Abstract
Diacylglycerol kinase (ATP:1,2-diacylglycerol 3-phosphotransferase, EC 2.7.1.107) from suspension-cultured Catharanthus roseus cells was extracted from a membrane fraction with 0.6% Triton X-100 and 150 millimolar NaCl and was purified about 900-fold by DEAE-cellulose, blue Sepharose, gel permeation, and phenyl-Sepharose chromatography. The enzyme is obviously membrane bound as activity in the cytosol could not be detected. In the presence of detergents such as Triton X-100 (3-[3-cholamidopropyl]dimethylamino)-1-propanesulfonate (Chaps), or deoxycholate, a molecular weight of about 250,000 was determined by gel filtration. In glycerol density gradients, the enzyme sedimented slightly more slowly than bovine serum albumin, indicating a molecular weight of less than 68,000. On sodium dodecyl sulfate-polyacrylamide gel electrophoresis enzyme activity could be assigned to a protein of 51,000 daltons. As found previously for bacterial and animal diacylglycerol kinases, the purified enzyme was completely devoid of activity without the addition of phospholipids or deoxycholate. Cardiolipin was found to be most effective, whereas higher amounts of detergent were inhibitory. The enzyme needs divalent cations for activity, with Mg2+ ions being the most effective. Apparent Km values for ATP and diacylglycerol were determined as 100 and 250 micromolar, respectively.
Full text
PDF
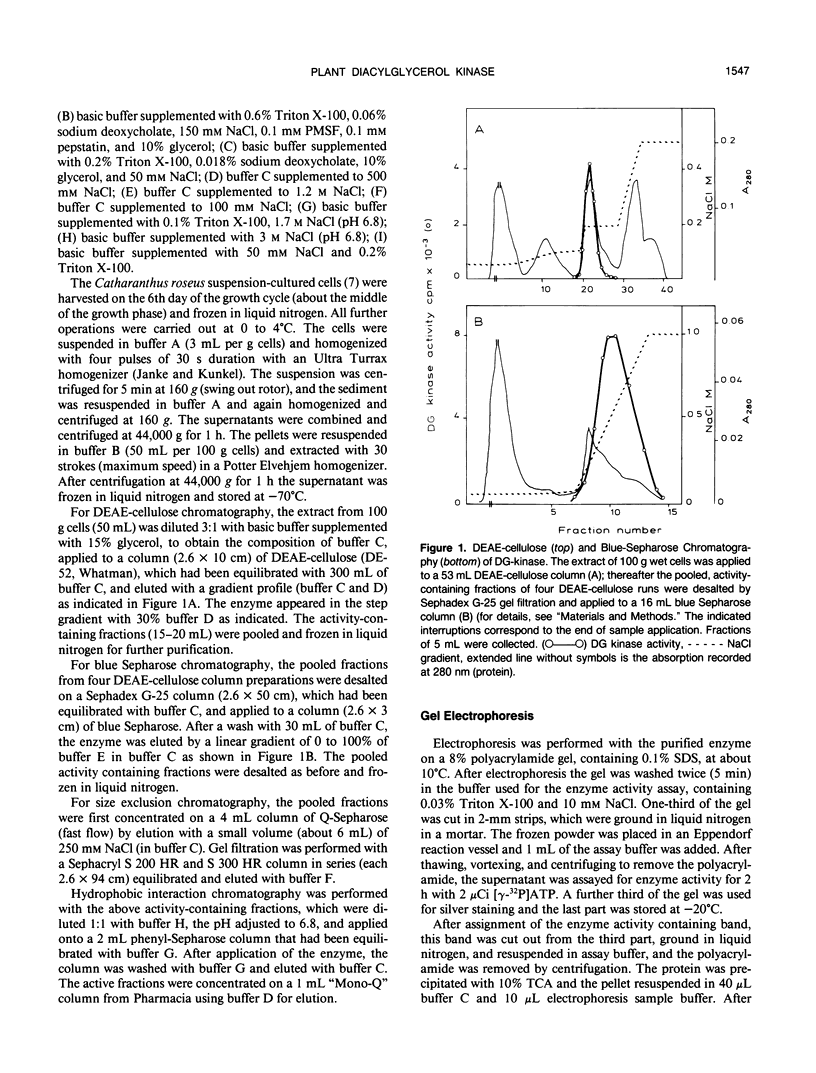

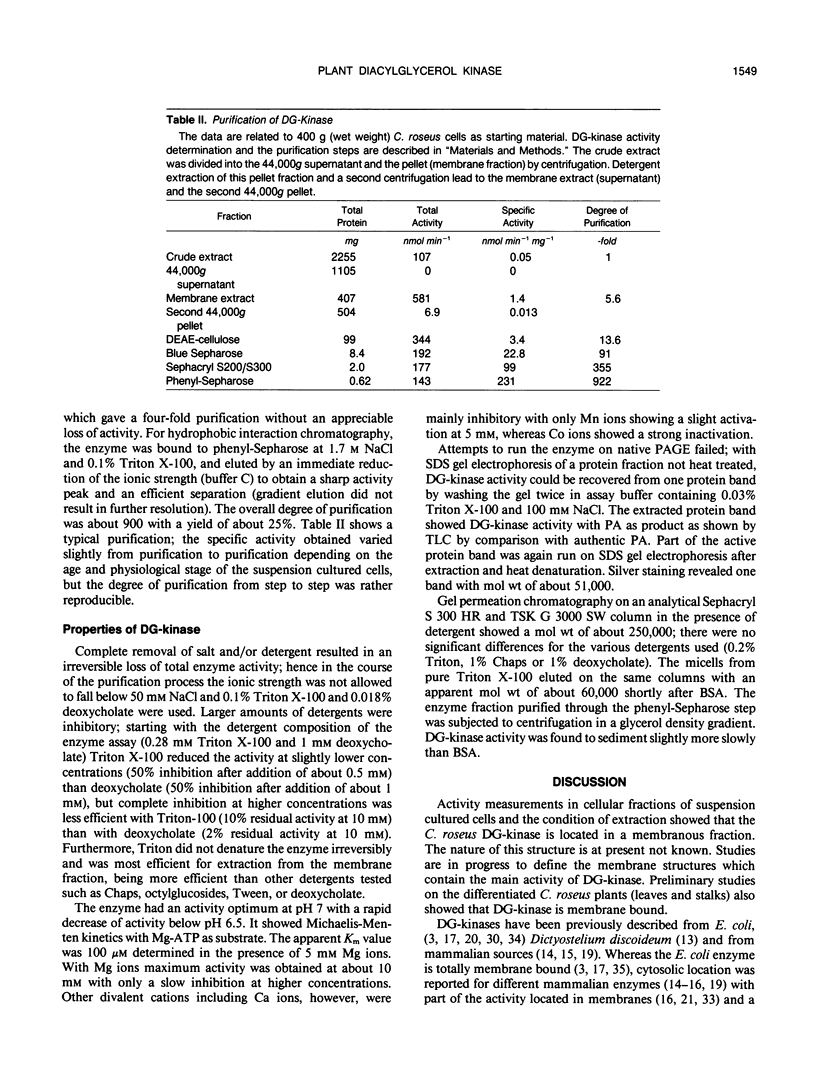


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Berridge M. J., Irvine R. F. Inositol trisphosphate, a novel second messenger in cellular signal transduction. Nature. 1984 Nov 22;312(5992):315–321. doi: 10.1038/312315a0. [DOI] [PubMed] [Google Scholar]
- Besterman J. M., Pollenz R. S., Booker E. L., Jr, Cuatrecasas P. Diacylglycerol-induced translocation of diacylglycerol kinase: use of affinity-purified enzyme in a reconstitution system. Proc Natl Acad Sci U S A. 1986 Dec;83(24):9378–9382. doi: 10.1073/pnas.83.24.9378. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bohnenberger E., Sandermann H., Jr Lipid dependence of diacylglycerol kinase from Escherichia coli. Eur J Biochem. 1983 May 16;132(3):645–650. doi: 10.1111/j.1432-1033.1983.tb07412.x. [DOI] [PubMed] [Google Scholar]
- Hasin M., Kennedy E. P. Role of phosphatidylethanolamine in the biosynthesis of pyrophosphoethanolamine residues in the lipopolysaccharide of Escherichia coli. J Biol Chem. 1982 Nov 10;257(21):12475–12477. [PubMed] [Google Scholar]
