Abstract
Efficient differentiation of embryonic stem cells (ESC) into hematopoietic progenitor cells (HPCs) is crucial for the establishment of stem cell-based therapies targeting the treatment of immunological and hematological disorders. However, so far, it has not been possible to induce long-term survival of murine ESC-derived HPCs without the overexpression of HoxB4, a homeobox transcription factor that confers self-renewal properties to hematopoietic cells. Yet it has not been feasible to generate T cells from HoxB4-expressing HPCs, a problem that has been attributed to HoxB4. Here, we show that Notch1 signaling in HoxB4-transduced ESCs leads to efficient derivation of T cells that survive long term. These T cells display a normal T-cell Vβ repertoire, respond to mitogen stimulation and induce lethal graft-versus-host disease. Thymic selection in fetal thymic organ cultures (FTOCs) allowed negative selection and generation of T cells tolerant to ‘self’ and capable of rejecting MHC-mismatched skin allografts. Our data show that ESC-derived T cells, despite high expression of HoxB4, are fully immunocompetent.
Keywords: Acute graft-versus-host disease, HoxB4, stem cells, skin allograft, T cells
Introduction
One of the characteristic features of embryonic stem cells (ESCs) is their ability to generate tissues of all three germ layers under appropriate differentiation stimuli. In hematopoietic cells, this property has many advantages for the derivation and study of lineage-specific cells allowing studies on immune responses to specific pathogens. For example, newly generated T, B and NK cells could be applied in immunodeficient mouse models for the study of pathogen control. Similarly, in humans, many immunodeficient syndromes could be treated by generating healthy hematopoietic progenitor cells (HPCs) that could be transplanted in patients, restoring normal immune responses.
The generation of HPCs from ESCs has been best studied in murine models. However, it has been difficult to derive T, B and NK cells from ESCs, making it impossible to determine the functional capacity of ESC-derived lymphocytes. More importantly, ESC-derived HPCs fail to self-renew and thus are incapable of long-term engraftment (1,2). Recent reports have shown that ESCs can be differentiated into HPCs after transduction with HoxB4, a homeobox transcription factor that confers self-renewal properties to hematopoietic stem cells (3–7). Although the differentiation of ESCs into hematopoietic cells is equally efficient with non-HoxB4-transduced cells (8), the progenitors do not self-renew and are usually nondetectable in vivo in both syngeneic and immune-incompetent mice within 3–4 weeks (2). In contrast, after transduction of ESCs with HoxB4, permanent engraftment and long-term chimerism can be achieved (1,2,9). Unfortunately, these mouse progenitor cells predominantly differentiate into myeloid cells with very few cells differentiating into lymphoid cells hampering immunological studies (1). This previous report by Daley’s group involved the transduction of HoxB4 into embryoid bodies, whereas we transduced HoxB4 into ESCs before their differentiation into T cells (3). Others have used conditional expression of HoxB4 to avoid permanent expression of HoxB4 or combined HoxB4 with caudal genes (10) to enforce lymphocytic cell development. These approaches have not led to increased lymphocyte development.
During T-cell development, hematopoietic precursor cells enter the outer cortex of the thymus to become lymphoid progenitors. These cells become CD44+CD25− and are known as DN (double negative) stage 1 cells. They further develop into the DN2 cells expressing both CD44 and CD25. In the next phase, they lose CD44 expression becoming DN3 and then DN4 cells that are CD25−CD44− (11). These developmental stages require specific signaling cues to drive T-cell specification. In particular, Notch1 signaling is crucial for the successful development of T cells. The DN4 cells become double positive for CD4 and CD8, engaging with cortical epithelial cells to become single CD4- or CD8-expressing cells. After positive selection, mature single positive T cells emerge out of the medulla into the periphery. To recapitulate this process in vitro, the stromal cell line OP9 has been mostly used for such studies (12,13). This cell line was established from bone marrow cells and is MHC-class I positive, but class II negative, which means, T-cell development favors the generation of CD8+ T cells and not that of CD4+ cells. For Notch1 signaling, the developing cells on the OP9 cell cultures are transferred to the OP9-DL1 cell line, which express the Notch1 ligand Delta-like 1 (DL1) (14).
Here, we wondered whether Notch1 signaling in HoxB4-expressing ESC-derived HPCs can direct the differentiation of progenitors toward functional T cells that are responsive to antigen stimulation. Others have previously shown that Notch1 signaling in non-HoxB4-transduced ESCs directed their differentiation into T cells (14). These T cells do not survive long term in vivo because they lack self-renewal properties. The rationale for our studies is if we can coax HoxB4-transduced ESCs into T cells, we can exploit the self-renewal properties of this transcription factor to study the effectiveness of ESC-derived T cells in correcting immunological disorders. Herein, we show that T cells can be derived in large numbers in vitro from HoxB4-expressing murine ESCs. The T cells display a normal TcR diversity, respond to mitogen stimuli and rapidly induce severe lethal graft-versus-host disease (GVHD). In contrast, when T-cell progenitors were cultured in fetal thymic organ cultures (FTOCs), they were tolerant to “self” and rejected MHC-mismatched skin allografts.
Materials and Methods
Mice and ESCs
Four- to six-week-old 129SvJ mice were purchased from the Jackson Laboratory (Bar Harbor, ME, USA). All mice were bred in the Animal Facility at the VAMC, Iowa City. Animal procedures were approved by the IACUC and in accordance with NIH guidelines. The HM1 murine ESC line (Open Biosystems, Huntsville, AL, USA) was derived from the 129SvJ mouse strain.
In vitro differentiation of T cells from HoxB4-transduced ESCs
For T-cell differentiation, a modified protocol previously described by Schmitt et al. (14) was used. Briefly, ESCs were plated onto OP9 cells on day 0. After 5 days, recovered cells were plated onto OP9 cells with α-MEM containing Flt3L (5 ng/mL). On day 8, cells were transferred onto new OP9-DL1 cells using T-cell medium containing α-MEM with 20% FBS, 5 ng/mL Flt3L and 1 ng/mL IL-7. Cells were maintained in culture till day 30.
FTOCs and reconstitution of thymic lobes with T-cell precursors
Fetal thymic lobes were recovered from E14.5 to E15.5 embryos and cultured on 0.8-μm membranes (Millipore, Billerica, MA, USA) supported with Gelatin Sponge in the DMEM medium containing 1.35 mM 2′-deoxyguanosine (Sigma, St. Louis, MO, USA) to deplete hematopoietic cells for 5–7 days. After washing, lobes were reconstituted with T-cell progenitors (2 × 104 cells/lobe) using hanging drops in Terasaki plates for 48 h. Reconstituted lobes were cultured on a filter for 14 days and were either subcutaneously transplanted in 129SvJ mice or gently pressed to isolate T cells for phenotyping.
Transplantation of HoxB4-T cells
Four- to six-week-old mice were sublethally irradiated (800 cGy) 24 h prior to transplantation of T cells. To study T-cell engraftment, 0.1 to 4×106 non-FTOC T cells were injected intravenously. Thymic lobes containing FTOC-T cells were transplanted subcutaneously on the back of 129SvJ mice as previously described (14).
Skin grafting and immunohistochemistry
Tail skin of allogenic MRL mice (H2k) and syngenic 129 mice (H2b) were transplanted on the backs of Rag2−/−γc−/− mice (H2b), respectively, as we previously reported (15). After 4 weeks of engraftment, 106 FTOC-T cells were injected subcutaneously around the grafts (n = 6). Control grafts were injected with PBS. Skin graft rejection was noted when >75% of the skin had turned dark brown and began peeling off.
