Abstract
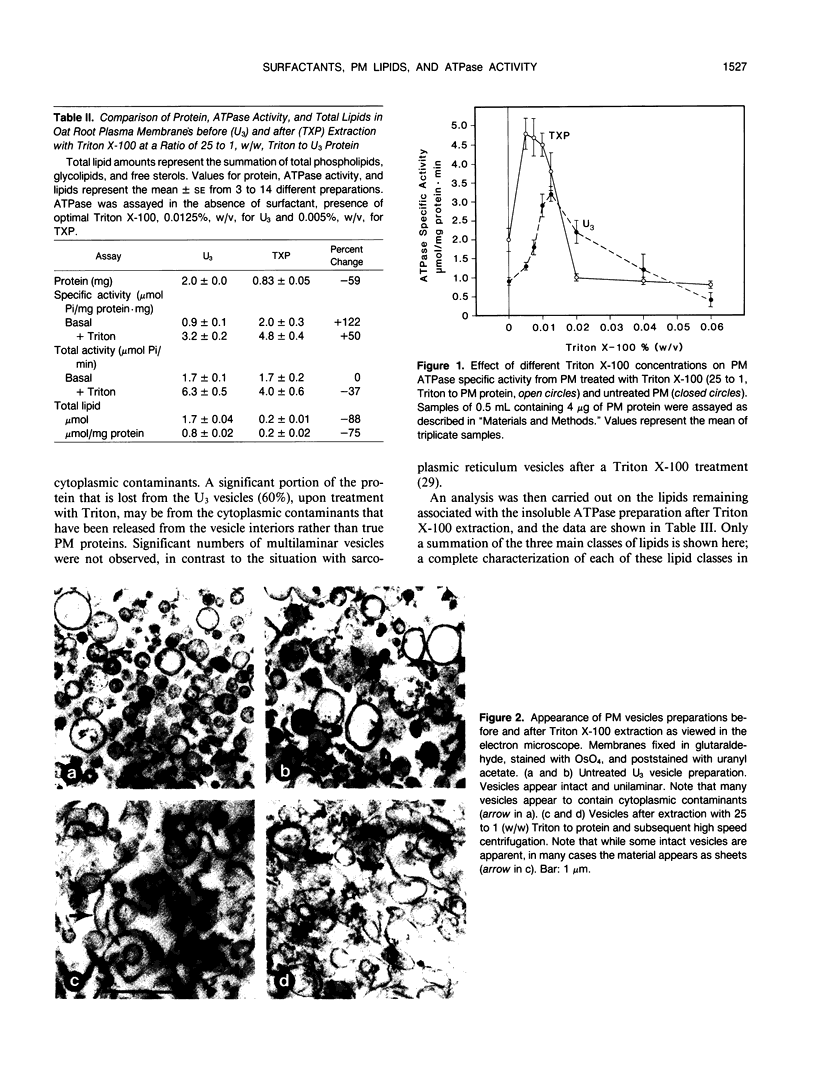
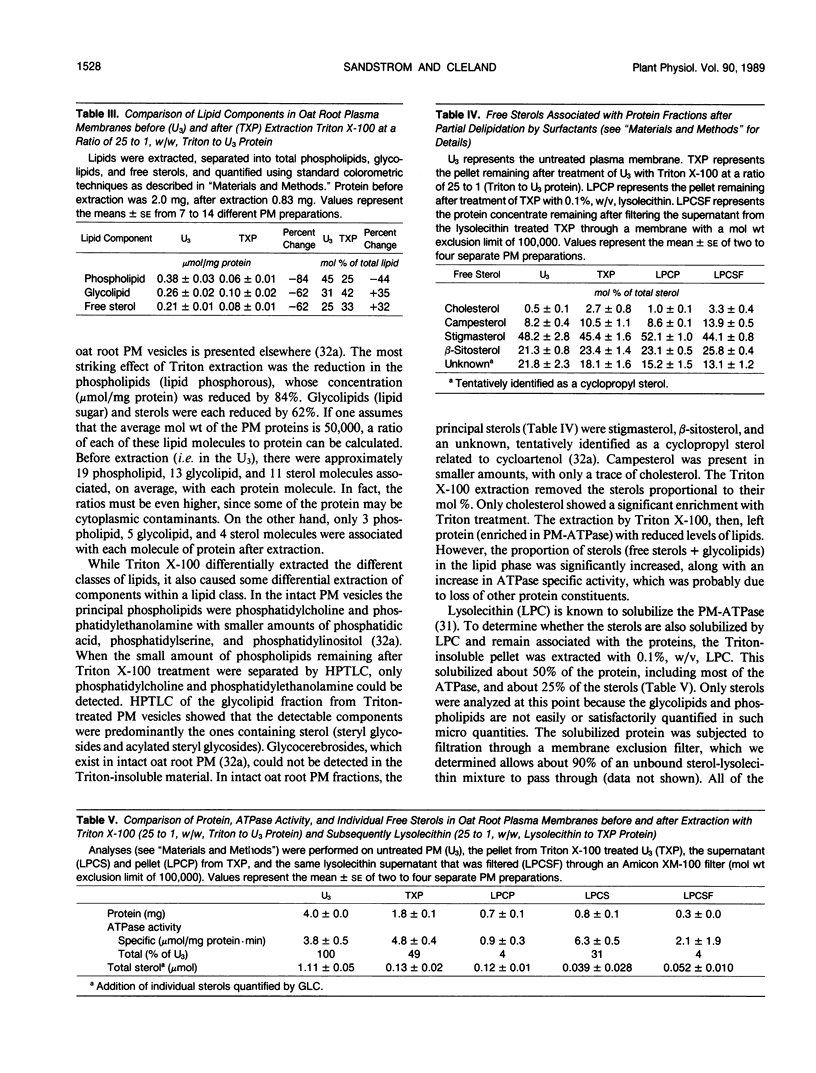
The influence of plasma membrane lipid components on the activity of the H+-ATPase has been studied by determining the effect of surfactants on membrane lipids and ATPase activity of oat (Avena sativa L.) root plasma membrane vesicles purified by a two-phase partitioning procedure. Triton X-100, at 25 to 1 (weight/weight) Triton to plasma membrane protein, an amount that causes maximal activation of the ATPase in the ATPase assay, extracted 59% of the membrane protein but did not solubilize the bulk of the ATPase. The Triton-insoluble proteins had associated with them, on a micromole per milligram protein basis, only 14% as much phospholipid, but 38% of the glycolipids and sterols, as compared with the native membranes. The Triton insoluble ATPase could still be activated by Triton X-100. When solubilized by lysolecithin, there were still sterols associated with the ATPase fraction. Free sterols were found associated with the ATPase in the same relative proportions, whether treated with surfactants or not. We suggest that surfactants activate the ATPase by altering the hydrophobic environment around the enzyme. We propose that sterols, through their interaction with the ATPase, may be essential for ATPase activity.
Full text
PDF





Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Anthon G. E., Spanswick R. M. Purification and properties of the h-translocating ATPase from the plasma membrane of tomato roots. Plant Physiol. 1986 Aug;81(4):1080–1085. doi: 10.1104/pp.81.4.1080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- BLIGH E. G., DYER W. J. A rapid method of total lipid extraction and purification. Can J Biochem Physiol. 1959 Aug;37(8):911–917. doi: 10.1139/o59-099. [DOI] [PubMed] [Google Scholar]
- Bigelow D. J., Thomas D. D. Rotational dynamics of lipid and the Ca-ATPase in sarcoplasmic reticulum. The molecular basis of activation by diethyl ether. J Biol Chem. 1987 Oct 5;262(28):13449–13456. [PubMed] [Google Scholar]
- Cocucci M., Ballarin-Denti A. Effect of polar lipids on ATPase activity of membrane preparations from germinating radish seeds. Plant Physiol. 1981 Aug;68(2):377–381. doi: 10.1104/pp.68.2.377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dufour J. P., Goffeau A. Phospholipid reactivation of the purified plasma membrane ATPase of yeast. J Biol Chem. 1980 Nov 25;255(22):10591–10598. [PubMed] [Google Scholar]