- Heim S., Wagner K. G. Evidence of phosphorylated phosphatidylinositols in the growth cycle of suspension cultured plant cells. Biochem Biophys Res Commun. 1986 Feb 13;134(3):1175–1181. doi: 10.1016/0006-291x(86)90374-8. [DOI] [PubMed] [Google Scholar]
- Irvine R. F., Letcher A. J., Dawson R. M. Phosphatidylinositol phosphodiesterase in higher plants. Biochem J. 1980 Oct 15;192(1):279–283. doi: 10.1042/bj1920279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ishitoya J., Yamakawa A., Takenawa T. Translocation of diacylglycerol kinase in response to chemotactic peptide and phorbol ester in neutrophils. Biochem Biophys Res Commun. 1987 Apr 29;144(2):1025–1030. doi: 10.1016/s0006-291x(87)80066-9. [DOI] [PubMed] [Google Scholar]
- Jímenez B., Van Lookeren Campagne M. M., Pestaña A., Fernández-Renart M. Regulation of diacylglycerol kinase in the transition from quiescence to proliferation in Dictyostelium discoideum. Biochem Biophys Res Commun. 1988 Jan 15;150(1):118–125. doi: 10.1016/0006-291x(88)90494-9. [DOI] [PubMed] [Google Scholar]
- Kanoh H., Iwata T., Ono T., Suzuki T. Immunological characterization of sn-1,2-diacylglycerol and sn-2-monoacylglycerol kinase from pig brain. J Biol Chem. 1986 Apr 25;261(12):5597–5602. [PubMed] [Google Scholar]
- Kanoh H., Kondoh H., Ono T. Diacylglycerol kinase from pig brain. Purification and phospholipid dependencies. J Biol Chem. 1983 Feb 10;258(3):1767–1774. [PubMed] [Google Scholar]
- Kanoh H., Ohno K. Partial purification and properties of diacylglycerol kinase from rat liver cytosol. Arch Biochem Biophys. 1981 Jun;209(1):266–275. doi: 10.1016/0003-9861(81)90280-0. [DOI] [PubMed] [Google Scholar]
- Kurosaki F., Tsurusawa Y., Nishi A. Breakdown of Phosphatidylinositol during the Elicitation of Phytoalexin Production in Cultured Carrot Cells. Plant Physiol. 1987 Nov;85(3):601–604. doi: 10.1104/pp.85.3.601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- König B., Bohnenberger E., Sandermann H., Jr Hexamethylphosphoric triamide as a solubilizing agent. Purification and reactivation of diglyceride kinase. FEBS Lett. 1981 Jul 6;129(2):301–304. doi: 10.1016/0014-5793(81)80188-3. [DOI] [PubMed] [Google Scholar]
- Lin C. H., Bishop H., Strickland K. P. Properties of diacylglycerol kinase purified from bovine brain. Lipids. 1986 Mar;21(3):206–211. doi: 10.1007/BF02534823. [DOI] [PubMed] [Google Scholar]
