Abstract
The objective was to investigate the possibility of using ExacTrac X-ray (ETX) for 6D image guidance in stereotactic body radiation therapy (SBRT) of bone metastasis and to propose a patient management protocol. The analyses were first obtained from measurements on a pelvic phantom and on 19 patients treated for bone metastasis. The phantom study consisted of applying known offsets and evaluating the ETX level of accuracy, where results were compared with kV-cone beam computed tomography (kV-CBCT). Two groups of patients, 10 spinal and 9 nonspinal SBRT cases, were analyzed to evaluate ETX imaging for different bone localisations. A comparison was made between kV-CBCT and ETX prior to the treatment fractions. During treatments, two other kV-CBCT/ETX image pairs were also acquired and a total of 224 shifts were compared. A second study, using the ETX monitoring module, analyzed the intrafraction motion of 8 other patients. In the phantom study, the root mean square (RMS) of the translational and rotational discrepancies between ETX and kV-CBCT were < 0.6 mm and < 0.4°, respectively. For both groups of patients, the RMS of the discrepancies observed between the two imaging systems were greater than the phantom experiment while still remaining < 1 mm and < 0.7°. In the nonspinal group, three patients (2 scapulas and 1 humerus) did not have consistent shift values with ETX due to a lack of anatomical information. When ETX monitoring was used during irradiation, the setup errors measured were on average less than 1 mm/1°. The results obtained validated the use of ETX for 6D image guidance during bone SBRT. Real-time tracking of the target position improves the accuracy of the irradiation. This strategy allowed for faster correction of out-of-tolerance positioning errors. The registration of bone lesions with poor anatomical information is a limitation of this 2D-kV imaging system.
Keywords: bone SBRT, kV-CBCT, intra-fraction motion, ExacTrac X-ray 6D, IGRT
Introduction
Stereotactic body radiotherapy (SBRT) is a treatment option based on the concept of image-guided radiation therapy (IGRT) with millimetric precision that delivers high doses. It may benefit from strong gradients between target volumes, with subsequently reduced margins and dose to adjacent organs at risk. The objective is to obtain a highly biologically effective dose (BED) in a limited number of fractions to deliver ablative radiation doses to the target, especially in radioresistant histology types. The most frequent protocols used are 16–24 Gy in 1 fraction, 24 Gy in 2 fractions, 24–27 Gy in 3 fractions and 30–35 Gy in 5 fractions. Several prospective and retrospective studies showed that a high BED provides local control rates of about 80–90% in 1 year. 1
The treatment of bone metastases, spinal or nonspinal, by this irradiation technique fundamentally requires the use of precise multimodal imaging, 2 a dosimetric plan using intensity modulation to allow for high conformity when irradiating concave targets and an image guidance strategy, often coupled with an immobilization system, to guarantee a high precision for the radiation delivery. The level of precision required, depending on the proximity of the organs at risk, can be of the order of 1 mm/2°. 3
The use of noninvasive positioning systems for the treatment of bone metastases raises the question of the relevance of intrafraction imaging. This question has been investigated by a few teams, but only for vertebral metastases by using data from different imaging systems, generally kV-cone beam computed tomography (kV-CBCT) and kV-2D4-7 and not for treatments of other bone locations. Megavoltage computed tomography is essentially used on specific installations such as tomotherapy (Accuray, Sunnyvale, CA) and very rarely on Linacs. This last system was not discussed here. Depending on the treatment machine and the techniques used, the treatment times reported in the literature range from 10 to 60 min. 8 The link between treatment time and the amplitude of positioning errors has been clearly established by various studies. For example, Ma et al. 9 recommend intrafractional imaging every 5 min to guarantee an accuracy of 1 mm/2°.
An analysis that was performed at our institution from 2018 to 2020 using data from kV-CBCT imaging acquired before each radiation beam revisited this finding. 10 The correlation between treatment time and intrafraction motion encouraged us to move toward faster imaging modalities that allow for a reduction in fraction time.
