Abstract
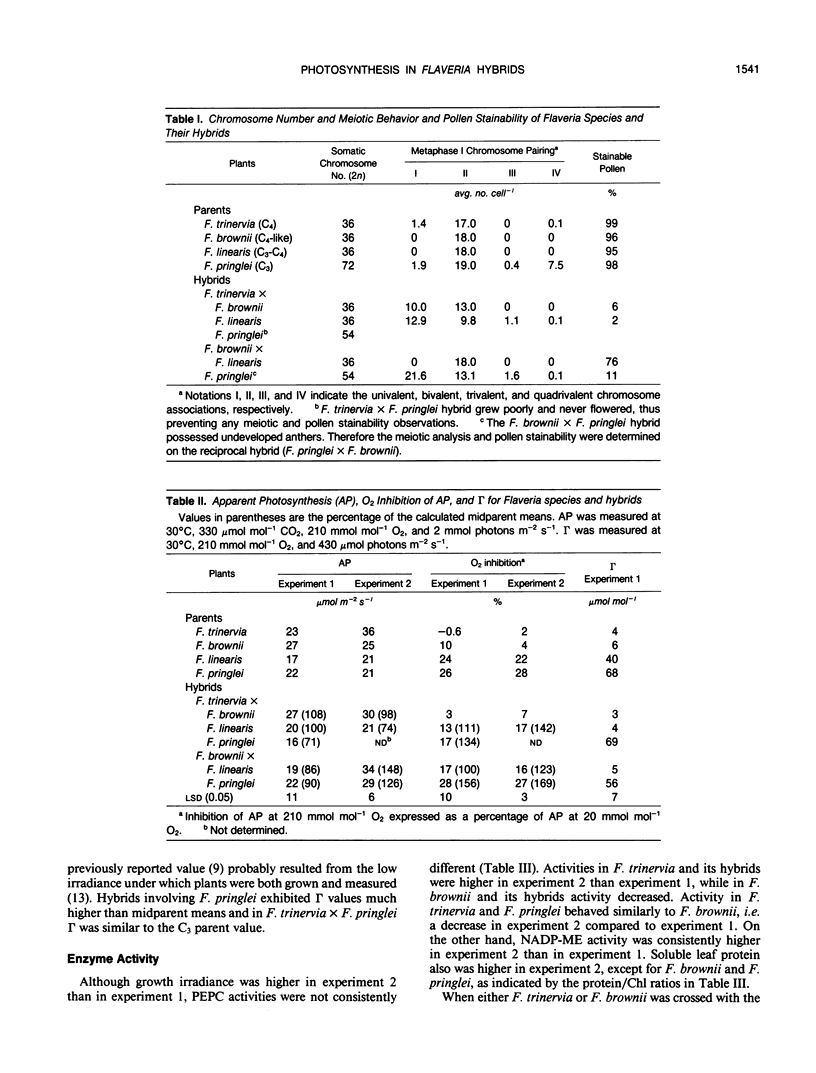
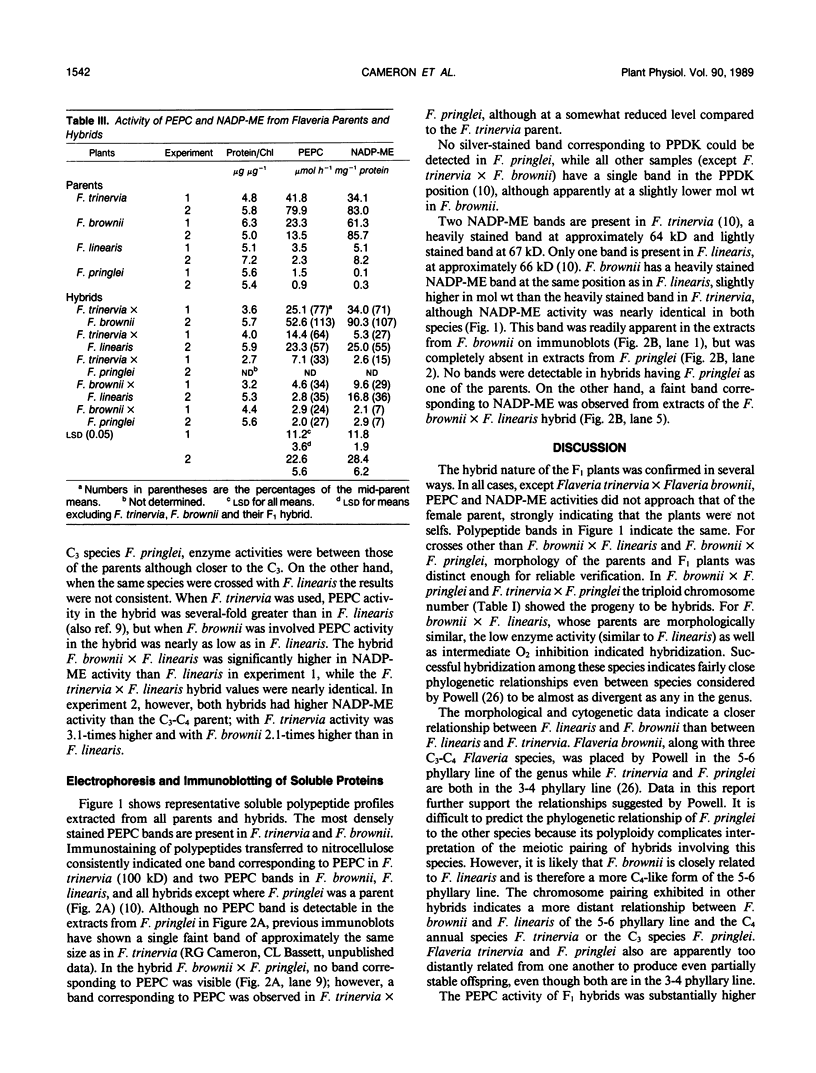
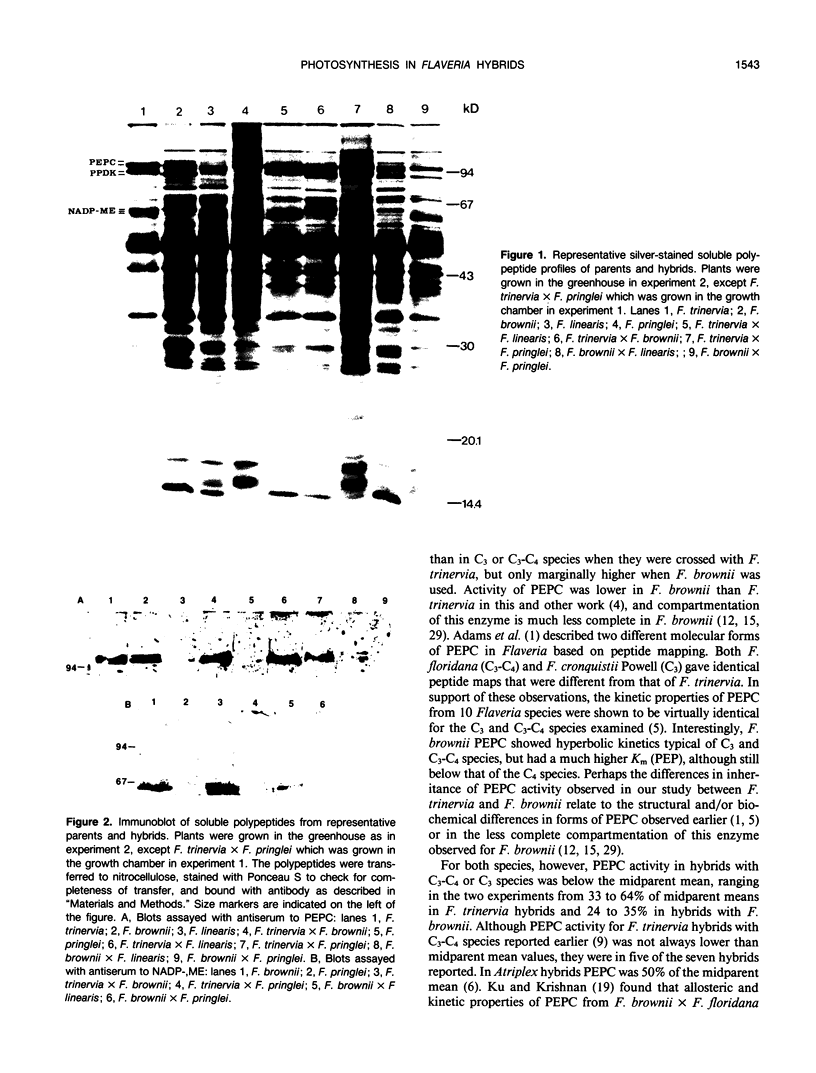
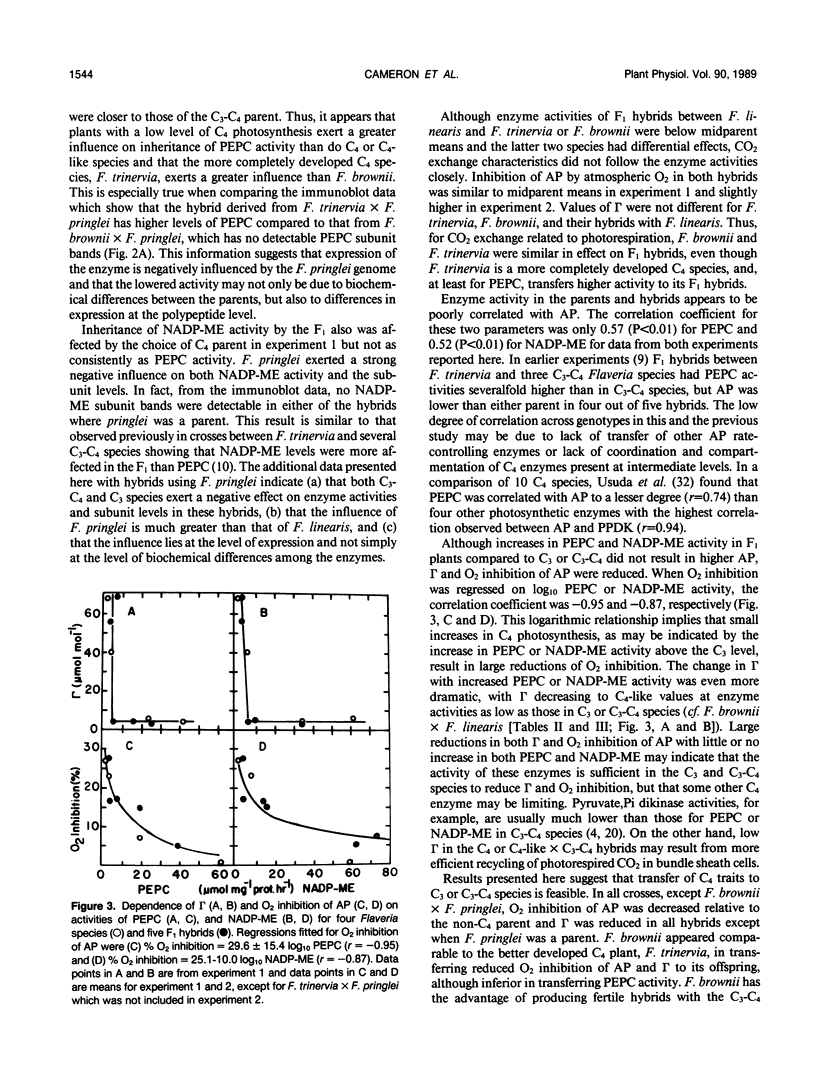
Transfer of C4 photosynthetic traits was studied through hybridization of Flaveria trinervia (Spreng.) Mohr (C4) and Flaveria brownii A.M. Powell (C4-like) with Flaveria linearis Lag. (C3-C4) and the C3 species Flaveria pringlei Gandoger (C3). Fertility was low, based on irregular chromosome pairing and low pollen stainability, except in F. brownii × F. linearis which had bivalent pairing and 76% stainable pollen. Hybrids had apparent photosynthesis values of 71 to 148% of the midparental means, while the CO2 compensation concentration was similar to the C4 or C4-like parent, except in hybrids having the C3 species F. pringlei as a parent. Inhibition of apparent photosynthesis by O2, and phosphoenolpyruvate carboxylase and NADP-malic enzyme activities and subunit levels in the hybrids were closer to the C3 or C3-C4 parent. The species F. brownii and F. trinervia were equal in their capacity to transfer reduced O2 inhibition of AP and CO2 compensation concentration values to hybrids with F. linearis (C3-C4), although hybrids with