Abstract
Objectives:
Our study aimed to compare the efficacy of transdermal dihydrotestosterone and testosterone enanthate in treating idiopathic micropenis.
Patients and methods:
It’s a comparative randomized study of 49 patients with idiopathic micropenis who are followed up in the Endocrinology-Diabetology and Nutrition Department of Mohammed VI University Hospital Center of Oujda, Morocco. The study was conducted from December 2019 to April 2021. All patients received a clinical examination including measurement of penis size before and after hormonal treatment. The patients were divided into two random groups, each group received a different drug, the first arm was treated with transdermal dihydrotestosterone (27 patients) and the second arm was treated with testosterone enanthate (22 patients). The Trial registration number was researchregistry7745.
Results:
The majority of the patients were children. The mean age was 9.7 ± 4.4 years. In the first arm, the mean penile size increased from −2.42 SD to −0.7 SD with a gain of 2.37 cm on average. In the second arm, the mean size increased from −2.48 SD to −0.69 SD, with a gain of 1.82 cm on average. The increase in penile size in the first arm was significantly greater than in the second arm (P = .008). No side effects were detected in both arms.
Discussion and conclusion:
In the present study, we demonstrated the superiority of transdermal DHT compared to injectable exogenous testosterone in the treatment of idiopathic micropenis. According to the age subgroups, there was no significant difference between the 2 treatments in each age group.
Keywords: Micropenis, transdermal dihydrotestosterone, testosterone enanthate
Introduction
Micropenis is a relatively common phenomenon that can occur either in an isolated form or as part of a disorder of male sexual development.1,2 It is defined as an abnormally short and anatomically correct penis. There are several methods to estimate penile length, including flaccid (non-stretched penile length), erected, and Ultrasonography. However, the length of the stretched penis is the most valid measurement, since it correlates more closely with erectile length than does the length of the relaxed penis.3,4 It is the best diagnostic indicator and should be compared with the values of reference tables such as the Schönefeld and Flatta curve, which is still the most widely used in daily practice. 5 A value of −2.5 standard deviation (SD) has been used as the lower limit for normal penile length by some authors,1,2,7 −2 SD for others.5 -9 Its incidence in the United States is 1.5/10000 boys between 1997 and 2000. 10
It can occur as an endocrine disorder defining a genetic origin or can be “idiopathic” when no cause can be found. The diagnosis must be made early in life to ensure that appropriate androgenic treatment can be initiated as soon as possible to avoid the psychological consequences which are sometimes dramatic.
The treatment is based either on the substitution of dihydrotestosterone (DHT) or exogenous testosterone which will be converted by 5 alpha-reductase type 2 to DHT. Several studies have been carried out using a single drug to prove its effectiveness.3,8,11,12 However, we couldn’t find any previous study comparing the response of DHT and TE in patients with micropenis, especially in children and adolescents. In this light, we aimed to compare the efficacy of transdermal dihydrotestosterone (DHT) and testosterone enanthate (TE) in treating idiopathic micropenis in the young population.
Patients and Methods
Study design and population
This is a controlled randomized trial, conducted over 2 years (from December 2019 to April 2021) in the Department of Endocrinology-Diabetology and Nutrition of Mohammed VI University Hospital Center of Oujda, Morocco. All enrolled patients had complete medical records and gave their oral consent to participate in this study. For children and adolescents, verbal consent was obtained from the parents and/or a legal representative. The ethical review committee at our faculty of Medicine approved the study design and protocol under the number 22/2020. The Trial registration number was researchregistry7745.
Eligible participants were patients aged less than 18 years old, who underwent a complete physical examination and etiological investigation to rule out the other etiologies of micropenis.
Exclusion criteria were: family history of sterility, delayed puberty, non-genital anomalies, abnormal genitalia (Hypospadias, abnormal scrotal shape, cryptorchidism, anorchidism, delayed tanner stage), malnutrition, and associated comorbidities. Abnormal testicular function (Assessed with baseline plasma testosterone and interpreted for tanner stage and/or HCG test: normal response if baseline testosterone is multiplied by 4), abnormal gonadotrophin (baseline levels and/or after LHRH stimulation test in case of delayed puberty), abnormal blood karyotype, and abnormal adrenal function (morning serum cortisol <180 ng/ml). Other investigations were carried out according to the clinical context before retaining the diagnosis of idiopathic micropenis (Pituitary MRI, pelvic ultrasound, and genetic testing in case of associated abnormal genitalia and dysmorphia).