In our center, SBRT is performed in two rooms equipped with Truebeam Stx systems (Varian Medical Systems, Palo Alto, CA, USA) dedicated to treatments in stereotactic conditions and equipped with a 120HD MLC (High Definition), an On-Board Imager (OBI) allowing for kV-CBCT acquisitions and a PerfectPitch table with 6 degrees of freedom. These treatment rooms are each equipped with ExacTrac X-ray (ETX) 6D imaging systems (Brainlab AG, Munich, Germany) which are room-based and integrated with, but not restricted to, the accelerator. This system allows to verify the patient's position in 6 directions of freedom (longitudinal, lateral, vertical, pitch, yaw, and roll) from two kV-2D oblique acquisitions made from 2 X-ray sources and 2 detectors installed on the floor and ceiling of the treatment room respectively. This device is described, for example, in detail by Montgomery et al. 11
The two imaging devices mentioned have their respective advantages and limitations reported in the literature as well as in our clinical experience. The kV-CBCT (3D) acquisition allows for better visualization of anatomical structures, in particular soft tissues, than a planar kV-2D acquisition. It also allows to avoid localization errors in the case of vertebral treatments, for example. However, the patient's irradiation often starts only several minutes after the acquisition of this volumetric image, inducing a significant lapse of time without managing the patient's position. On the contrary, the nonembedded kV-2D device allows the acquisition of images and an automatic registration with the reference image in real time with or without interruption of the irradiation. This type of acquisition can be limited by the lack of anatomical information or the superposition of several structures for the same plane. The reduction of dose delivered by this technique is also a benefit of kV-2D imaging. 12
Moreover, and for our team this point raises questions, during kV-CBCT imaging for very lateralized treatments (> 10 cm from the reference of the CT scanning), the treatment table is recentered before acquisition and then moved back to the isocenter for irradiation. In other words, imaging in this case is not performed in the treatment position.
In this article a first study on a phantom and on patients allowed us to define the accuracy level of this system and to compare it to the kV-CBCT system generally used as reference imaging in IGRT.
The second part of this work also allowed us to analyze the positioning errors measured by 3 to 4 images acquired per-arc by the ExacTrac kV-2D system. Only a few studies have been based on such a high imaging frequency.12-14 Most of them used data from images acquired before and after the treatment fraction, sometimes between each irradiation beam.4-6
To our knowledge, no study has shared an IGRT methodology for the treatment of bone metastasis outside of spinal sites. Finally, this study provided an analysis of intrafraction motion and proposes IGRT workflows for linear accelerator (linac) systems equipped with a nonembedded kV-2D imaging system, for a more exhaustive list of sites involved in bone stereotactic treatment.
Methods
Phantom Study
Accuracy of ETX 6D: Phantom Study and Comparison With kV-CBCT Imaging
Prior to clinical routine use, an anthropomorphic phantom study was performed based on the works of Hazelaar et al 14 and Chang et al 15 for spinal lesions or Li et al, 16 Oh et al, 17 and Ma et al 18 for intracranial stereotactic treatments.
A pelvic phantom (Brainlab, Munich, Germany) with bony structures was scanned in helical mode on a GE Optima 580 scanner with a protocol used in clinical routine for bone SBRT. CT images were acquired with a slice thickness of 1.25 mm and a pixel resolution of 0.94 mm.
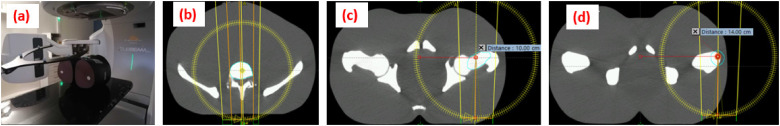
Three treatment plans were created in our Eclipse v15.6 Treatment Planning System (Varian, Medical Systems, Palo Alto, CA, USA) with isocenters shifted from the CT reference by 0, 10 and 14 cm (Figure 1). The value 14 cm matched this configuration with no risk of collision. The other 2 values, 0 and 10 cm, corresponded to cases of spinal and nonspinal treatment without table movement during the kV-CBCT acquisition.
Figure 1.
Description of the setup using a pelvic phantom (a) to assess the accuracy of the ExacTrac X-ray system and compare it to kV-CBCT for 3 different isocenters (b, c, d). kV-CBCT, kV-cone beam computed tomography. kV-CBCT, kV-cone beam computed tomography.
