Abstract
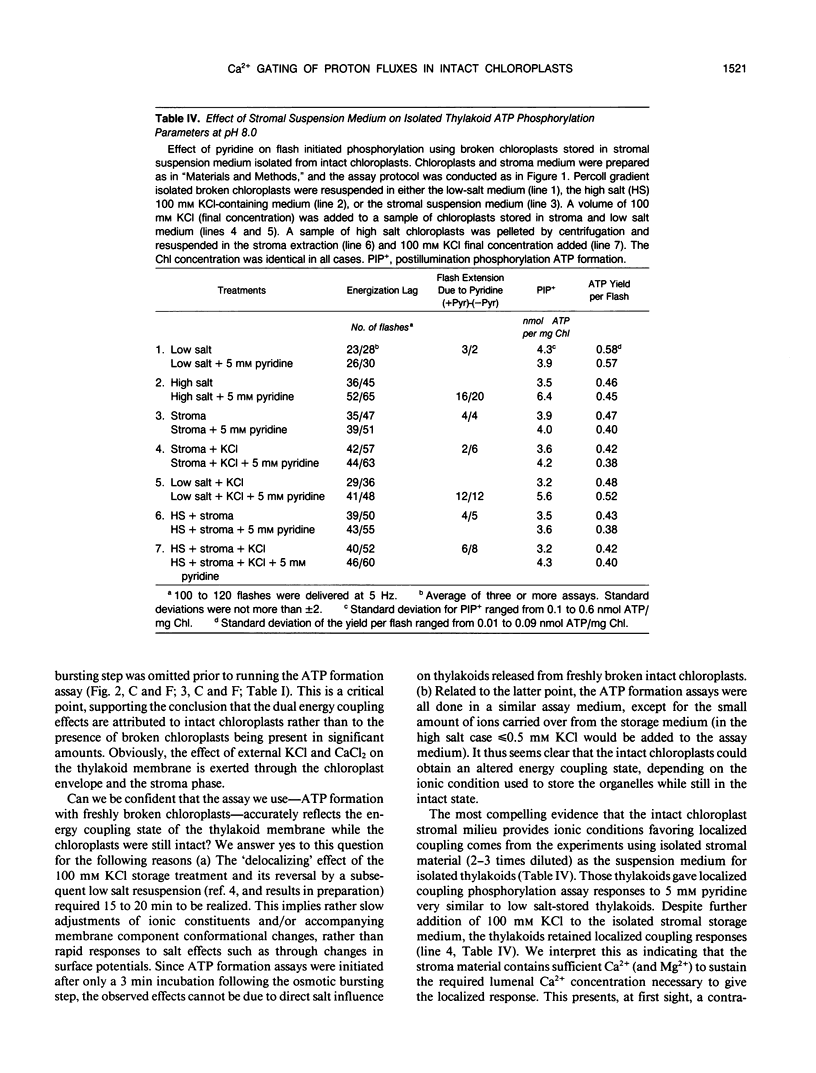
Intact chloroplasts were compared to isolated thylakoids as to whether storage of the organelle in high KCl medium caused the energy coupling reactions to show a delocalized or a localized proton gradient energy coupling response. With isolated thylakoids, the occurrence of one or the other energy coupling mode can be reversibly controlled by the concentration of mono- and divalent cations used for the thylakoid storage media. Calcium was shown to be the key ion and previous evidence suggested a Ca2+-controlled gating of H+ fluxes in the thylakoid membrane system (G Chiang, RA Dilley [1987] Biochemistry 26: 4911-4916). Isolated, intact chloroplasts, which retained the outer envelope membranes during the 30 min or longer storage treatments in various concentrations of KCl and CaCl2 (with sorbitol to maintain iso-osmotic conditions), were osmotically burst in a reaction cuvette and within 3 minutes were assayed for either a localized or a delocalized proton gradient energy coupling (ATP formation) mode. The intact chloroplast system was analogous to isolated thylakoids, with regard to the effects of KCl and CaCl2 on the energy coupling mode. For example, adding 100 millimolar KCl to the intact organelle storage medium resulted in the subsequent ATP formation assay showing delocalized proton gradient coupling just as with isolated thylakoids. Adding 5 millimolar CaCl2 to the 100 millimolar KCl storage medium resulted in a localized proton gradient coupling mode. Suspending thylakoids in stromal material previously isolated from intact chloroplast preparations and testing the energy coupling response showed that the stromal milieu has enough Ca2+ to cause the localized coupling response even though there was about 80 millimolar K+ in the intact chloroplasts used in this study (determined by atomic absorption spectrophotometry). Extrapolating the intact chloroplast data to the whole leaf level, we suggest that proton gradient energy coupling is normally of the localized mode, but under certain conditions it could be either localized or delocalized, depending on factors that affect the putative Ca2+-regulated proton flux gating function.