Out of the 82 patients who presented to our department with micropenis, 49 patients met the inclusion criteria. The age of management of the patients in this study was highly variable and corresponded to the time they were referred to our department.
Study protocol
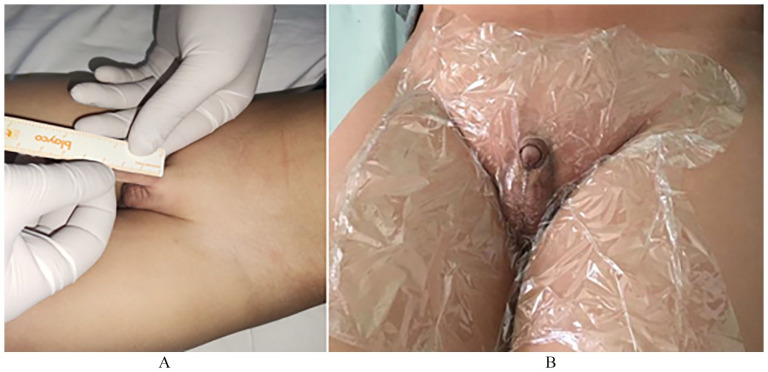
The diagnosis of idiopathic micropenis was made after the exclusion of all other causes of micropenis. The length of the penis was measured (in centimeters) on the dorsal side with a rigid ruler, from the pubis, after depressing the prepubic fat and pulling the penis, to the tip of the glans (Figure 1A). The results were expressed in standard deviation (SD) according to the Schönefeld reference curve. 5
Figure 1.
(A) Measuring technique of the penile length and (B) image showing the precautions to take before applying the treatment.
After inclusion, we identified 49 patients with isolated idiopathic micropenis, the patients were divided into 2 random arms. The 2 groups were paired for age, gonadotropin status, and circulating titers of testosterone. Table 1 resumes the characteristics of the enrolled patients (Table 1).
Table 1.
Epidemiologic, clinical, and biological characteristics of the 2 groups.
| Transdermal DHT | Testosterone Enanthate | P value | |
|---|---|---|---|
| Number of patients | 27 | 22 | |
| Age at consultation (Years) | 8.4 ± 3.7 | 10 ± 3.4 | .1 |
| Medical and familial history | No | No | |
| Initial size of micropenis (Cm) | 2.9 ± 0.7 cm (−2.42 SD) | 3 ± 0.9 cm (−2.2 SD) | .74 |
| Mean of FSH (mUi/ml) | 3.1 ± 2 | 1.3 ± 0.9 | .29 |
| Mean of LH (mUi/ml) | 3.4 ± 1 | 1.4 ± 1 | .44 |
| Mean of Testosterone (ng/ml) | 0.12 ± 0.1 | 0.23 ± 0.15 | .29 |
Abbreviations: SD, standard deviation; Cm, centimeters; FSH, follicle stimulating hormone; LH, luteinizing hormone.
The first arm was treated with the local treatment: transdermal DHT (ANDRACTIM®). Our protocol was based on a daily application of 5 mg, either in the evening or in the morning after washing. It was applied on the penis sheath while avoiding direct application on the mucous membranes (alcoholic gel – 45°), and let dry before putting a piece of clothing on the application area. It was continued for 5 weeks and renewed 1 to 2 times if no normalization of the penis size or in case of great regression of the size after normalization (<− 2SD) (Figure 1B).
The second arm was treated with the systemic treatment: testosterone enanthate (ANDROTARDYL 250 mg®). Patients received an intramuscular injection of 50 mg of Testosterone enanthate per month for 3 months, repeated only once if necessary.
The groups were also divided into 4 age groups in to study the efficacy of the treatment according to the pubertal stage (Figure 2).
Figure 2.