The setup procedure on the linac, which was identical for the three isocenters, consisted first of prepositioning the phantom on the markers set up during the CT imaging. Thereafter, a kV-CBCT was acquired with a 360° arm rotation (120 kV, 80 mA), a slice thickness of 1.98 mm and a pixel resolution of 0.91 mm, thereby allowing for comparison with the simulation CT scan. The kV-CBCT shifts were applied in 6 degrees of freedom. At this reference position, 2 oblique kV-2D images were acquired using ETX with a pixel resolution of 194 µm. For the three prepared plans, known shifts for both translations and rotations were applied together with the acquisition of a pair of kV-CBCT/ETX images. For translations, the offsets corresponded to 1, 2, 5, and 10 mm in anterior-posterior (AP), left-right (LR), and superior-inferior (SI) directions, applied in only one direction at the time. Rotations of 0.5°, 1°, 1.5°, and 2° were also simulated in the three directions; ie, pitch (RX), yaw (RY), and roll (RZ). In this case also, only one variable was tested while keeping all others fixed. The registration values given by the two imaging systems, kV-CBCT and ETX 6D, were taken from the Offline review module of ARIA (15.6, Varian Medical Systems, Palo Alto, CA) and ExacTrac module (v6.5.2, Brainlab, Munich, Germany) respectively, and compared. The ETX accuracy was obtained by comparing the registration values with the known offsets for the 6 translational and rotational movements. The total set represented a sample of 90 measurements.
Patient Study
Our retrospective monocenter study received the institutional consent of Centre Henri Becquerel necessary for its realization (reference number: 2018-155, May 3, 2018). Patients received a letter of consent in accordance with the French recommendations of the MR004 regulations and the study was registered institutionally under the name “SPINE SRS.” The clinical data analyzed were from 24 patients treated at our center with stereotactic radiotherapy for one or more bone lesions (30 in total) at a dose of 35 Gy in 5 fractions of 7 Gy, three times a week. The period covered is from November 2020 to September 2021.
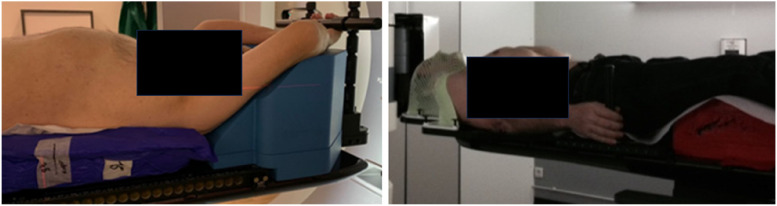
The patients were positioned, for locations below T4, on an integrated solution consisting of an SBRT table, an OrfitTM AIO system (Orfit Industries NV, Wijnegem, Belgium), and a half-body vacuum bag. Above the T4 vertebra, a thermoplastic mask was used (Figure 2).
Figure 2.
Patient positioning systems. Left: AIO Orfit™ system and vacuum cushion. Right: thermoplastic mask above T4 vertebra.
Accuracy of ETX 6D: Patient Study and Comparison With kV-CBCT Imaging
Two separate groups, 10 spinal and 9 nonspinal tumors, were studied in order to evaluate the feasibility of ETX imaging for repositioning different bone locations. A comparison was made between the patient setup correction proposed by a pretreatment kV-CBCT (CBCT_pTT) applied and a subsequent ETX 6D shift performed (ETX_pTT). Between each of our three intensity-modulated irradiation arcs, corresponding to our usual dosimetric protocol, 2 other pairs of kV-CBCT (CBCT_12 and CBCT_23) and ETX (ETX_12 and ETX_23) images were also acquired in the same order. A total of 224 6D shifts proposed by the 2 imaging systems were compared. More specifically, for the group including the 10 patients treated for spinal lesions, 143 ETX 6D/kV-CBCT comparisons were performed (Table 1, targets 1 to 10). For the other group, 6 patients treated for nonspinal tumors (Table 1, targets 11 to 16), 81 paired images were acquired and analyzed. On average 3 ETX 6D/kV-CBCT paired images were acquired per fraction.
Table 1.
Types of Intrafraction IGRT Used for the Localization of Different Bone Tumors. The Average Treatment Time was Calculated From the Beginning of the Acquisition of the Prepositioning kV-CBCT.
| Target | Location | IGRT intrafractions | Mean (SD) treatment time |
|---|---|---|---|
| 1 | T10 | (kV-CBCT + ExacTrac X-ray) Intra-arcs | 17 ± 4 min |
| 2 | T2 | ||
| 3 | L1 | ||
| 4 | T3 | ||
| 5 | L1 | ||
| 6 | L5 | ||
| 7 | T11 | ||
| 8 | T11 | ||
| 9 | C6 | ||
| 10 | L3 | ||
| 11 | Left iliac bone | ||
| 12 | Left femoral neck | ||
| 13 | sacrum | ||
| 14 | sacrum | ||
| 15 | Right iliac bone | ||
| 16 | Right femoral head | ||
| 17 | Right humerus | kV-CBCT Intra-arcs | 17 ± 4 min |
| 18 | Right scapula | ||
| 19 | Left scapula | ||
| 20 | L1 | ExacTrac monitoring during treatment | 10 ± 3 min |
| 21 | L4 | ||
| 22 | T8 | ||
| 23 | C1 | ||
| 24 | T12 | ||
| 25 | T10 | ||
| 26 | T4 | ||
| 27 | Right iliac bone | ||
| 28 | L4 | ||
| 29 | L2 | ||
| 30 | Left ischion |
kV-CBCT, kV-cone beam computed tomography; IGRT, image-guided radiation therapy; SD = standard deviation.