To determine the histology of the spleens, small intestines and thyroid glands of GVHD animals, organs were fixated with 10% formalin for 48 h and embedded in paraffin for staining with hematoxylin and eosin. For immunohistochemistry, small intestine sections were stained with anti-GFP rabbit IgG primary antibody (1:100, Invitrogen, Carlsbad, CA, USA) for 2 h at room temperature. For secondary antibody, antirabbit HRP was used. The substrate (Dako DAB Plus, Carpinteria, CA, USA) was applied according to the manufacturer’s directions.
Further methods are described in the Supplementary Materials and Methods section.
Results
Notch1 signaling promotes the generation of T cells from HoxB4-expressing ESCs
To derive T cells, we used a modified protocol based on Schmitt et al. (14). HM1–ESCs were either transduced with GFP or with HoxB4-GFP as reported by others (16). As expected, GFP-transduced and HoxB4-GFP-transduced ESCs expressed Nanog and Oct4 transcription factors before differentiation into T cells (Figure 1A). To derive T cells, HM1-ESCs were grown on OP9 stromal cells for 8 days and were then transferred to OP9-DL1 cells, which express DL1, a ligand for Notch1 (14). To monitor T-cell development, T-cell progenitors were regularly analyzed for T-cell surface marker expression. By day 5, the cells had significantly increased Flk-1 expression, a receptor for vascular endothelial growth factor, and the earliest mesodermal differentiation marker for endothelial cells and blood cells (17,18). By day 8, the cells expressed CD45 and CD41, markers of hematopoietic cells and HPCs, respectively. The expression levels of Oct4, Nanog, Flk-1, CD41 and CD45 at days 0, 5 and 8 are tabulated in Table S1, indicating that as the cells differentiated into hematopoietic cells, they lost markers of pluripotency. After transferring the cells onto OP9-DL1 cells, early T-cell development became apparent as the cells acquired expression of CD44 and CD25. Surprisingly, there were no characteristic differences in the stages of T-cell development between HoxB4 and non-HoxB4 progenitors as reflected by immunophenotyping results. The cells progressed into the DN1 stage, expressing CD44, but not CD25 (Figure 1B). By day 16, there were DN1, DN2, DN3 and DN4 cells as detected by flow cytometric analysis of CD25 and CD44 expression. Finally, on day 22, the cells were double positive (DP) for CD4 and CD8, suggesting normal T-cell development. Again, there was no difference in the phenotype of T-cell precursors between HoxB4- and non-HoxB4-T cells, suggesting that HoxB4 did not perturb T-cell development under Notch1 signaling. However, HoxB4-T cells expanded 40- to 50-fold compared to non-HoxB4-T cells, demonstrating the capability of HoxB4 to confer self-renewal and proliferative properties to hematopoietic cells. Statistical analysis of these data is shown in Figure S1. For comparison, 129SvJ mouse thymocytes were analyzed for CD4 and CD8 expression (Figure 1C).
Figure 1: Phenotypic changes during T-cell development.
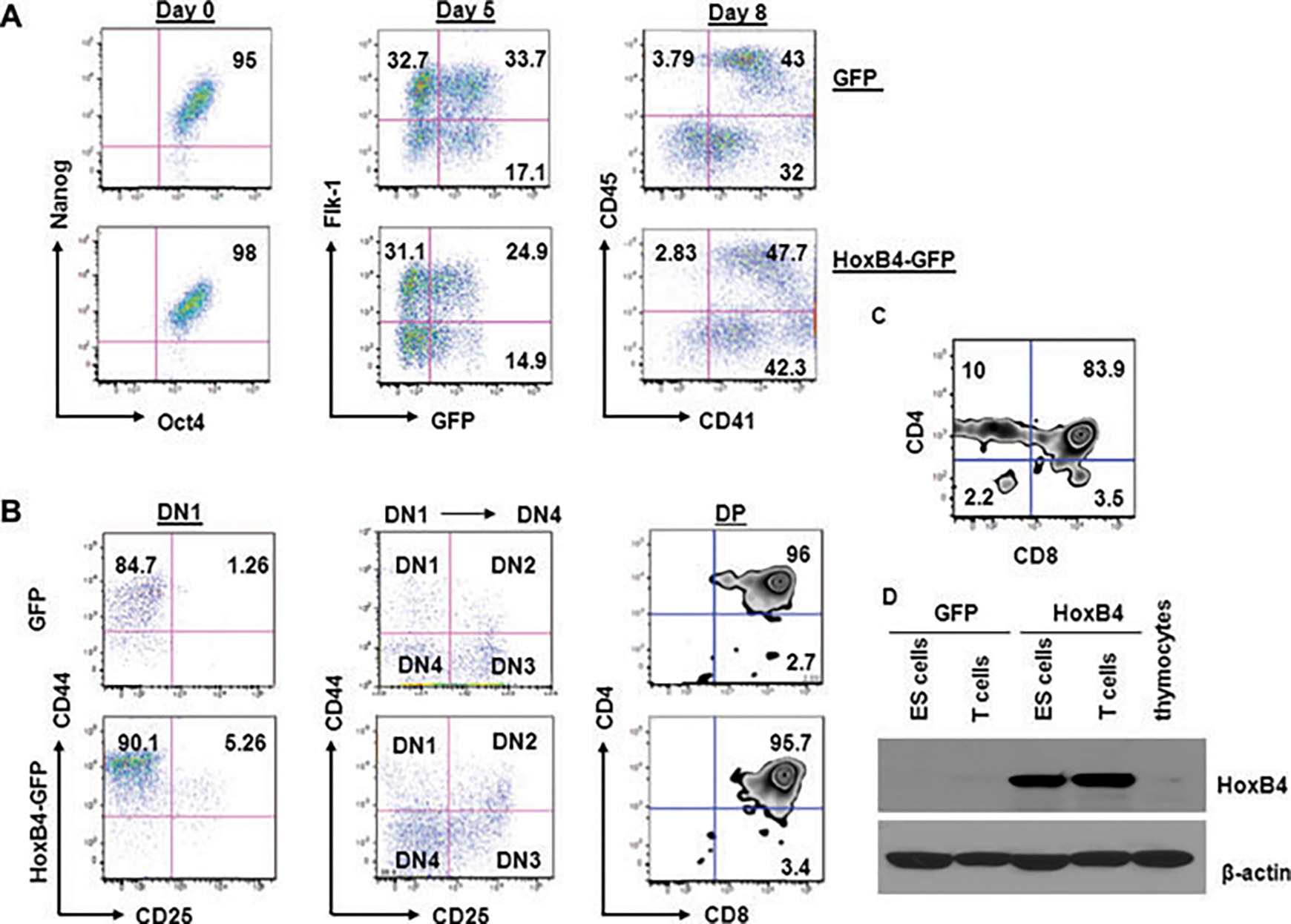
(A) HM1 ESCs transduced with either GFP or with a GFP-HoxB4 construct, respectively, were analyzed for Nanog1 and Oct4 expression by flow cytometry to confirm pluripotency. For differentiation into HPCs the cells were cultured on monolayers of OP9 stromal cells. By day 5, the cells were expressing Flk-1. CD45 and CD41 emerged by day 8. (B) After transferring the cells onto OP9-DL1 cells, the cells transitioned into the DN1, DN2, DN3 and DN4 stages becoming CD4+CD8+ DP T cells by day 22. There were no characteristic differences between the HoxB4 and non-HoxB4 cultures. (C) As controls, thymocytes were phenotyped for CD4 and CD8 expression. (D) To confirm HoxB4 expression, cell lysates were analyzed by Western blotting. Only cell lysates of transduced cells were positive for HoxB4. Thymocytes were used as controls.