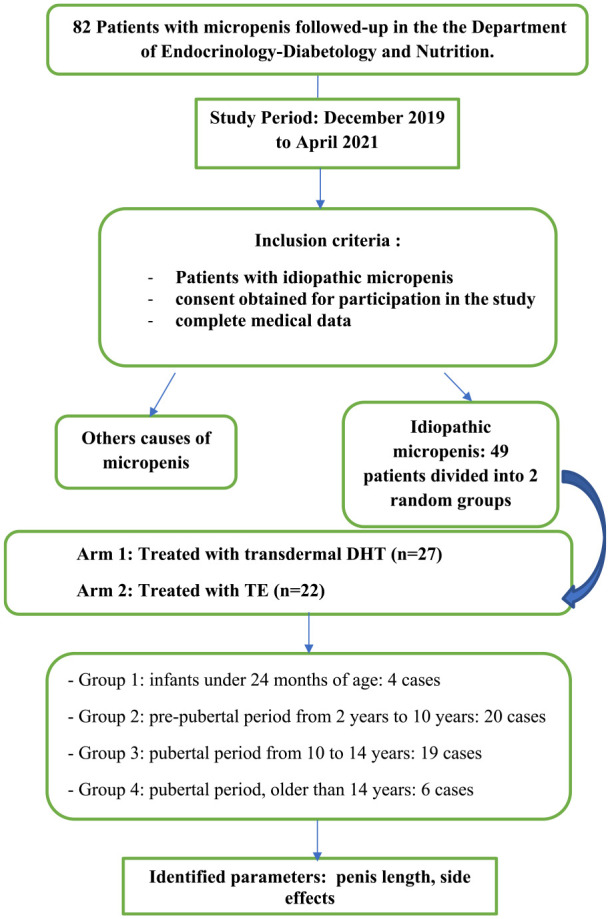
Design of the study.
Outcomes
The primary outcome of our study was to determine the efficacy of the 2 drugs (DHT and TE) on increasing the penis length at the end of the treatment and at a distance, that is, at least 12 weeks later. The according to the pubertal stage. Secondary outcomes included the evaluation of the clinical response according to pubertal stages and identification of possible side effects (allergic reaction, appearance of pubic pilosity, acceleration of bone maturation, gynecomastia, acne, nervousness, and aggressiveness) during follow-up.
Statistical analysis
We used the Statistical Package for the Social Sciences, version 21 (IBM, Armonk, NY) for all analyses. The data were expressed as means ±SD and were analyzed using the student t-test, the chi-square test, and the Fisher exact test. A critical value of P < .05 indicated statistical significance.
Results
Eighty-two patients with micropenis were enrolled in our study. After eliminating (testicular dysgenesis, hypogonadotropic hypogonadism, defects in testosterone synthesis, androgen resistance (5α-reductase deficiency or partial androgen insensitivity), and other rare causes like growth hormone deficiency), the diagnosis of idiopathic micropenis was retained in 49 patients. The majority of our patients were children with a mean age of 9.7 ± 4.4 years. The discovery of micropenis was incidental during a routine endocrine examination in 86% of cases. Micropenis was isolated in all patients with a negative etiological investigation.
In the first arm, the mean size before treatment with transdermal DHT was 2.9 ± 0.7 cm corresponding to an average of −2.42 SD. After an average number of days of treatment estimated to be 65 ± 43 days, the mean size increased to 5.3 ± 1cm corresponding to −0.7 SD on average, with a mean gain of 2.37 cm (Figure 3). In the second arm, the mean size before treatment was 3 cm ±0.9 corresponding to an average of −2.48 SD. After an average number of injections estimated to 2.6 ± 0.6 injections, the mean size increased to 4.8 cm ±1.2 corresponding to −0.69 SD, with a gain of 1.82 cm on average. The increase in penile size in the first arm was significantly greater than in the second arm (P = .008) (Table 2).
