Abstract
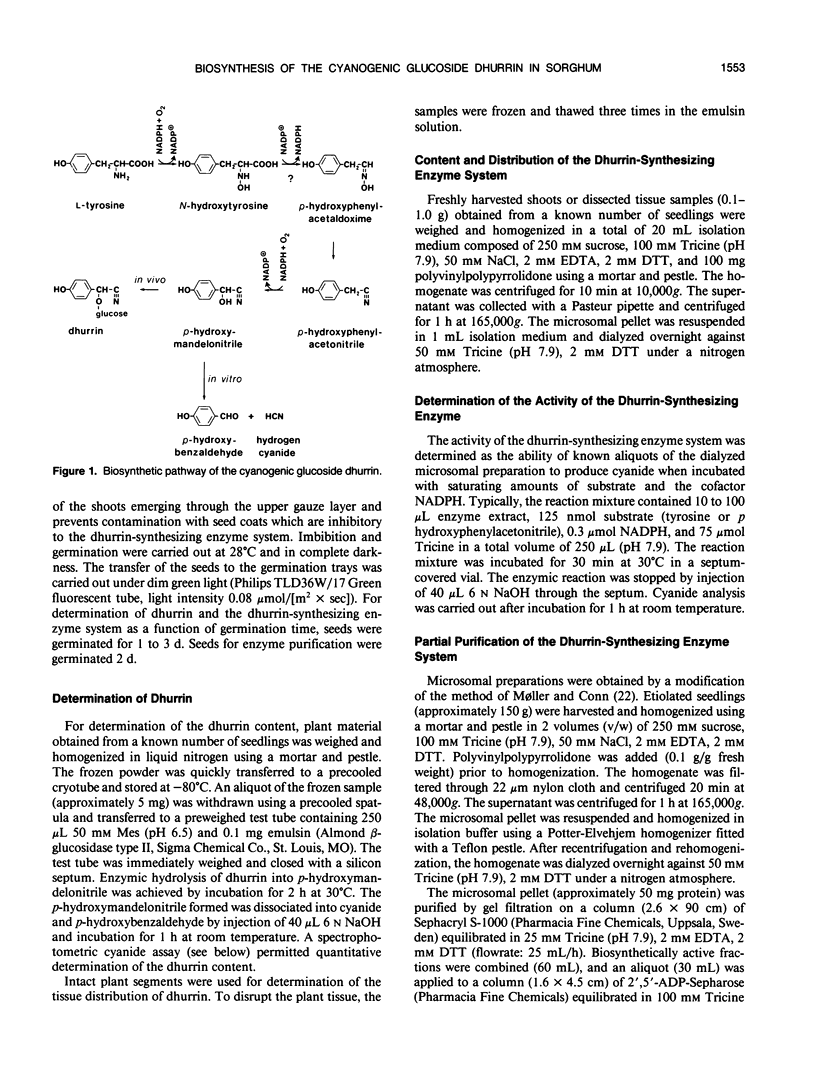
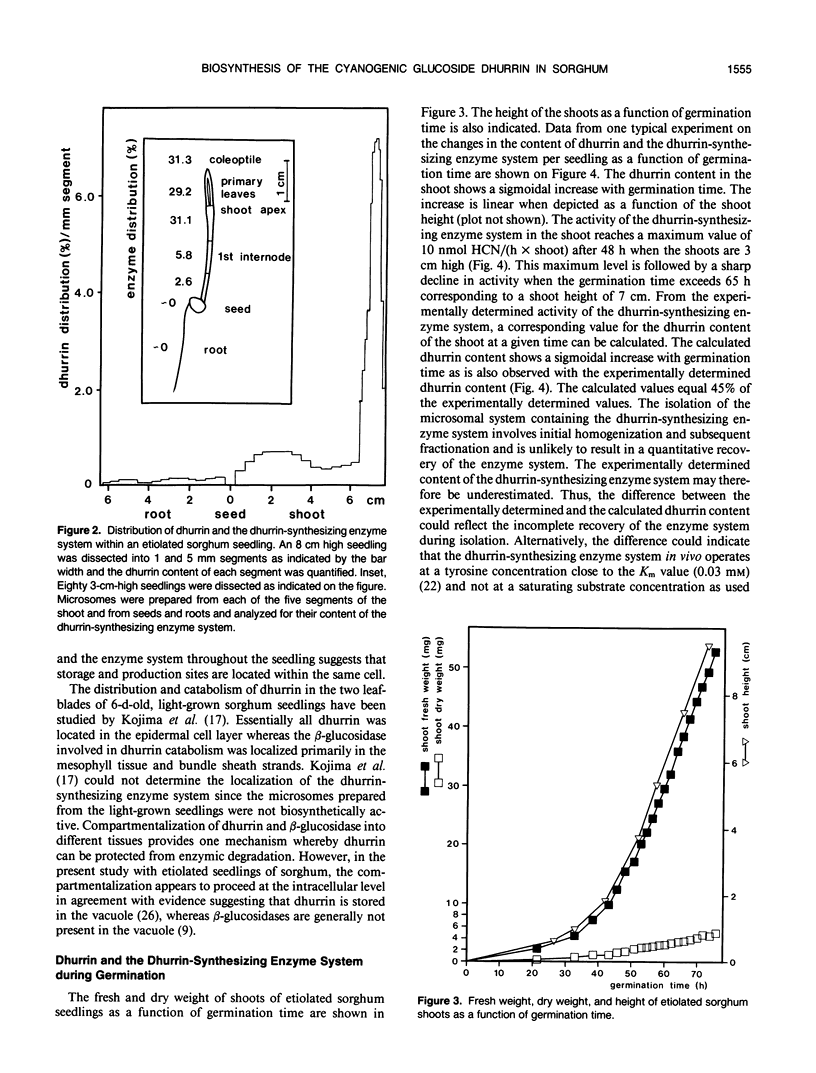
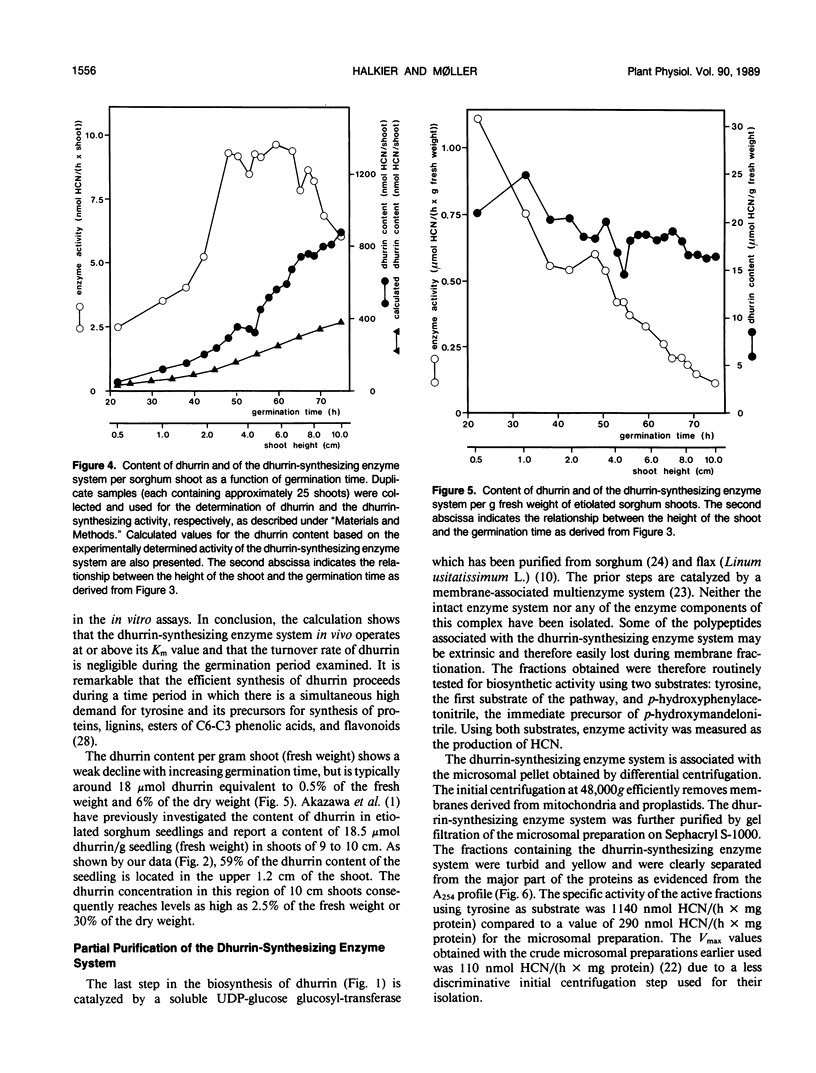
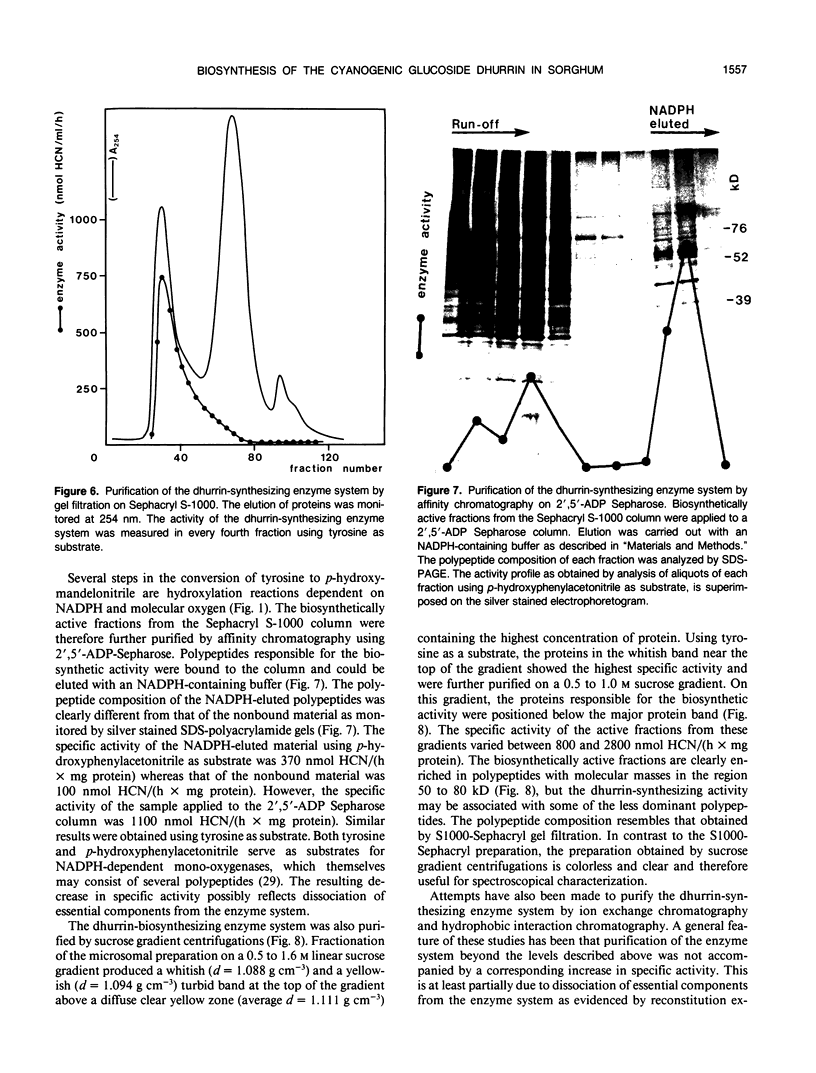
The cyanogenic glucoside dhurrin is rapidly synthesized in etiolated seedlings of Sorghum bicolor (L.) Moench. The dhurrin content of the seedlings increases sigmoidally with the germination time. Shoots of 10 centimeters height contain 850 nanomoles of dhurrin per shoot corresponding to 6% of the dry weight. The biosynthetic activity sharply rises upon germination and reaches a maximum level of 10 nanomoles dhurrin/(hour × shoot) after 48 hours when the shoots are 3 centimeters high. This maximum level is followed by a sharp decline in activity when germination time exceeds 65 hours. Dhurrin and the dhurrin-synthesizing enzyme system are primarily located in the upper part of the etiolated shoot where both are evenly distributed between the coleoptile, the primary leaves and the upper 0.5 centimeter of the first internode including the shoot apex. Dhurrin constitutes 30% of the dry weight of the upper 1.2 centimeter of 10 centimeter high shoots. The seed and root contain neither dhurrin nor the dhurrin-synthesizing enzyme system. The codistribution of dhurrin and the enzyme system throughout the seedling indicates that production and storage sites are located within the same cell. Purification of the dhurrin-synthesizing enzyme by gel filtration or by sucrose gradient centrifugations results in a tenfold increase in specific activity. Further purification is accompained by a decline in specific activity due to loss of essential components as demonstrated by reconstitution experiments.