To confirm the expression of HoxB4 in the ESCs and T cells, we prepared cell lysates from non-HoxB4- and HoxB4-cells and analyzed them by western blotting. Indeed, HoxB4-transduced cells expressed HoxB4 (Figure 1D). This result confirmed that our ESCs expressed HoxB4, and that expression was maintained in fully developed T cells. In contrast, non-HoxB4-T cells and thymocytes only showed very weak expression of endogenous HoxB4.
ESC-derived T cells display Vβ diversity and respond to mitogen stimulation
To determine TcR diversity in newly developed DP T cells, we stained the T cells with antibodies against Vβ regions. Four representative Vβ stains are shown (Figure 2). For comparison, ex vivo thymocytes are also shown. There were no significant differences between in vitro generated T cells and thymocytes, independent of HoxB4 expression. Furthermore, statistical analysis of the experimental repeats demonstrated that this finding was reproducible and that the T-cell repertoire was not significantly different (p > 0.05) between thymocytes and in vitro generated T cells (Table S2). Thus, ESC-derived T cells show normal TcR diversity and HoxB4 did not seem to influence TcR specification. This result was not expected as prior data using a cytokine-based protocol published by us and others had shown that the derivation of T cells in HoxB4-expressing murine ESCs was inefficient (1,3,4). We attribute the efficient T-cell generation to Notch 1 signaling conveyed by the OP9-DL1 cells.
Figure 2: HoxB4 does not affect T-cell diversity in ESC-derived T cells.
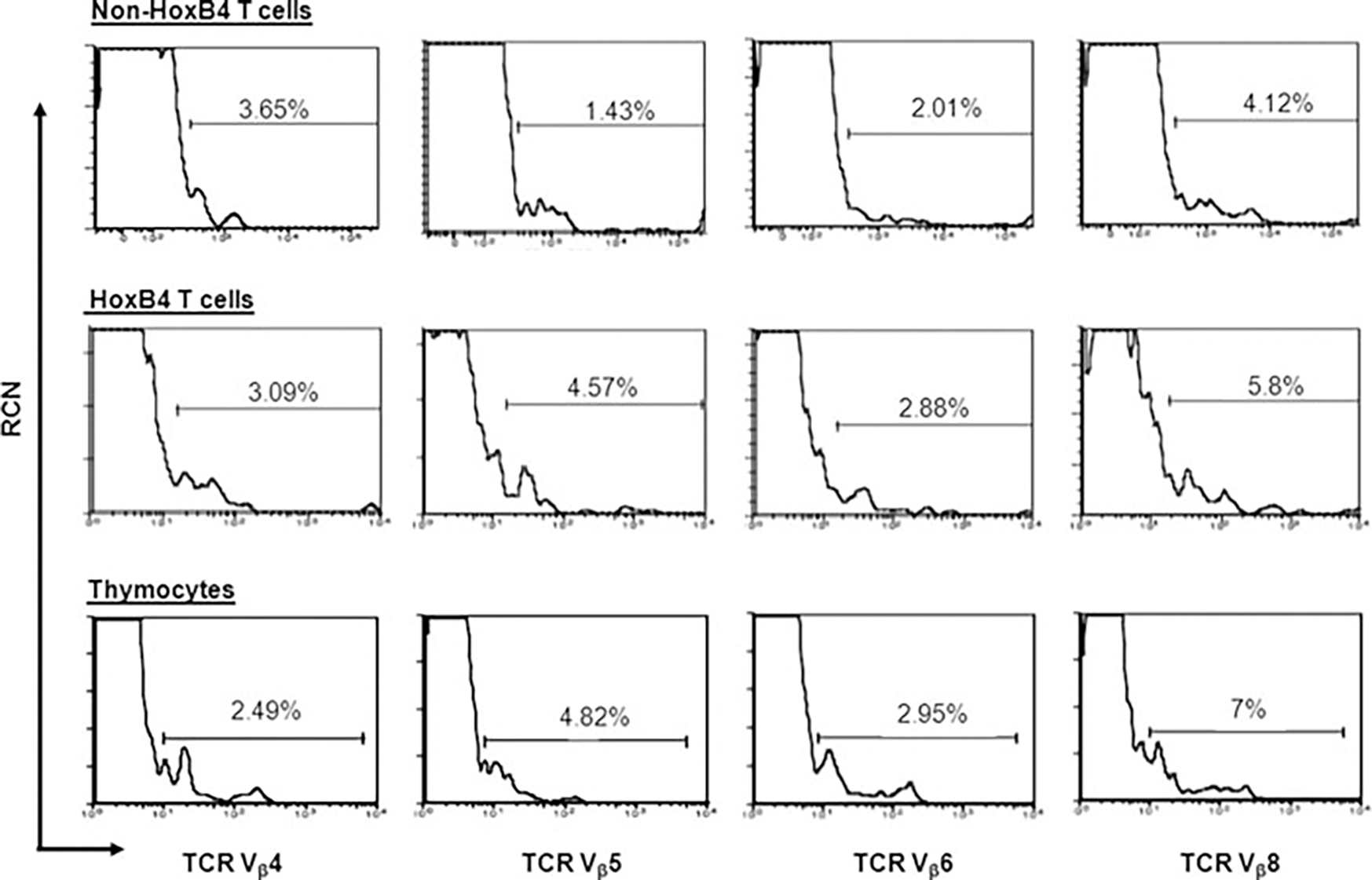
HoxB4- and non-HoxB4-T cells were stained with a panel of 9 Vβ-specific antibodies to determine T-cell diversity. Here are four representative Vβs showing no apparent differences between these diversity regions of the TcRs. Thymocytes from adult mice were used as controls. These experiments were repeated three times. Data shown here are representative of a typical experiment.
Having established no apparent differences between the HoxB4- and non-HoxB4-T cells, we pursued to further characterize HoxB4-T cells for their functionality and response to mitogen stimulation. The yield of the non-HoxB4-T cells was too low to allow more extensive functional studies. However, studies by others already show that non-HoxB4-T cells respond to antigenic and mitogen stimulation (14,19). Newly generated HoxB4-T cells were stained for CD45, CD3, CD4, CD8, αβ- and γδ-TcRs. Our findings confirm that the cells were CD45+, CD3+, TcR+ and CD4+CD8+. Most interestingly, 7.3% of the T cells express γδ T-cell receptors (TcR) (Figure 3A), which is consistent with earlier findings (14). Another observation was that in addition to the CD4+CD8+ T cells, we detected a robust population of single CD8+ T cells but not that of single CD4+ cells. The absence of single CD4+ T cells can be explained by the lack of MHC-class II antigens on OP9 cells, suggesting that they cannot mediate CD4+ T-cell selection.
Figure 3: ESC-derived T cells respond normally to stimulation.