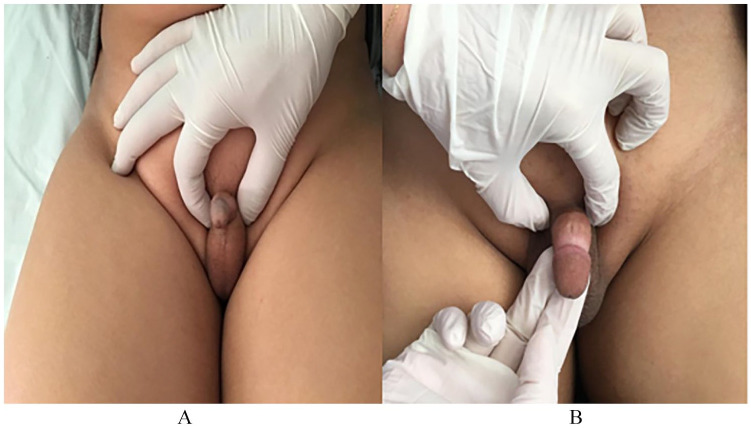
Figure 3.
Image showing the evolution of a micropenis before (A) and after application of Transdermal DHT (B).
Table 2.
Penile length at admission and outcomes after treatment.
| The average size at inclusion | The average size after treatment | Mean gain | P-value | |
|---|---|---|---|---|
| Transdermal DHT | 2.9 ± 0.7 cm (−2.42 SD) | 5.3 ± 1 cm (−0.7 SD) | 2.37 cm | .008 |
| Testosterone Enanthate | 3 cm ±0.9 ( −2.48 SD) | 4.8 cm ±1.2 (−0.69 SD) | 1.82 cm |
When comparing the gain in cm by age group, we could not find a statistically significant difference (P = .409 for the first arm and P = .144 for the second arm). Furthermore, we found a better response during the peripubertal period in the patients treated with transdermal DHT (Figure 4).
Figure 4.
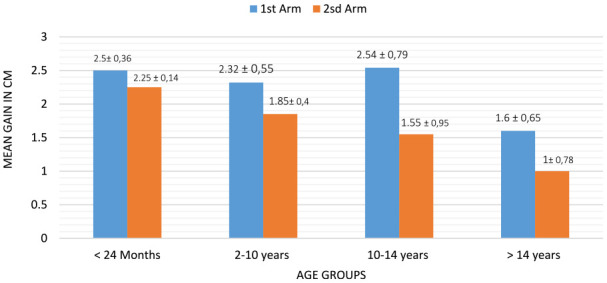
The comparison of the mean gain in both arms according to age groups.
We did not observe any side effects of hormonal therapy during the follow-up of our patients with idiopathic micropenis in the 2 arms.
Discussion
In the present study, the transdermal DHT appeared to be more effective than the TE in the treatment of idiopathic micropenis (P = .008). According to the age subgroups, there was no significant difference between the 2 treatments in each age group. However, we noted a better response to transdermal DHT during the peripubertal period.
Micropenis is the clinical expression of insufficient secretion or defectuous action of testosterone in utero between 12 weeks of amenorrhea and birth. Similarly, a primary testicular disorder that causes insufficient production of testosterone during late gestation or its metabolite or its receptivity can also result in insufficient penile growth. When no etiology can be found, the micropenis is considered “idiopathic.” 11 Furthermore, obese boys may suffer from micropenis, unstable bladder, poor body image, decreased quality of life, anxiety and sexual problems. 13
In our research, we chose to use transdermal DHT and injectable TE. These 2 therapeutic approaches represent the mainstay of the treatment of micropenis. The treatment is based either on the substitution of dihydrotestosterone (DHT) or exogenous testosterone which will be converted by 5 alpha-reductase type 2 to DHT. DHT exerts its physiological functions through androgenic receptors in the penis. 14 Several studies have been carried out using a single drug to prove its effectiveness, Choi et al investigated the response to a local treatment after 4 weeks of use and found an elevation rate of penile length of 153 ± 17% 12 (Table 3). However, we couldn’t find any previous study comparing the response of DHT and TE in patients with micropenis, especially in children and adolescents.
Table 3.