Full text
PDF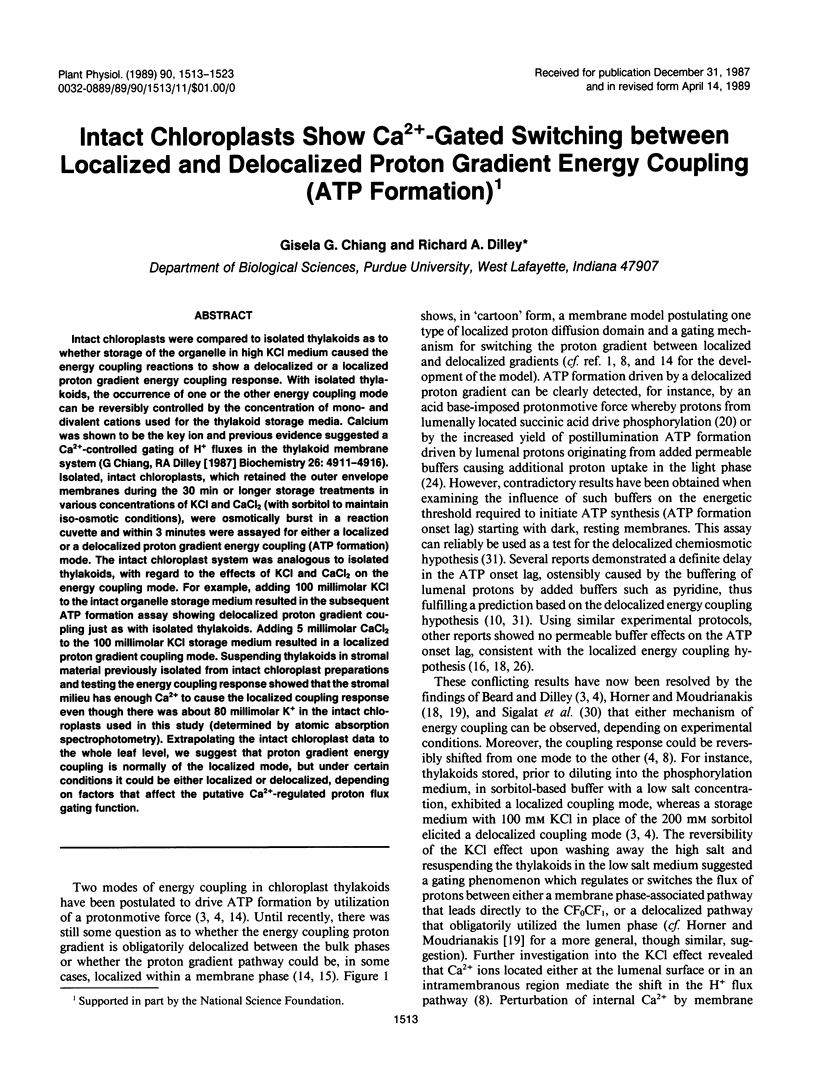






Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Beard W. A., Chiang G., Dilley R. A. ATP formation onset lag and post-illumination phosphorylation initiated with single-turnover flashes. II. Two modes of post-illumination phosphorylation driven by either delocalized or localized proton gradient coupling. J Bioenerg Biomembr. 1988 Feb;20(1):107–128. doi: 10.1007/BF00762140. [DOI] [PubMed] [Google Scholar]
- Beard W. A., Dilley R. A. ATP formation onset lag and post-illumination phosphorylation initiated with single-turnover flashes. I. An assay using luciferin-luciferase luminescence. J Bioenerg Biomembr. 1988 Feb;20(1):85–106. doi: 10.1007/BF00762139. [DOI] [PubMed] [Google Scholar]
- Beard W. A., Dilley R. A. ATP formation onset lag and post-illumination phosphorylation initiated with single-turnover flashes. III. Characterization of the ATP formation onset lag and post-illumination phosphorylation for thylakoids exhibiting localized or bulk-phase delocalized energy coupling. J Bioenerg Biomembr. 1988 Feb;20(1):129–154. doi: 10.1007/BF00762141. [DOI] [PubMed] [Google Scholar]
- Davenport J. W., McCarty R. E. The onset of photophosphorylation correlates with the rise in transmembrane electrochemical proton gradients. Biochim Biophys Acta. 1980 Feb 8;589(2):353–357. doi: 10.1016/0005-2728(80)90051-1. [DOI] [PubMed] [Google Scholar]
- Dilley R. A., Vernon L. P. Ion and water transport processes related to the light-dependent shrinkage of spinach chloroplasts. Arch Biochem Biophys. 1965 Aug;111(2):365–375. doi: 10.1016/0003-9861(65)90198-0. [DOI] [PubMed] [Google Scholar]
- Heber U., Santarius K. A. Direct and indirect transfer of ATP and ADP across the chloroplast envelope. Z Naturforsch B. 1970 Jul;25(7):718–728. doi: 10.1515/znb-1970-0714. [DOI] [PubMed] [Google Scholar]
- Horner R. D., Moudrianakis E. N. Effects of permeant buffers on the initiation of photosynchronous phosphorylation and postillumination phosphorylation in chloroplasts. J Biol Chem. 1986 Oct 15;261(29):13408–13414. [PubMed] [Google Scholar]