- Dulley J. R. Determination of inorganic phosphate in the presence of detergents or protein. Anal Biochem. 1975 Jul;67(1):91–96. doi: 10.1016/0003-2697(75)90275-4. [DOI] [PubMed] [Google Scholar]
- Galtier N., Belver A., Gibrat R., Grouzis J. P., Rigaud J., Grignon C. Preparation of Corn Root Plasmalemma with Low Mg-ATPase Latency and High Electrogenic H Pumping Activity after Phase Partitioning. Plant Physiol. 1988 Jun;87(2):491–497. doi: 10.1104/pp.87.2.491. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hamilton J. G., Comai K. Separation of neutral lipids and free fatty acids by high-performance liquid chromatography using low wavelength ultraviolet detection. J Lipid Res. 1984 Oct;25(10):1142–1148. [PubMed] [Google Scholar]
- Hidalgo C., Thomas D. D., Ikemoto N. Effect of the lipid environment on protein motion and enzymatic activity of sarcoplasmic reticulum calcium ATPase. J Biol Chem. 1978 Oct 10;253(19):6879–6887. [PubMed] [Google Scholar]
- Imbrie C. W., Murphy T. M. Photoinactivation of Detergent-Solubilized Plasma Membrane ATPase from Rosa damascena: Action Spectra. Plant Physiol. 1984 Mar;74(3):617–621. doi: 10.1104/pp.74.3.617. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kasamo K., Nouchi I. The Role of Phospholipids in Plasma Membrane ATPase Activity in Vigna radiata L. (Mung Bean) Roots and Hypocotyls. Plant Physiol. 1987 Feb;83(2):323–328. doi: 10.1104/pp.83.2.323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- Le Maire M., Moller J. V., Tanford C. Retention of enzyme activity by detergent-solubilized sarcoplasmic Ca2+ -ATPase. Biochemistry. 1976 Jun 1;15(11):2336–2342. doi: 10.1021/bi00656a014. [DOI] [PubMed] [Google Scholar]
- Lynch D. V., Steponkus P. L. Plasma Membrane Lipid Alterations Associated with Cold Acclimation of Winter Rye Seedlings (Secale cereale L. cv Puma). Plant Physiol. 1987 Apr;83(4):761–767. doi: 10.1104/pp.83.4.761. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Markwell M. A., Haas S. M., Bieber L. L., Tolbert N. E. A modification of the Lowry procedure to simplify protein determination in membrane and lipoprotein samples. Anal Biochem. 1978 Jun 15;87(1):206–210. doi: 10.1016/0003-2697(78)90586-9. [DOI] [PubMed] [Google Scholar]
- McIntosh D. B., Ross D. C. Role of phospholipid and protein-protein associations in activation and stabilization of soluble Ca2+-ATPase of sarcoplasmic reticulum. Biochemistry. 1985 Feb 26;24(5):1244–1251. doi: 10.1021/bi00326a029. [DOI] [PubMed] [Google Scholar]