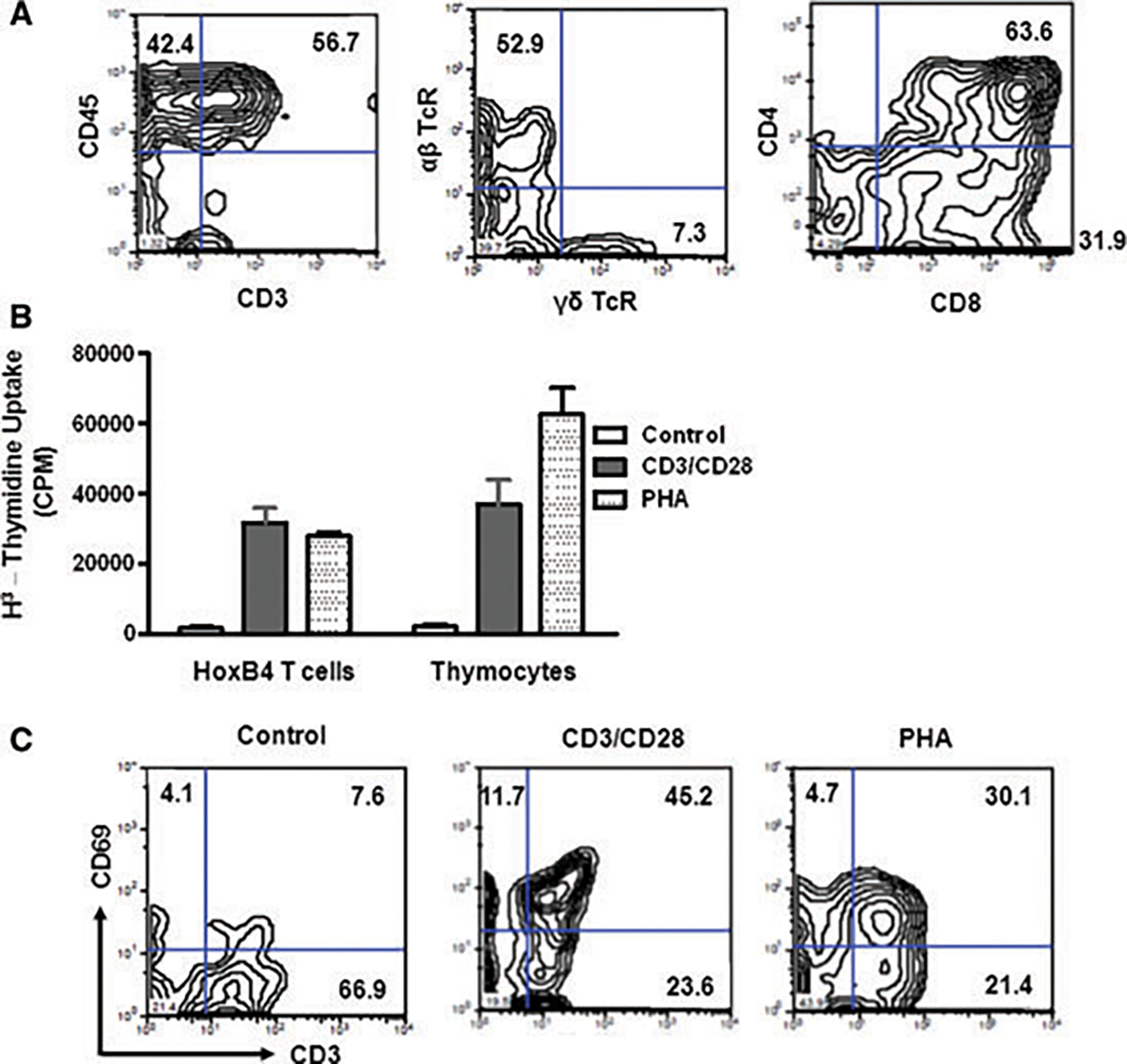
(A) In vitro generated T cells were immunophenotyped by flow cytometry (n = 6). The cells were shown to express CD45, CD3, αβ TcRs and γδ TcRs. Single CD8+, but no single CD4+ T cells were detectable. (B) To determine T-cell responses to stimuli, HoxB4-T cells and thymocytes were stimulated with antibodies against CD3 and CD28 or with PHA (n = 5). Both cell types responded well to mitogenic stimulation. (C) We determined whether the HoxB4-T cells express markers of activation. T cells were stimulated with either CD3/CD28 antibodies or with PHA and stained for CD69 after 48 hours. CD69 was upregulated in both cases confirming their responses to stimulation. These experiments were repeated at least 6 times.
Next, to establish T-cell function, we studied the response of HoxB4-T cells to mitogen stimulation. Microtiter plates were coated with antibodies against CD3 and CD28 or with an isotype control. T cells or thymocytes were cultured on the plates for 48 h, and pulsed with 3H-thymidine before recovery. Both HoxB4-T cells and thymocytes robustly responded to CD3/CD28 stimulation (Figure 3B). Proliferation in response to PHA was higher in thymocytes. Finally, T-cell activation was determined by monitoring CD69 expression. Our data indicate significant CD69 upregulation after CD3/CD28 or PHA mitogen stimulation, suggesting normal T-cell responses (Figure 3C).
Non-thymic selected T cells induce lethal graft-versus-host disease
Next, we studied the engraftment pattern of these HoxB4-T cells that had not undergone thymic selection by infusing them into the 129SvJ mice, the parental strain from which the ESCs were derived. Mice were sub-lethally irradiated to create hematopoietic space. 0.1 to 4×106 nonthymic selected T cells, termed non-FTOC T cells, were infused intravenously. These mice rapidly developed a hunched posture, diarrhea and bleeding on the paws, typical signs of GVHD (Figure 4A). The animals lost weight and appeared much smaller than control mice or mice transplanted with T cells that had undergone thymic selection (FTOC-T cells, Figure 4B). Macroscopically, GVHD was further confirmed by significant gut obstruction and thinning (Figure 4C). These mice experienced significant weight loss (Figure 4D) and died within 12 days. Onset of disease and lethality were cell number dependent (Figure 4E). As expected, an equivalent cell number of FTOC-T cells had no impact on the health of the mice. Thus, our data show that non-FTOC T cells generated from ESCs induced severe GVHD in syngeneic recipients as a consequence of nonselection of T cells tolerant to self.
Figure 4: T cells generated in vitro without thymic selection induce lethal GVHD.