Full text
PDF


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Akazawa T., Miljanich P., Conn E. E. Studies on Cyanogenic Glycoside of Sorghum Vulgare. Plant Physiol. 1960 Jul;35(4):535–538. doi: 10.1104/pp.35.4.535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Benveniste I., Gabriac B., Durst F. Purification and characterization of the NADPH-cytochrome P-450 (cytochrome c) reductase from higher-plant microsomal fraction. Biochem J. 1986 Apr 15;235(2):365–373. doi: 10.1042/bj2350365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bradford M. M. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem. 1976 May 7;72:248–254. doi: 10.1016/0003-2697(76)90527-3. [DOI] [PubMed] [Google Scholar]
- Fling S. P., Gregerson D. S. Peptide and protein molecular weight determination by electrophoresis using a high-molarity tris buffer system without urea. Anal Biochem. 1986 May 15;155(1):83–88. doi: 10.1016/0003-2697(86)90228-9. [DOI] [PubMed] [Google Scholar]
- Frehner M., Conn E. E. The Linamarin beta-Glucosidase in Costa Rican Wild Lima Beans (Phaseolus lunatus L.) Is Apoplastic. Plant Physiol. 1987 Aug;84(4):1296–1300. doi: 10.1104/pp.84.4.1296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hahlbrock K., Conn E. E. The biosynthesis of cyanogenic glycosides in higher plants. I. Purification and properties of a uridine diphosphate-glucose-ketone cyanohydrin beta-glucosyltransferase from Linum usitatissimum L. J Biol Chem. 1970 Mar 10;245(5):917–922. [PubMed] [Google Scholar]
- Hughes M. A., Stirling J. D., Collinge D. B. The inheritance of cyanoglucoside content in Trifolium repens L. Biochem Genet. 1984 Feb;22(1-2):139–151. doi: 10.1007/BF00499294. [DOI] [PubMed] [Google Scholar]
- Kojima M., Poulton J. E., Thayer S. S., Conn E. E. Tissue Distributions of Dhurrin and of Enzymes Involved in Its Metabolism in Leaves of Sorghum bicolor. Plant Physiol. 1979 Jun;63(6):1022–1028. doi: 10.1104/pp.63.6.1022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lambeth J. D., Kamin H. Adrenodoxin reductase and adrenodoxin. Mechanisms of reduction of ferricyanide and cytochrome c. J Biol Chem. 1977 May 10;252(9):2908–2917. [PubMed] [Google Scholar]
- MacFarlane I. J., Lees E. M., Conn E. E. The in vitro biosynthesis of dhurrin, the cyanogenic glycoside of Sorghum bicolor. J Biol Chem. 1975 Jun 25;250(12):4708–4713. [PubMed] [Google Scholar]
- Merril C. R., Goldman D., Sedman S. A., Ebert M. H. Ultrasensitive stain for proteins in polyacrylamide gels shows regional variation in cerebrospinal fluid proteins. Science. 1981 Mar 27;211(4489):1437–1438. doi: 10.1126/science.6162199. [DOI] [PubMed] [Google Scholar]
- Møller B. L., Conn E. E. The biosynthesis of cyanogenic glucosides in higher plants. Channeling of intermediates in dhurrin biosynthesis by a microsomal system from Sorghum bicolor (linn) Moench. J Biol Chem. 1980 Apr 10;255(7):3049–3056. [PubMed] [Google Scholar]
- Møller B. L., Conn E. E. The biosynthesis of cyanogenic glucosides in higher plants. N-Hydroxytyrosine as an intermediate in the biosynthesis of dhurrin by Sorghum bicolor (Linn) Moench. J Biol Chem. 1979 Sep 10;254(17):8575–8583. [PubMed] [Google Scholar]
- Pichichero M. E., Disney F. A., Aronovitz G. H., Talpey W. B., Green J. L., Francis A. B. Randomized, single-blind evaluation of cefadroxil and phenoxymethyl penicillin in the treatment of streptococcal pharyngitis. Antimicrob Agents Chemother. 1987 Jun;31(6):903–906. doi: 10.1128/aac.31.6.903. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reay P. F., Conn E. E. The purification and properties of a uridine diphosphate glucose: aldehyde cyanohydrin beta-glucosyltransferase from sorghum seedlings. J Biol Chem. 1974 Sep 25;249(18):5826–5830. [PubMed] [Google Scholar]
- Saunders J. A., Conn E. E. Subcellular localization of the cyanogenic glucoside of sorghum by autoradiography. Plant Physiol. 1977 Apr;59(4):647–652. doi: 10.1104/pp.59.4.647. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shimada M., Conn E. E. The enzymatic conversion of p-hydroxyphenylacetaldoxime to p-hydroxymandelonitrile. Arch Biochem Biophys. 1977 Apr 15;180(1):199–207. doi: 10.1016/0003-9861(77)90026-1. [DOI] [PubMed] [Google Scholar]
- Stafford H. A. Biosynthesis of phenolic compounds in first internodes of sorghum: lignin and related products. Plant Physiol. 1967 Mar;42(3):450–455. doi: 10.1104/pp.42.3.450. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Woodland M. P., Dalton H. Purification and characterization of component A of the methane monooxygenase from Methylococcus capsulatus (Bath). J Biol Chem. 1984 Jan 10;259(1):53–59. [PubMed] [Google Scholar]