- Prado A., Arrondo J. L., Villena A., Goñi F. M., Macarulla J. M. Membrane-surfactant interactions. The effect of Triton X-100 on sarcoplasmic reticulum vesicles. Biochim Biophys Acta. 1983 Aug 24;733(1):163–171. doi: 10.1016/0005-2736(83)90102-5. [DOI] [PubMed] [Google Scholar]
- Proverbio F., Proverbio T., Rawlins F. Efecto de la sustitución parcial del colesterol de membrana por otros esteroles sobre la actividad ATPásica estimulada por Na+ + K+ en fracciones microsomales de corteza cerebral de rata. Acta Cient Venez. 1980;31(3):219–223. [PubMed] [Google Scholar]
- Rochester C. P., Kjellbom P., Andersson B., Larsson C. Lipid composition of plasma membranes isolated from light-grown barley (Hordeum vulgare) leaves: identification of cerebroside as a major component. Arch Biochem Biophys. 1987 Jun;255(2):385–391. doi: 10.1016/0003-9861(87)90406-1. [DOI] [PubMed] [Google Scholar]
- Roughan P. G., Batt R. D. Quantitative analysis of sulfolipid (sulfoquinovosyl diglyceride) and galactolipids (monogalactosyl and digalactosyl diglycerides) in plant tissues. Anal Biochem. 1968 Jan;22(1):74–88. doi: 10.1016/0003-2697(68)90261-3. [DOI] [PubMed] [Google Scholar]
- Sandstrom R. P., Deboer A. H., Lomax T. L., Cleland R. E. Latency of Plasma Membrane H-ATPase in Vesicles Isolated by Aqueous Phase Partitioning : Increased substrate Accessibility or Enzyme Activation. Plant Physiol. 1987 Nov;85(3):693–698. doi: 10.1104/pp.85.3.693. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Serrano R. Purification of the proton pumping ATPase from plant plasma membranes. Biochem Biophys Res Commun. 1984 Jun 15;121(2):735–740. doi: 10.1016/0006-291x(84)90243-2. [DOI] [PubMed] [Google Scholar]
- Xie X. S., Tsai S. J., Stone D. K. Lipid requirements for reconstitution of the proton-translocating complex of clathrin-coated vesicles. Proc Natl Acad Sci U S A. 1986 Dec;83(23):8913–8917. doi: 10.1073/pnas.83.23.8913. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yoda S., Yoda A. Phosphorylated intermediates of Na,K-ATPase proteoliposomes controlled by bilayer cholesterol. Interaction with cardiac steroid. J Biol Chem. 1987 Jan 5;262(1):103–109. [PubMed] [Google Scholar]
- Zlatkis A., Zak B. Study of a new cholesterol reagent. Anal Biochem. 1969 Apr 11;29(1):143–148. doi: 10.1016/0003-2697(69)90017-7. [DOI] [PubMed] [Google Scholar]