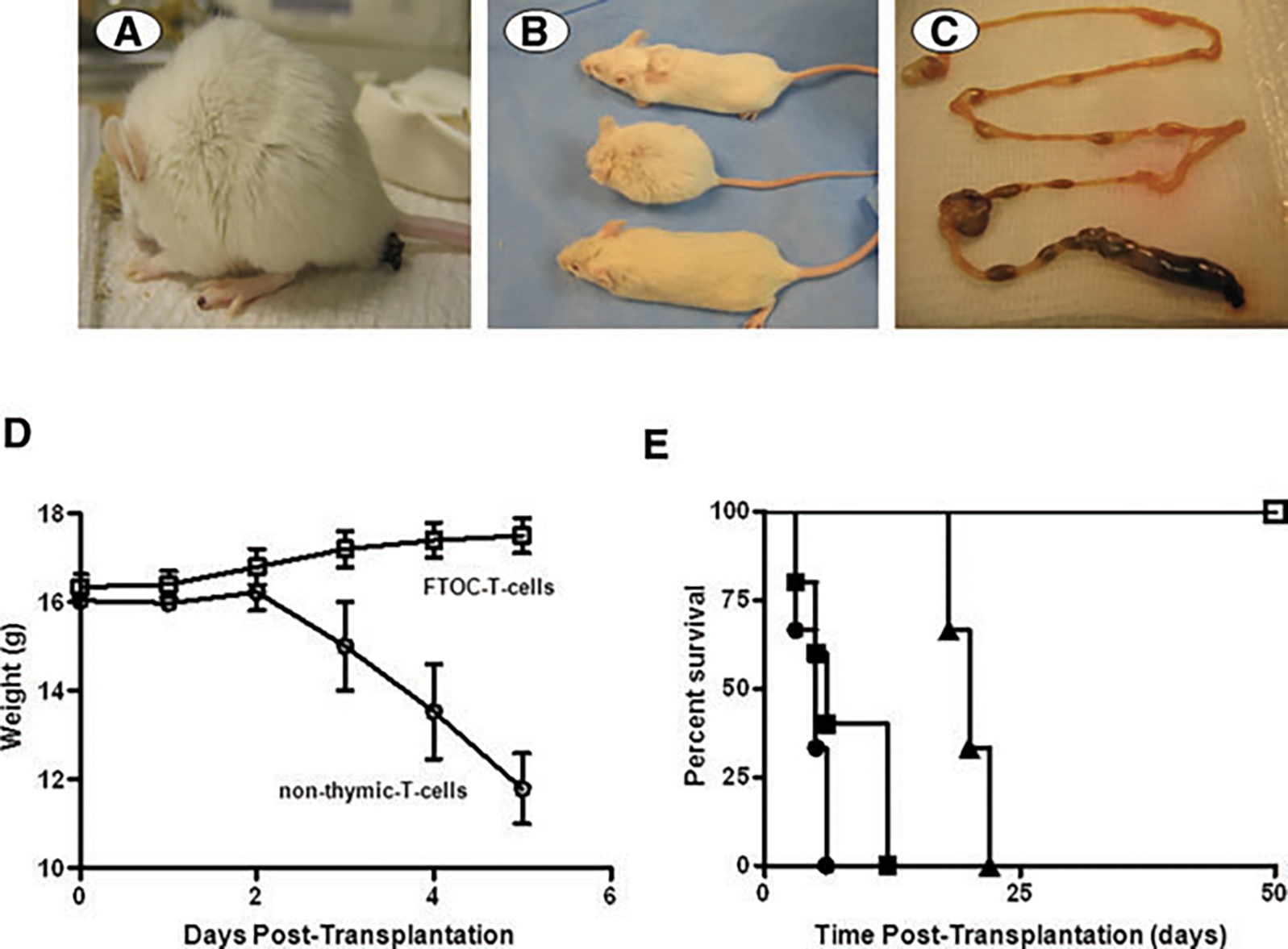
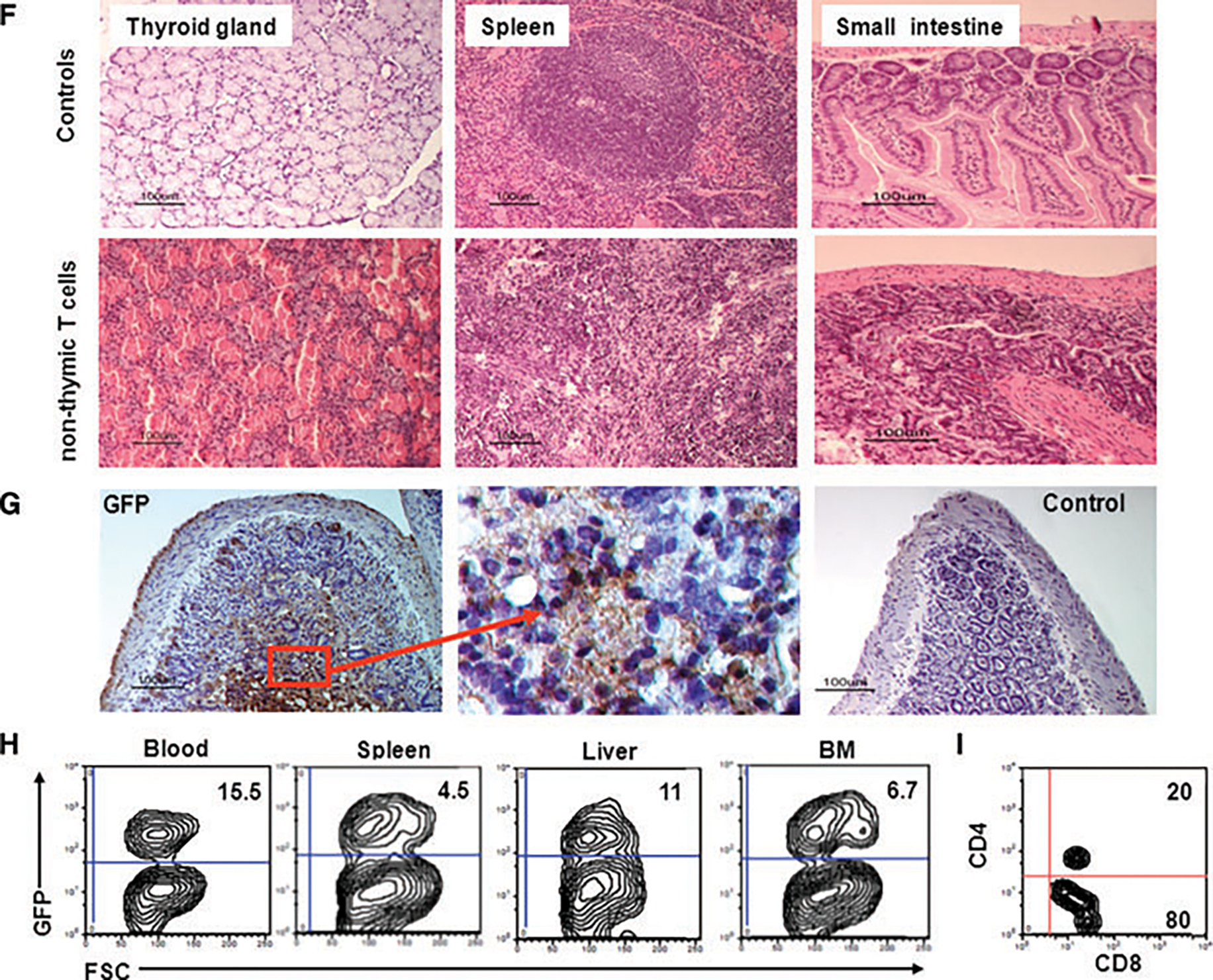
(A) Six to eight-week-old 129SvJ mice were infused with 4 × 106 T cells generated without thymic selection. Mice (n = 6) developed restricted cage movements within 2 days. They developed a hunched back posture, diarrhea and lesions around the eyes and paws, consistent with GVHD. (B) Mice that received T cells generated through thymic selection via the FTOCs looked healthy (top) and comparable to their healthy littermates (bottom). In contrast, mice undergoing GVHD (middle) looked smaller and had a hunched posture. (C) The gut of the GVHD mice showed obstruction and thinning. (D) Mice injected with non-thymic T cells (2 × 106 cells) (n = 6) lost weight rapidly whereas mice transplanted with FTOC-T cells maintained and gained some weight within the observation period. (E) The onset and severity of disease was cell number dependent. Here we used 4 × 106 (•), 2 × 106 cells (■) or 105 (▴) non-thymic T cells (n = 6 each). For comparison, we infused six mice each with 105 FTOC-T cells (), all of which thrived. The FTOC-T cells were still detectable by flow cytometry 3 months after transplantation, showing that they survived long term, without causing disease. (F) To confirm GVHD, the gut, spleen and thyroid gland of a representative mouse were examined by H&E staining. The thyroid gland showed heavy mononuclear cell infiltration and obliteration of the glandular structure. Similarly, the spleen had lost the follicular structure and also showed evidence of heavy mononuclear cell infiltration. The small intestines showed infiltration and loss of the crypts and villi, typical of GVHD (n = 6). (G) To confirm that the tissue damage was caused by the infused GFP-expressing T cells, histological sections were stained for GFP by immunohistochemistry. The sections showed dense stains for GFP, confirming that the infused T cells were responsible for the tissue insult (n = 6). (H and I) GFP+ T cells are detectable in peripheral organs during GVHD. Using flow cytometry, we identified robust hematopoietic cell chimerism in the blood, spleen, liver and bone marrow. To characterize for CD4 and CD8, we isolated the splenocytes and gated on the GFP-expressing cells and analyzed them for CD4 and CD8 expression (n = 6) (I). Our data show 80% CD8+ T cells and 20% DP, but no single CD4+ T cells.