- Horner R. D., Moudrianakis E. N. The effect of permeant buffers on initial ATP synthesis by chloroplasts using rapid mix-quench techniques. J Biol Chem. 1983 Oct 10;258(19):11643–11647. [PubMed] [Google Scholar]
- Jagendorf A. T., Uribe E. ATP formation caused by acid-base transition of spinach chloroplasts. Proc Natl Acad Sci U S A. 1966 Jan;55(1):170–177. doi: 10.1073/pnas.55.1.170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kreimer G., Melkonian M., Holtum J. A., Latzko E. Stromal free calcium concentration and light-mediated activation of chloroplast fructose-1,6-bisphosphatase. Plant Physiol. 1988 Feb;86(2):423–428. doi: 10.1104/pp.86.2.423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nakatani H. Y., Barber J., Minski M. J. The influence of the thylakoid membrane surface properties on the distribution of ions in chloroplasts. Biochim Biophys Acta. 1979 Jan 11;545(1):24–35. doi: 10.1016/0005-2728(79)90110-5. [DOI] [PubMed] [Google Scholar]
- Nobel P. S. Light-induced changes in the ionic content of chloroplasts in Pisum sativum. Biochim Biophys Acta. 1969 Jan 14;172(1):134–143. doi: 10.1016/0005-2728(69)90098-x. [DOI] [PubMed] [Google Scholar]
- Ort D. R., Dilley R. A., Good N. E. Photophosphorylation as a function of illumination time. II. Effects of permeant buffers. Biochim Biophys Acta. 1976 Oct 13;449(1):108–124. doi: 10.1016/0005-2728(76)90011-6. [DOI] [PubMed] [Google Scholar]
- Portis A. R., Jr, Heldt H. W. Light-dependent changes of the Mg2+ concentration in the stroma in relation to the Mg2+ dependency of CO2 fixation in intact chloroplasts. Biochim Biophys Acta. 1976 Dec 6;449(3):434–436. doi: 10.1016/0005-2728(76)90154-7. [DOI] [PubMed] [Google Scholar]
- Schröppel-Meier G., Kaiser W. M. Ion Homeostasis in Chloroplasts under Salinity and Mineral Deficiency : I. Solute Concentrations in Leaves and Chloroplasts from Spinach Plants under NaCl or NaNO(3) Salinity. Plant Physiol. 1988 Aug;87(4):822–827. doi: 10.1104/pp.87.4.822. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vinkler C., Avron M., Boyer P. D. Effects of permeant buffers on the initial time course of photophosphorylation and postillumination phosphorylation. J Biol Chem. 1980 Mar 25;255(6):2263–2266. [PubMed] [Google Scholar]