F. trinervia had higher PEPC activity. The O2 inhibition of AP was correlated with the logarithm of activities of phosphoenolpyruvate carboxylase (r = −0.95) and NADP-malic enzyme (r = −0.87). These results confirm that C4 traits can be transferred by hybridization of C3-C4 and C4 or C4-like species, with a higher degree of C4 photosynthesis than exists in C3-C4 species, and at least in F. brownii × F. linearis, fertile progeny are obtained.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Alexander M. P. Differential staining of aborted and nonaborted pollen. Stain Technol. 1969 May;44(3):117–122. doi: 10.3109/10520296909063335. [DOI] [PubMed] [Google Scholar]
- Bauwe H., Chollet R. Kinetic properties of phosphoenolpyruvate carboxylase from c(3), c(4), and c(3)-c(4) intermediate species of flaveria (asteraceae). Plant Physiol. 1986 Nov;82(3):695–699. doi: 10.1104/pp.82.3.695. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bradford M. M. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem. 1976 May 7;72:248–254. doi: 10.1006/abio.1976.9999. [DOI] [PubMed] [Google Scholar]
- Brown R. H., Bassett C. L., Cameron R. G., Evans P. T., Bouton J. H., Black C. C., Sternberg L. O., Deniro M. J. Photosynthesis of F(1) Hybrids between C(4) and C(3)-C(4) Species of Flaveria. Plant Physiol. 1986 Sep;82(1):211–217. doi: 10.1104/pp.82.1.211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cameron R. G., Bassett C. L. Inheritance of c(4) enzymes associated with carbon fixation in flaveria species. Plant Physiol. 1988 Nov;88(3):532–536. doi: 10.1104/pp.88.3.532. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cheng S. H., Moore B. D., Edwards G. E., Ku M. S. Photosynthesis in Flaveria brownii, a C(4)-Like Species: Leaf Anatomy, Characteristics of CO(2) Exchange, Compartmentation of Photosynthetic Enzymes, and Metabolism of CO(2). Plant Physiol. 1988 Aug;87(4):867–873. doi: 10.1104/pp.87.4.867. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holaday A. S., Brown R. H., Bartlett J. M., Sandlin E. A., Jackson R. C. Enzymic and Photosynthetic Characteristics of Reciprocal F(1) Hybrids of Flaveria pringlei (C(3)) and Flaveria brownii (C(4)-Like Species). Plant Physiol. 1988 Jun;87(2):484–490. doi: 10.1104/pp.87.2.484. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huber W. E., Brown R. H., Bouton J. H., Sternberg L. O. CO(2) Exchange, Cytogenetics, and Leaf Anatomy of Hybrids between Photosynthetically Distinct Flaveria Species. Plant Physiol. 