Three other patients, 2 scapulae and 1 humerus (Table 1, targets 17-19), were excluded from the comparison due to ETX not identifying distinct anatomy and sufficient bony landmarks to propose a consistent registration.
Real-Time Tracking of Target Position: Using ExacTrac Monitoring Module
The ExacTrac monitoring module enabled the acquisition of kV-2D image pairs during treatment beam delivery. The patient management process consisted first of a prepositioning kV-CBCT, to avoid any possibility of vertebral error, for example, followed by ExacTrac kV-2D verification imaging. Then, the intrafractional acquisitions in real time and the online registration with the reference DRR image could be performed by placing the irradiation beam on pause. The radiotherapy technologists were then instructed either to stop the treatment and apply the proposed shifts when these were greater than a certain threshold value, here fixed at 1 mm/2° 2 in any direction, or to continue the irradiation when this was not the case. The manual acquisition of kV-2D images was only allowed by the Exactrac v6.5.2 module when the positions of the accelerator's arms were within the particular angulations 0°, 90°, 180°, and 270° (to within ±10°). On average, 3 to 4 images per-arc were performed manually by the radiotherapy technologists. The results of this study were based on postprocessing measurements of 44 treatment fractions of 8 patients and 11 different locations (Table 1, targets 20-30). A total of 472 intrafraction shifts were analyzed. The average duration of a treatment fraction was 10 ± 3 min, counted from the start of the pretreatment kV-CBCT acquisition. For each patient, after pretreatment kV-CBCT, the kV-2D image acquisition frequency averaged 11 image pairs per fraction with minimum and maximum values of 7 and 17 acquisitions, respectively.
Statistical Analysis
The significance of the differences in positioning errors measured with the ETX 6D and those proposed by the KV-CBCT, was assessed using a nonparametric Mann–Whitney test. The results were associated with a p-value and a threshold of .05 below which the difference was considered significant. The results are presented by giving the root mean square error (RMS), the associated standard deviation (SD), and the maximum value (Max) for each direction.
Results
Phantom Study
The discrepancies (RMS, SD, Max) obtained between the proposed ETX 6D offsets after applying known offsets for the 6 translational and rotational movements are summarized in Table 2. The RMS measured in any direction was < 0.2 mm in translation and < 0.2° in rotation. Even considering the largest deviations, the alignment errors proposed by ETX remained < 0.7 mm/0.4°. The RMS differences between the translational and rotational errors measured by ETX and kV-CBCT were < 0.6 mm and < 0.4°, respectively. The maximum deviation between the two systems remained in all cases < 1 mm/1°. Table 2 summarizes the observed statistically significant differences for all directions of movement.
Table 2.
The Anthropomorphic Phantom Study. (Left) Setup Discrepancies Between ExacTrac X-ray and kV-CBCT and (Right) ExacTrac X-ray Accuracy When Known Offsets for the 6 Translational and Rotational Movements Were Applied.
| Motions | Discrepancies ExacTrac X-ray versus kV-CBCT phantom study | ExacTrac X-ray accuracy phantom study | |||||
|---|---|---|---|---|---|---|---|
| RMS | SD | Max | p-value | RMS | SD | Max | |
| Translations(mm) | |||||||
| Vert. (AP) | 0.23 | 0.14 | 0.61 | .042 | 0.17 | 0.10 | 0.38 |
| Long. (SI) | 0.47 | 0.23 | 0.84 | <.001 | 0.13 | 0.10 | 0.47 |
| Lat. (LR) | 0.52 | 0.19 | 0.97 | <.001 | 0.11 | 0.06 | 0.68 |
| Rotations (°) | |||||||
| Pitch (Rx) | 0.20 | 0.12 | 0.48 | <.001 | 0.11 | 0.06 | 0.25 |
| Roll (Rz) | 0.31 | 0.18 | 0.68 | .005 | 0.19 | 0.11 | 0.37 |
| Yaw (Ry) | 0.30 | 0.14 | 0.53 | <.001 | 0.12 | 0.07 | 0.25 |
kV-CBCT, kV-cone beam computed tomography; RMS = root-mean-square.