To study the histology of these animals, we euthanized some mice on day 5 and prepared paraffin or frozen sections of the thyroid gland, spleen and small intestines. Mice that did not receive any ESC-derived T cells were used as controls. As expected, organs derived from mice transplanted with non-FTOC T cells showed massive cell infiltration (Figure 4F). Of particular interest was the severe thyroiditis characterized by mononuclear cell infiltration with destruction of the epithelium. These results are consistent with previous observations of GVHD (20).
Similarly, the spleens were generally smaller than those of healthy mice and showed marked lymphoid atrophy, structural disorganization and obliteration of follicular structure. In addition, the gut showed signs of edema, epithelial cell necrosis and marked mucosa wall thickening. Finally, to confirm that the cells infiltrating the organs were indeed GFP+ T cells, we stained the histological sections for GFP. Representative of other tissues, our results on the small intestines show that the tissue was heavily infiltrated with GFP+ T cells (Figure 4G), suggesting that the observed disease was a consequence of the infused T cells. Control sections from these mice were stained with an isotype control antibody.
Furthermore, we tracked GFP+ T cells in peripheral blood, spleen and bone marrow in mice undergoing GVHD. T cells were detected in all tissues with the highest numbers in peripheral blood (Figure 4H), indicating that the T cells circulate throughout peripheral lymphoid organs. We are therefore confident that in vitro generated T cells are functionally active and that, without thymic selection, they induce lethal GVHD. We then wondered whether the phenotype of the non-FTOC T cells had changed in vivo during GVHD. Further characterization of the circulating T cells revealed that the majority of these cells were now single CD8+. However, no single CD4+ T cells were detectable (Figure 4I). This finding is consistent with our hypothesis that GVHD is induced by cytotoxic single CD8+ T cells, which expanded in vivo after interacting with self-tissue antigens.
FTOC-T cells are tolerant to self
Our data showed thus far that non-FTOC T cells displayed the normal phenotype of T cells and responded to mitogen stimulation. However, these T cells had not undergone thymic selection and thus induced GVHD. Therefore, to replicate T-cell development in the thymus, we utilized FTOCs. Briefly, 2′-deoxyguanosine treated thymic lobes from E14.5 to 15.5 fetuses were infused with DN2 HoxB4-T-cell precursors that had been cultured on OP9-DL1 cells. Cells were recovered after 21 days of culture and studied for T-cell marker expression. FTOC-T cells had both single CD4+ and CD8+ T cells in addition to the DP population (Figure 5A). As expected, these T cells also expressed CD3 and the αβ TcRs (Figure 5B).
Figure 5: FTOC-derived T cells reject MHC-mismatched skin allografts.
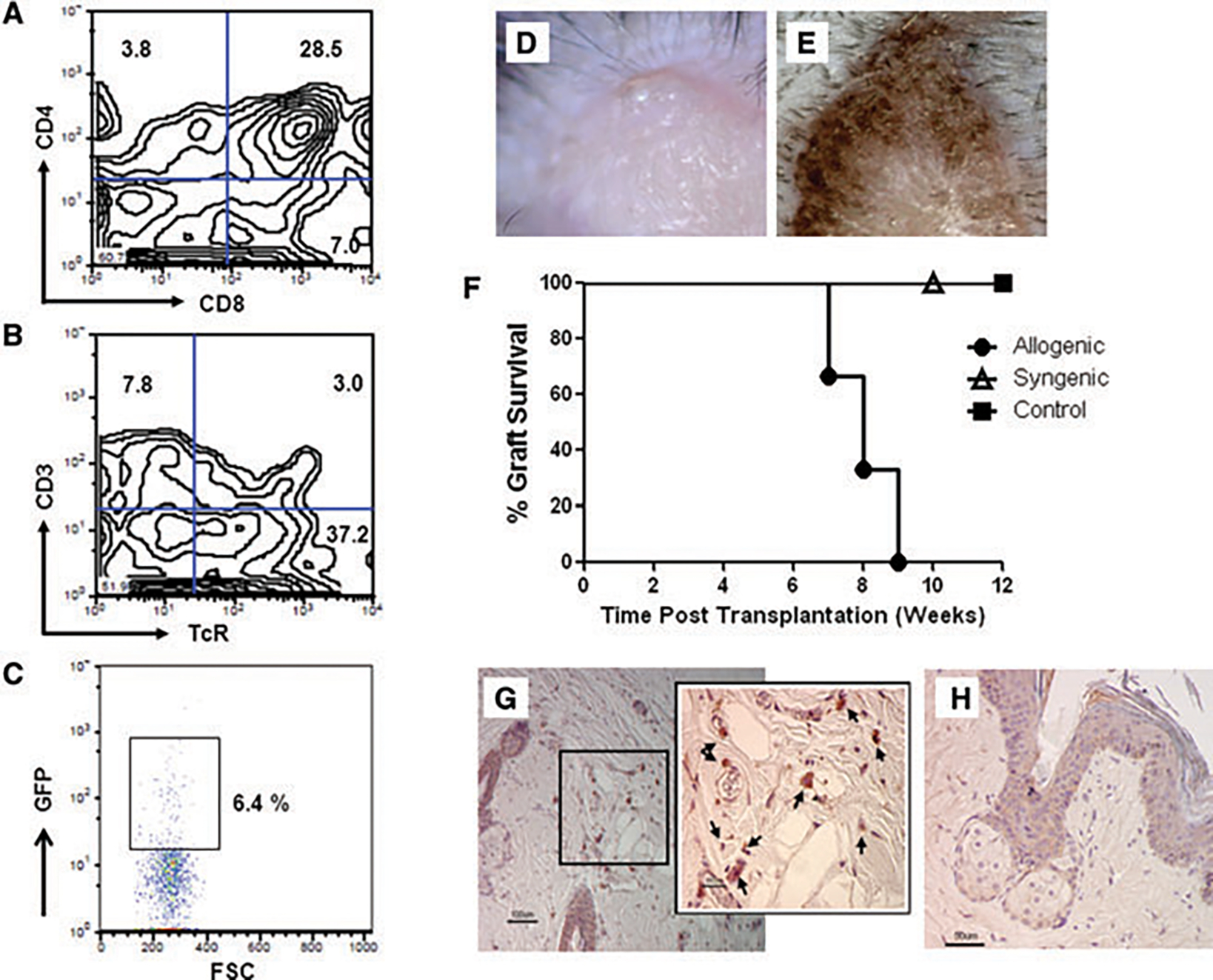
(A–C) To determine whether HoxB4-transduced ESCs differentiate into T cells in fetal thymic lobes, T-cell precursors in the DN2 stage were infused in E14.5-E15.5 fetal thymic lobes and allowed to differentiate. On immunophenotyping the cells after 14 days of culture, we identified single CD4+, single CD8+ and DP cells (A). These cells expressed αβ TcR and CD3 (B). When the thymic lobes infused with T-cell progenitors were transplanted under the kidney capsules of sublethally irradiated 129SvJ mice, the GFP-expressing T cells were detectable in the thymus (C), n = 7. (D–G) To determine if FTOC-T cells were responsive to alloantigen stimulation, MRL (H2k) tail skin allografts and autologous 129 SvJ tail skin grafts were transplanted onto Rag2−/−γc−/− mice, respectively (n = 6). The grafts engrafted well and were vascularized. To determine whether thymic selected T cells rejected skin allografts, 106 FTOC-T cells were infused subcutaneously around the MRL skin grafts. Skin discoloration (E) of the allografts (•) was first observed after 3 weeks post-T-cell infusion, darkening further until the grafts were rejected after another 4 weeks. Graft survival data are depicted in (F). Allogenic grafts, but not syngeneic () or nontreated controls (■), were rejected. Each group comprises six animals. Finally, rejected skin histological sections were stained for GFP. Single GFP-positive cells were detected within the graft but not in the controls.