1989 Mar;89(3):839–844. doi: 10.1104/pp.89.3.839. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Inskeep W. P., Bloom P. R. Extinction coefficients of chlorophyll a and B in n,n-dimethylformamide and 80% acetone. Plant Physiol. 1985 Feb;77(2):483–485. doi: 10.1104/pp.77.2.483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ku M. S., Monson R. K., Littlejohn R. O., Nakamoto H., Fisher D. B., Edwards G. E. Photosynthetic Characteristics of C(3)-C(4) Intermediate Flaveria Species : I. Leaf Anatomy, Photosynthetic Responses to O(2) and CO(2), and Activities of Key Enzymes in the C(3) and C(4) Pathways. Plant Physiol. 1983 Apr;71(4):944–948. doi: 10.1104/pp.71.4.944. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Moran R., Porath D. Chlorophyll determination in intact tissues using n,n-dimethylformamide. Plant Physiol. 1980 Mar;65(3):478–479. doi: 10.1104/pp.65.3.478. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Uedan K., Sugiyama T. Purification and characterization of phosphoenolpyruvate carboxylase from maize leaves. Plant Physiol. 1976 Jun;57(6):906–910. doi: 10.1104/pp.57.6.906. [DOI] [PMC free article] [PubMed] [Google Scholar]