Reported research studies using transdermal DHT and TE.
| Choi et al 12 | Hatipoğlu et al 11 | Nerli et al. 3 | Velasquez-Urzola et al 8 | |
|---|---|---|---|---|
| Number of cases | 7 | 6 | 11 | 16 |
| Age (Years) | 9.7 | 1.9-8.3 | 5 ± 1.4 | 4.5 ± 2.5 |
| Etiologies of micropenis | Idiopathic | All etiologies | Idiopathic | Idiopathic |
| Therapy regimens | Transdermal DHT | Transdermal DHT | Testosterone enanthate 25 mg/ month | Testosterone heptylate (100 mg/m2) every 2 wk |
To the best of our knowledge, our research is considered the first of its kind in the existing literature. There is no consensus on the exact protocol to use when using DHT or TE in children and adolescents. We established our own protocol, detailed previously in the methods section, based on our experience in treating micropenis.
Through our study, we proved the efficacy and safety of Transdermal DHT, this efficacy has been shown on all age groups, with an average gain of 2.37 cm (P = .008). Sakhri and Gooren 14 showed that DHT is biologically 8 times more active than testosterone, thus, DHT has superiority over intramuscular testosterone because it cannot be converted to estradiol in vivo; therefore, it is less likely to cause gynecomastia. They also reported an association of exogenous testosterone with premature epiphyseal fusion. 14 Choi et al 12 also reported satisfactory results in patients treated with DHT even in those who had shown no response to previous testosterone therapy (Table 3).
At post-pubertal age, studies proved that hormonal treatment is not satisfying regardless of the etiology of micropenis. This can be explained by the decrease in androgen receptors at this period of life. 15 The same finding was noted in our study by the insufficient response as expected in group 4 regardless of the medical treatment used (Figure 4).
The main strength of our study is the fact that it is the first research of its kind to ever compare these 2 drugs in large randomized groups, according to our best knowledge. Moreover, our study gives an insight into the possible response of each drug and thus helps choose the best therapeutic approach to use for each patient depending on their age and clinical data.
There are some limits of the study that may have undermined the validity of our results. One possible bias was related to the accuracy of idiopathic micropenis diagnosis. In the majority of cases, the etiology of isolated micropenis remain unknown and is considered “idiopathic.” However, idiopathic micropenis can also be related to undetected genetic abnormalities and heterogeneous mutations, as shown by Paris et al, 16 in a study conducted in 2010; where they discovered 5 alpha-reductase type 2 gene mutations, androgen receptor gene mutations and SF1 (Steroidogenic factor 1) gene mutations in patients with isolated micropenis and normal testosteronemia. 16 The existence of such undetected molecular genetic defects may have influenced the response to the tested treatments. Unfortunately, genetic molecular analysis is unavailable in our country and was not performed in all of our patients. On another note, body mass index was not studied in our patients, so the response to the treatment may have been influenced in overweight or obese patients. Another limit was the power analysis for sample size calculation and blinding that were unfortunately not done.
Conclusion
Our study compares the 2 most commonly used therapeutic approaches in the literature. Through our research, we demonstrated the superiority of transdermal DHT compared to injectable exogenous testosterone in the treatment of idiopathic micropenis. Transdermal DHT also seems more effective during the peripubertal period. However, larger studies are needed on patients with genetically confirmed diagnoses to confirm the findings of our research.
Acknowledgments
The authors would like to acknowledge all medical and paramedical staff involved in the management of the patients
Footnotes
ORCID iDs: Najoua Messaoudi  https://orcid.org/0000-0002-6262-6995
https://orcid.org/0000-0002-6262-6995
Imane Assarrar  https://orcid.org/0000-0002-0074-7986
https://orcid.org/0000-0002-0074-7986
Declarations
Ethics Approval and Consent to Participate: The ethical review committee at the faculty of Medicine, Mohamed first university of Oujda (CERBO), approved the study design and protocol under the number 22/2020.
Consent for Publication: A verbal consent was obtained from the parents and/or a legal representative, for the publication of the results of the current study.
Author Contributions: Marouan Karrou: Conceptualization; Data curation; Formal analysis; Investigation; Methodology; Writing – original draft. Najoua Messaoudi: Conceptualization; Data curation; Formal analysis; Investigation; Methodology; Writing – original draft. Imane Assarrar: Data curation; Investigation; Writing – original draft. Achwak Alla: Data curation; Investigation; Writing – original draft. Siham Rouf: Conceptualization; Methodology; Supervision; Writing – review & editing. Hanane Latrech: Conceptualization; Methodology; Supervision; Writing – review & editing.