To determine whether the FTOC-T cells survive long term in vivo and are functional, thymic lobes containing ESC-derived T cells were transplanted under the kidney capsules of sublethally irradiated 129SvJ mice. In these mice, we failed to detect peripheral chimerism over 3 months posttransplantation. We argued that this was due to the low cell numbers in the transplanted thymic lobes. However, transplanted animals remained healthy and gained weight. The animals were finally killed after 3 months and the GFP-expressing T cells tracked by flow cytometry in peripheral lymphoid organs. We observed a robust population of 6.4% GFP-expressing T cells in the thymuses of the mice (Figure 5C) but not in the spleen, bone marrow or peripheral blood (data not shown). Thus, these experiments confirmed that T cells derived from HoxB4-transduced ESCs persist long term in vivo and are tolerant to ‘self’.
FTOC-T cells reject MHC-mismatched skin allografts
To determine whether FTOC-T cells were functional in vivo, naive FTOC-T cells were recovered after 22 days in culture. Rag2−/−γc−/− mice (H-2b) were transplanted with MRL (H-2k) skin grafts. These grafts are not rejected in this mouse strain because the Rag2−/−γc−/− mice are immunodeficient, lacking NK, T and B cells. The recovered FTOC-T cells were subcutaneously infused around the skin graft (Figure 5D) 4 weeks posttransplantation to determine if the T cells recognized and responded to the allogenic MRL skin grafts. Three to four weeks after the injection of T cells, the allogenic skin grafts were rejected (n = 6; Figures 5E and F). Rejection was defined as graft necrosis of >75% as shown by the darkening and peeling off of the graft. The rejection timeline of these grafts is long in contrast to the rejection of allografts in fully competent mice as we previously reported (15). The slow rejection kinetics observed here can be attributed to the relatively low cell numbers of the T cells recovered and to the fact that most of the T cells were DP and therefore immature. Single CD4+ and CD8+ T cells only constituted 3.8% and 7%, respectively (Figure 5A) at the time of transplantation. Nonetheless, the data show that our cells are tolerant to self and are functional and capable of rejecting a highly immunogenic allograft.
Finally, to determine that our T cells were tolerant to self, we transplanted Rag2−/−γc−/− mice with syngeneic 129SvJ skin grafts. These grafts were not rejected when the FTOC-T cells were injected around the grafts, showing that the T cells were tolerant to self (Figure 5F). As expected, control MRL tail skin grafts that did not receive any T cells were also not rejected (Figure 5F).
Lastly, immunohistochemistry of the rejected allografts was performed to detect the infused T cells by staining for GFP. The rejected tail skin allograft (Figure 5G) showed the presence of GFP-expressing cells, which were not detectable in control mice (Figure 5H).
Our data show that HoxB4 does not abrogate the generation of T cells, but rather that Notch 1 signaling is required for T cells to differentiate.
Discussion
Our data show for the first time that functional T cells can be successfully generated from HoxB4-transduced ESCs. This approach allows studies on whether ESC-generated T lymphocytes can restore immunity in immunodeficient mice, a step which has not been feasible up to now. Although other authors (14,22) successfully derived T cells from ESCs, their cells were not HoxB4 transduced and allowed only short-term in vivo studies. We have previously reported that non-HoxB4-transduced hematopoietic cells do not survive long term in vivo due to their lack of self-renewal properties (2). Thus, when transplanted, such T cells only allow short-term studies of 1–3 weeks. Our approach now allows long-term studies on ESC-derived T cells in both infection and transplantation models. ESC-derived T cells were still detectable in the spleen of transplanted mice 3 months post-transplantation. The observation that FTOC-derived T cells reject skin allografts suggests that these T cells are immunocompetent.
Comparing T cells derived from non-HoxB4 ESCs with those from HoxB4 ESCs, we observed no differences with regard to their differentiation efficiency, Vβ diversity or any other phenotypic characteristics. Further, when compared to thymocytes, we concluded that HoxB4 does not interfere with T-cell development under Notch1 signaling. We attribute this finding to Notch1 signaling, because we and others were previously unsuccessful in deriving T cells using cytokine-based protocols (1,3). This was not because the progenitor cells were unable to migrate to the thymus after transplantation, because we showed that when transplanted in the Rag2−/−γc−/−, the ESC-derived HPCs successfully populated the thymus (1). Instead, the cells were not able to transition into adult T cells in sufficient numbers. We concluded in those experiments that the transplanted T-cell progenitors were likely past the developmental stage where in vivo Notch1 signaling could have directed T-cell development. Additionally, the thymic epithelium in Rag2−/−γc−/− mice is notoriously poorly developed, which might have contributed to poor T-cell development. However, when these progenitors were infused in immunocompetent mice, T-cell development did not improve (2,9). Our T cells expressed αβ-, γδ-TcRs and CD3, which are important T-cell markers. These T-cells formed single CD8+ but not CD4+ T cells. In contrast, T cells derived from FTOCs showed both cell populations. Lastly, when T cells were stimulated with CD3/CD28 antibodies or with PHA, their proliferation was comparable to that of thymocytes.
Non-FTOC T cells induced lethal GVHD when transplanted into syngeneic recipients, underlining the requirement for thymic selection. The development of disease was cell number dependent. For example, 1 to 2×106 T cells required at least 6–8 days to induce clinical signs of disease. These mice succumbed within 12–14 days. In contrast, when 4×106 T cells were transplanted, signs of GVHD were observed as early as 2 days post-transplantation and the animals died within 5–6 days. When 1×105 non-FTOC T cells were injected, mice began to show symptoms of GVHD within 13–15 days, suggesting that the occurrence of symptoms was dependent on the infused cell numbers. We show here for the first time that ESC-derived T cells induce GVHD. When studied by H&E staining, peripheral organs were heavily infiltrated by mononuclear cells. To confirm that our transplanted T cells were involved in the inflammation process, we stained for GFP, which confirmed the presence of a high population of GFP+ cells. We further confirmed the presence of the transplanted T cells in peripheral organs by flow cytometry. GFP-expressing cells were detected in peripheral blood, spleen, liver and bone marrow. Interestingly, the T cells were now predominantly CD8+ in addition to DP cells. The presence of this high percentage of CD8+ T cells suggests clonal expansion of the CD8+ T cells after interaction with self-antigens, suggesting that they were not tolerant to self.
In contrast to the non-FTOC T cells, FTOC-T cells displayed both single CD4+ and CD8+ T cells. The ability to form CD4+ T cells can be explained by the expression of MHC-class II molecules on thymic epithelium in contrast to OP9 stromal cells, which are negative for class II molecules. When thymic T cells were transplanted in syngeneic 129SvJ mice, GVHD was not observed. The animals thrived, adding on weight (Figure 4D) and remained healthy over 3 months (Figure 4E), suggesting that thymic selection was effective in eliminating high affinity T cells directed at self-antigens. Finally, these T cells rejected allogenic skin allografts when transferred to Rag2−/−γc−/− mice, suggesting that they were responsive to antigenic stimulation and were therefore functional. The rejection of the allografts was observed 30 days posttransplantation. We attribute this delay in rejection to low cell numbers that were transferred but also to the fact that the cells were immature as most of them were DP rather than single positive. Additionally, these experiments were performed in immunodeficient mice, making the environment less favorable for strong immunological responses. In contrast, our earlier observation had shown that allogenic skin grafts were swiftly rejected in immunocompetent 129SvJ mice (15) with the same recipient strain used here. Altogether, our data show efficient T-cell derivation from HoxB4-transduced ESCs and that these T cells are immunocompetent, responding to both antigen and mitogen stimulation. HoxB4 appears to confer self-renewal properties to ESC-derived T cells, but does not interfere with their development or function. These studies now allow further interrogation of the cells for their ability to develop memory T cells and maintain long-term immunity.