- MacDonald M. L., Mack K. F., Williams B. W., King W. C., Glomset J. A. A membrane-bound diacylglycerol kinase that selectively phosphorylates arachidonoyl-diacylglycerol. Distinction from cytosolic diacylglycerol kinase and comparison with the membrane-bound enzyme from Escherichia coli. J Biol Chem. 1988 Jan 25;263(3):1584–1592. [PubMed] [Google Scholar]
- McMurray W. C., Irvine R. F. Phosphatidylinositol 4,5-bisphosphate phosphodiesterase in higher plants. Biochem J. 1988 Feb 1;249(3):877–881. doi: 10.1042/bj2490877. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Melin P. M., Sommarin M., Sandelius A. S., Jergil B. Identification of Ca2+-stimulated polyphosphoinositide phospholipase C in isolated plant plasma membranes. FEBS Lett. 1987 Oct 19;223(1):87–91. doi: 10.1016/0014-5793(87)80515-x. [DOI] [PubMed] [Google Scholar]
- Pfaffmann H., Hartmann E., Brightman A. O., Morré D. J. Phosphatidylinositol specific phospholipase C of plant stems : membrane associated activity concentrated in plasma membranes. Plant Physiol. 1987 Dec;85(4):1151–1155. doi: 10.1104/pp.85.4.1151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Poovaiah B. W., Reddy A. S., McFadden J. J. Calcium messenger system: role of protein phosphorylation and inositol bisphospholipids. Physiol Plant. 1987;69:569–573. doi: 10.1111/j.1399-3054.1987.tb09241.x. [DOI] [PubMed] [Google Scholar]
- Rotering H., Raetz C. R. Appearance of monoglyceride and triglyceride in the cell envelope of Escherichia coli mutants defective in diglyceride kinase. J Biol Chem. 1983 Jul 10;258(13):8068–8073. [PubMed] [Google Scholar]
- Russ E., Kaiser U., Sandermann H., Jr Lipid-dependent membrane enzymes. Purification to homogeneity and further characterization of diacylglycerol kinase from Escherichia coli. Eur J Biochem. 1988 Jan 15;171(1-2):335–342. doi: 10.1111/j.1432-1033.1988.tb13795.x. [DOI] [PubMed] [Google Scholar]
- Stubbs E. B., Jr, Kelleher J. A., Sun G. Y. Phosphatidylinositol kinase, phosphatidylinositol-4-phosphate kinase and diacylglycerol kinase activities in rat brain subcellular fractions. Biochim Biophys Acta. 1988 Feb 4;958(2):247–254. doi: 10.1016/0005-2760(88)90183-x. [DOI] [PubMed] [Google Scholar]
- Walsh J. P., Bell R. M. sn-1,2-Diacylglycerol kinase of Escherichia coli. Mixed micellar analysis of the phospholipid cofactor requirement and divalent cation dependence. J Biol Chem. 1986 May 15;261(14):6239–6247. [PubMed] [Google Scholar]
- Walsh J. P., Bell R. M. sn-1,2-Diacylglycerol kinase of Escherichia coli. Structural and kinetic analysis of the lipid cofactor dependence. J Biol Chem. 1986 Nov 15;261(32):15062–15069. [PubMed] [Google Scholar]