Funding: The author(s) received no financial support for the research, authorship, and/or publication of this article.
The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Availability of Data and Materials: The patient’s data used to support the findings of this study can be retrieved from the archives of the Department of Endocrinology-Diabetology and Nutrition, at the Mohammed VI University Hospital of Oujda, Morocco.
References
- 1. Lee PA, Mazur T, Danish R, et al. Micropenis. I. Criteria, etiologies and classification. Johns Hopkins Med J. 1980;146:156-163. [PubMed] [Google Scholar]
- 2. Elder JS. Congenital anomalies of the genitalia. Campbell’s Urology. Vol. 69. 7th ed. Hardcover; Publisher: W.B Saunders Company; 1998;2120; Chap.69: 2120. [Google Scholar]
- 3. Nerli RB, Guntaka AK, Patne PB, Hiremath MB. Penile growth in response to hormone treatment in children with micropenis. Indian J Urol. 2013;29:288-291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Rezakhaniha B, Siroosbakht S. Establishment of novel non-stretched penile length (Flaccid) cut-off point and normative data in Iranian prepubertal children and its significance: observational analytical study. J Compr Pediatr. 2022;13(3):e130095. [Google Scholar]
- 5. Schonfeld WA, Beebe GW. Normal growth and variation in the male genitalia from birth to maturity. J Urol. 1942;48:759-777. [Google Scholar]
- 6. Forest M, David M. Le micropenis: donnees etiologiques et traitement dans une serie de 88 cas. Rev Fr Endocrinol Clin. 1986;27:322-335. [Google Scholar]
- 7. Aigrain Y, Czemichow P. Mémoire original hypoplasie de la verge : diagnostic dtiologique et rbultat du traitement par testostbrone retard A Velisquez-Urzola. J Tiger. 1998;5(8):844-850. [DOI] [PubMed] [Google Scholar]
- 8. Velásquez-Urzola A, Léger J, Aigrain Y, Czernichow P. [Hypoplasia of the penis: etiologic diagnosis and results of treatment with delayed-action testosterone]. Arch Pediatr. 1998;5:844-850. [DOI] [PubMed] [Google Scholar]
- 9. Ludwig G. Micropenis and apparent micropenis–a diagnostic and therapeutic challenge. Andrologia. 1999;31 Suppl 1:27-30. [DOI] [PubMed] [Google Scholar]
- 10. Nelson CP, Park JM, Wan J, et al. The increasing incidence of congenital penile anomalies in the United States. J Urol. 2005;174:1573-1576. [DOI] [PubMed] [Google Scholar]
- 11. Hatipoğlu N, Kurtoğlu S. Micropenis: etiology, diagnosis and treatment approaches. J Clin Res Pediatr Endocrinol. 2013;5:217-223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Choi SK, Han SW, Kim DH, de Lignieres B. Transdermal dihydrotestosterone therapy and its effects on patients with microphallus. J Urol. 1993;150:657-660. [DOI] [PubMed] [Google Scholar]
- 13. Rezakhaniha S, Rezakhaniha B, Aarabi N, Siroosbakht S. Is it necessary to weight loss in obese boys with small penile length? A case-control study. J Compr Pediatr. 2020;11(4):e 107272. [Google Scholar]
- 14. Sakhri S, Gooren LJ. Safety aspects of androgen treatment with 5 a –dihydrotestosterone; 2007: 216–222. [DOI] [PubMed] [Google Scholar]
- 15. Charmandari E, Dattani MT, Perry LA, et al. Kinetics and effect of percutaneous administration of dihydrotestosterone in children. Horm Res. 2001;56:177-181. [DOI] [PubMed] [Google Scholar]
- 16. Paris F, De Ferran K, Bhangoo A, et al. Isolated 'idiopathic' micropenis: hidden genetic defects? Int J Androl. 2011;34:e518-e525. [DOI] [PubMed] [Google Scholar]