Patients Study
Accuracy of ETX 6D: Comparison With kV-CBCT Imaging
Table 3 summarizes the results of this section for the 2 groups of spinal and nonspinal patients. For both groups of patients, the RMS of the setup discrepancies observed between the imaging systems were greater than the phantom experiment while still remaining < 1 mm and < 1°. However, maximum values of discrepancies between the proposed ETX and kV-CBCT offsets were observed in translations and rotations > 1 mm and > 2° respectively. For the spinal group, 99% of the discrepancies between ETX and CBCT were ≤ 2 mm and ≤ 2°. 85% and 93% of the differences were ≤ 1 mm and ≤ 1°, respectively. For the nonspinal group, 98% of the discrepancies were ≤ 2 mm and ≤ 2° and 83% and 79% were ≤ 1 mm and ≤ 1°, respectively.
Table 3.
Setup Discrepancies Between ExacTrac X-ray 6D and CBCT for the Patient Study. Left, for the Spinal Patients Group and Right for the Nonspinal Cases.
| Motions | Discrepancies ExacTrac X-ray versus kV-CBCT spinal | Discrepancies ExacTrac X-ray versus kV-CBCT nonspinal | ||||||
|---|---|---|---|---|---|---|---|---|
| RMS | SD | Max | p-value | RMS | SD | Max | p-value | |
| Translations(mm) | ||||||||
| Vert. (AP) | 0.42 | 0.42 | 2.14 | .463 | 0.58 | 0.55 | 2.42 | <.0001 |
| Long. (SI) | 0.51 | 0.39 | 1.46 | <.0001 | 0.66 | 0.61 | 1.75 | <.0001 |
| Lat. (LR) | 0.75 | 0.47 | 1.58 | <.0001 | 0.73 | 0.70 | 2.4 | .099 |
| Rotations (°) | ||||||||
| Pitch (Rx) | 0.52 | 0.51 | 2.03 | .067 | 0.66 | 0.66 | 2.27 | .147 |
| Roll (Rz) | 0.44 | 0.38 | 1.31 | <.0001 | 0.77 | 0.67 | 1.93 | <.0001 |
| Yaw (Ry) | 0.57 | 0.42 | 1.92 | <.0001 | 0.65 | 0.59 | 2.23 | <.0001 |
kV-CBCT, kV-cone beam computed tomography.
In the nonspinal group of three patients (2 scapulas, 1 humerus), ETX could not provide consistent shift values due to a lack of anatomical information and was therefore excluded from the comparison study.
ExacTrac Monitoring Study
For this study, an average of 3 to 4 images were acquired per-arc for 44 treatment sessions with an average duration of 10 ± 3 min from the start of the prepositioning kV-CBCT scanning. The RMS, SD, and Max values of the positioning errors measured through the ExacTrac monitoring module are listed in Table 4. When this value was higher than the 1 mm/2° threshold, the radiotherapy technologists were instructed to apply the proposed offset.
Table 4.
The RMS, SD, and Max Values of the Positioning Errors Measured Through the ExacTrac Monitoring Module With on Average 3 to 4 Images Acquired Per-Arc.
| Motions | Vert. (AP) (mm) | Long. (SI) (mm) | Lat. (LR) (mm) | Pitch (°) | Roll (°) | Yaw (°) |
|---|---|---|---|---|---|---|
| RMS | 0.52 | 0.52 | 0.48 | 0.35 | 0.30 | 0.19 |
| SD | 0.36 | 0.31 | 0.31 | 0.26 | 0.22 | 0.12 |
| Max | 3.41 | 1.66 | 1.29 | 1.5 | 1.3 | 0.6 |
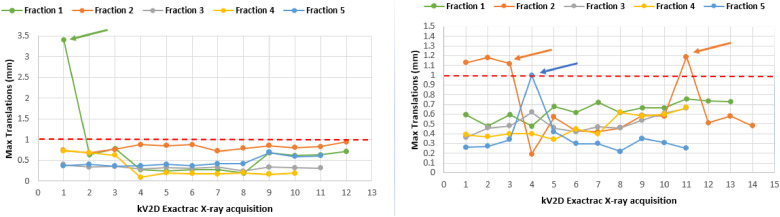
Whichever the direction for translations, the RMS remained well below 1 mm. On the other hand, the maximum values observed, and applied in this case, are greater than 1 mm. 87.3% of the translational offsets were less than 1 mm; 99.6% were less than 2 mm. Figure 3 shows the maximum translational positioning deviations during the 5 fractions of 2 different patients whose real-time imaging was performed with the ETX monitoring module. As an example, on the left in Figure 3, a positioning error in the anteroposterior (AP) direction of more than 3 mm was detected during the first treatment fraction and from the first ETX acquisition. The rest of the fractions showed no deviation greater than 1 mm.
