preventive-vaccine-clinical. [Google Scholar]
- 36.Creanga A, Gillespie RA, Fisher BE, Andrews SF, Lederhofer J, Yap C, Hatch L, Stephens T, Tsybovsky Y, Crank MC, Ledgerwood JE, McDermott AB, Mascola JR, Graham BS, Kanekiyo M, A comprehensive influenza reporter virus panel for high-throughput deep profiling of neutralizing antibodies. Nat. Commun. 12, 1722 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Shcherbo D, Murphy CS, Ermakova GV, Solovieva EA, Chepurnykh TV, Shcheglov AS, Verkhusha VV, Pletnev VZ, Hazelwood KL, Roche PM, Lukyanov S, Zaraisky AG, Davidson MW, Chudakov DM, Far-red fluorescent tags for protein imaging in living tissues. Biochem. J. 418, 567–574 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Yang Z-Y, Wei C-J, Kong W-P, Wu L, Xu L, Smith DF, Nabel GJ, Immunization by avian H5 influenza hemagglutinin mutants with altered receptor binding specificity. Science 317, 825–828 (2007). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Wei C-J, Boyington JC, McTamney PM, Kong W-P, Pearce MB, Xu L, Andersen H, Rao S, Tumpey TM, Yang Z-Y, Nabel GJ, Induction of broadly neutralizing H1N1 influenza antibodies by vaccination. Science 329, 1060–1064 (2010). [DOI] [PubMed] [Google Scholar]
- 40.Wei C-J, Boyington JC, Dai K, Houser KV, Pearce MB, Kong W-P, Yang Z-Y, Tumpey TM, Nabel GJ, Cross-neutralization of 1918 and 2009 influenza viruses: Role of glycans in viral evolution and vaccine design. Sci. Transl. Med. 2, 24ra21 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Ledgerwood JE, Wei C-J, Hu Z, Gordon IJ, Enama ME, Hendel CS, McTamney PM, Pearce MB, Yassine HM, Boyington JC, Bailer R, Tumpey TM, Koup RA, Mascola JR, Nabel GJ, Graham BS; VRC 306 Study Team, DNA priming and influenza vaccine immunogenicity: Two phase 1 open label randomised clinical trials. Lancet Infect. Dis. 11, 916–924 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Houser KV, Yamshchikov GV, Bellamy AR, May J, Enama ME, Sarwar U, Larkin B, Bailer RT, Koup R, Paskel M, Subbarao K, Anderson E, Bernstein DI, Creech B, Keyserling H, Spearman P, Wright PF, Graham BS, Ledgerwood JE; VRC 702 study team, DNAvaccine priming for seasonal influenza vaccine in children and adolescents 6 to 17 years of age: A phase 1 randomized clinical trial. PLOS ONE 13, e0206837 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
All data associated with this study are present in the paper or the Supplementary Materials. Data generated in this study are available as deidentified data at ClinicalTrials.gov, NCT03814720. The study protocol, statistical analysis plan, and informed consent form are available on ClinicalTrials.gov (www.clinicaltrials.gov/ProvidedDocs/20/NCT03814720/Prot_SAP_ICF_000.pdf). Individual participant data that underlie the results reported in this article are available, after de-identification, in the Supplementary Materials. Additional data may be made available upon reasonable request to the corresponding author and jstein@mail.nih.gov for MTA requests, for investigators whose proposed use of the data has been approved by the NIAID IRB. All unique materials used in this study are available from the authors upon reasonable request.