Supplementary Material
Figure S1: HoxB4 greatly enhances the efficiency of Tcell generation from ESCs.
Table S1: The cell marker expression profile of HoxB4-transduced ESCs during their differentiation
Table S2: In vitro generated T cells and thymocytes show similar expression of the Vβ repertoire
Acknowledgments
We acknowledge helpful discussion with Drs John Harty and Beverly Davidson (both at the University of Iowa) for useful suggestions. We are indebted to Dr. Hannes Klump (University of Essen, Germany) for providing the GFP and HoxB4-GFP vectors. We thank the Histology Core, Department of Pathology at the University of Iowa, for assisting us with the histological sections and stains. We thank Gohar Manzar for technical support and editing of this manuscript.
Funding source:
This study was funded through grants 5R01HL073015 and 3R01HL073015-04A1S1 awarded through the NIH/NHLBI.
Abbreviations:
- FTOC
fetal thymic organ cultures
- DN cells
double negative thymocyte cells
- DP
double positive thymocyte cells
- GVHD
graft-versus-host disease
- MHC
major histocompatibility complex
- HPC
hematopoietic progenitor cells
- DL1
delta-like 1
- ESC
embryonic stem cell
Footnotes
Disclosure
The authors of this manuscript have no conflicts of interest to disclose as described by the American Journal of Transplantation.
Supporting Information
Additional Supporting Information may be found in the online version of this article:
References
- 1.Chan KM, Bonde S, Klump H, Zavazava N. Hematopoiesis and immunity of HOXB4-transduced embryonic stem cell-derived hematopoietic progenitor cells. Blood 2008; 111: 2953–2961. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Bonde S, Zavazava N. Immunogenicity and engraftment of mouse embryonic stem cells in allogeneic recipients. Stem Cells 2006; 24: 2192–2201. [DOI] [PubMed] [Google Scholar]
- 3.Kyba M, Perlingeiro RCR, Daley GQ. HoxB4 confers definitive lymphoid-myeloid engraftment potential on embryonic stem cell and yolk sac hematopoietic progenitors. Cell 2002; 109: 29–37. [DOI] [PubMed] [Google Scholar]
- 4.Schiedlmeier B, Klump H, Will E, et al. High-level ectopic HOXB4 expression confers a profound in vivo competitive growth advantage on human cord blood CD34+ cells, but impairs lymphomyeloid differentiation. Blood 2003; 101: 1759–1768. [DOI] [PubMed] [Google Scholar]
- 5.Sauvageau G, Thorsteinsdottir U, Eaves CJ, et al. Overexpression of HOXB4 in hematopoietic cells causes the selective expansion of more primitive populations in vitro and in vivo. Genes Dev 1995; 9: 1753–1765. [DOI] [PubMed] [Google Scholar]
- 6.Antonchuk J, Sauvageau G, Humphries RK. HOXB4-induced expansion of adult hematopoietic stem cells ex vivo. Cell 2002; 109: 39–45. [DOI] [PubMed] [Google Scholar]
- 7.Helgason CD, Sauvageau G, Lawrence HJ, Largman C, Humphries RK. Overexpression of HOXB4 enhances the hematopoietic potential of embryonic stem cells differentiated in vitro. Blood 1996; 87: 2740–2749. [PubMed] [Google Scholar]
- 8.Tabayoyong WB, Salas JG, Bonde S, Zavazava N. HOXB4-transduced embryonic stem cell-derived Lin-c-kit +and Lin-Sca1+ hematopoietic progenitors express H60 and are targeted by NK cells. J Immunol 2009; 183: 5449–5457. [DOI] [PubMed] [Google Scholar]
- 9.Bonde S, Chan KM, Zavazava N. ES-cell derived hematopoietic cells induce transplantation tolerance. PLoS One 2008; 3: e3212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Wang Y, Yates F, Naveiras O, Ernst P, Daley GQ. Embryonic stem cell-derived hematopoietic stem cells. Proc Natl Acad Sci USA 2005; 102: 19081–19086. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Germain RN. T-cell development and the CD4-CD8 lineage decision. Nat Rev Immunol 2002; 2: 309–322. [DOI] [PubMed] [Google Scholar]
- 12.Nakano T, Kodama H, Honjo T. Generation of lymphohematopoietic cells from embryonic stem cells in culture 32. Science 1994; 265: 1098–1101. [DOI] [PubMed] [Google Scholar]
- 13.Nishikawa SI, Nishikawa S, Hirashima M, Matsuyoshi N, Kodama H. Progressive lineage analysis by cell sorting and culture identifies FLK1+cadherin+ cells at a diverging point of endothelial and hemopoietic lineages. Development 1998; 125: 1747–1757. [DOI] [PubMed] [Google Scholar]
- 14.Schmitt TM, de Pooter RF, Gronski MA, Cho SK, Ohashi PS, Zuniga-Pflucker JC. Induction of T cell development and establishment of T cell competence from embryonic stem cells differentiated in vitro. Nat Immunol 2004; 5: 410–417. [DOI] [PubMed] [Google Scholar]
- 15.Bonde S, Dowden AM, Chan KM, Tabayoyong WB, Zavazava N. HOXB4 but not BMP4 confers self-renewal properties to ES-derived hematopoietic progenitor cells. Transplantation 2008; 86: 1803–1809. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Pilat S, Carotta S, Schiedlmeier B, et al. HOXB4 enforces equivalent fates of ES-cell-derived and adult hematopoietic cells. Proc Natl Acad Sci USA 2005; 102: 12101–12106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Yamaguchi TP, Dumont DJ, Conlon RA, Breitman ML, Rossant J. Flk-1, an Flt-related receptor tyrosine kinase is an early marker for endothelial cell precursors. Development 1993; 118: 489–498. [DOI] [PubMed] [Google Scholar]
- 18.Yamashita J Flk1-positive cells derived from embryonic stem cells serve as vascular progenitors. Nature 2000; 408: 92–96. [DOI] [PubMed] [Google Scholar]
- 19.Schmitt TM, Zuniga-Pflucker JC. Induction of T cell development from hematopoietic progenitor cells by delta-like-1 in vitro. Immunity 2002; 17: 749–756. [DOI] [PubMed] [Google Scholar]
- 20.Fujiwara K, Peszkowski MJ, Larsson A, Yamasaki A, Toda S, Watanabe T. Mononuclear cell thyroiditis in rats with graft-versus-host disease. J Pathol 1995; 175: 349–355. [DOI] [PubMed] [Google Scholar]
- 21.Molinero LL, Zhou P, Wang Y, et al. Epidermal Langerhans cells promote skin allograft rejection in mice with NF-kappa B-impaired T cells. Am J Transplant 2008; 8: 21–31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Schmitt TM, Ciofani M, Petrie HT, Zuniga-Pflucker JC. Maintenance of T cell specification and differentiation requires recurrent notch receptor-ligand interactions. J Exp Med 2004; 200: 469–479. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Figure S1: HoxB4 greatly enhances the efficiency of Tcell generation from ESCs.
Table S1: The cell marker expression profile of HoxB4-transduced ESCs during their differentiation
Table S2: In vitro generated T cells and thymocytes show similar expression of the Vβ repertoire