Figure 3.
Measurement of the maximum translational intrafraction positioning error (3 to 4 kV-2D images per-arc) during 2 stereotactic treatments in 5 fractions. The arrows indicate shifts that were applied during the treatment fraction by the radiotherapy technologists. The correction threshold was set at 1 mm/2° (red dashed line).
The rotations observed during the treatment fractions were largely below our threshold of 2°, with a maximum of 1.5° around the Rx axis (pitch). For the rotations, 100% were lower than 2° and 97.4% lower than 1°.
Discussion
Our objective was first to validate the use of ETX imaging in the management of patients treated for bone SBRT, and to define its limitations.
The anthropomorphic phantom study showed a degree of accuracy for ETX well below a millimeter and a degree (Table 2). Compared to known positioning errors for translations or rotations, and whichever the position of the isocenter, the RMS values of the corrections proposed by ETX were of the order of 0.2 mm/0.2°. In comparison with kV-CBCT, the RMS discrepancies between the two systems were less than 0.6 mm/0.4° with maximum values < 1 mm/1°.
The results obtained in the patient study, both for the spinal and nonspinal groups, were less unequivocal. The RMS of the differences between the results given by the two imaging systems were less than 1 mm/1°, but could in some cases reach values > 2 mm/2°. This result has already been shown by Chang et al. 15 but with a version of the ExacTrac system that is over 10 years old. The more recent study by Park et al. 19 (2021) only compared ExacTrac and kV-CBCT for 3 translations and one rotation (pitch) for spinal metastases. Here we generalize these results for a more recent version of ExacTrac by comparing it with kV-CBCT in 6D and including locations outside the vertebrae. Chang et al (2010) 15 listed possible causes of these discrepancies. Mainly, the comparison was made between a planar image and a volumetric acquisition using different registration methods. Patient motion between the acquisitions of the 2 images, in the order of a few minutes, may also be a factor explaining the large differences. In addition, we can add that kV-CBCT acquisitions for very lateralized treatments imply table movements that could trigger patient motion and which is difficult to evaluate.
In the nonspinal group, three patients (2 scapulas and 1 humerus) did not benefit from ETX imaging and were excluded from the comparison with kV-CBCT. The lack of anatomical landmarks did not allow the ETX system to propose consistent offsets. However, for this type of localization, the intrafraction motion was still important and made it necessary to keep kV-CBCT imaging between each treatment beam. At the same time, a reconsideration of the positioning margins and/or the positioning itself must be carried out. The nonspinal cases (ie, bones without anatomical landmarks) that did not benefit from ETX as an IGRT system constituted about 25% of the 128 bone metastasis patients treated in our center to date (11/2021).
Wang et al 2 conducted a dosimetric study on 20 vertebral metastasis and recommended an accuracy of 1 mm/2° to keep the risk of loss of coverage of the target volume < 5% and an increase in dose to organs at risk < 25%. Consequently, with the use of the ExacTrac monitoring module, the radiotherapy technologists were instructed to stop the treatment and apply any offsets beyond this threshold. This methodology allowed to reach RMS of shifts < 1 mm and < 2°. Measured translational offsets were > 1 mm, in more than 10% of cases, but corrected to a frequency ≤ 1 min. Only 0.4% of offsets were > 2 mm. All rotational offsets were < 2°. Most of the studies on intrafraction motion are based on the analysis of data from images acquired between each radiation beam or at the end of the treatment. Few, such as Hirai et al 13 and Wu et al 20 have performed regular per-fraction acquisitions. These two studies for spine metastases reported results comparable to ours. In the Wu et al study, only 0.6% of cases required intrafraction repositioning > 2 mm. All intrafraction rotational movements were < 1.1°. Hirai et al utilized kV-2D X-ray intrafraction imaging acquisitions every 35 to 60 s for CyberKnife treatment and recommended to use 1 mm thresholds to check the target position every 4 to 6 min. Again, no rotational errors > 1° were observed when the treatment time was around 10 min. The 2 studies did not evaluate nonvertebral locations.
The work conducted by Ong et al 21 also confirmed our choice of an intrafraction IGRT strategy based on frequent and rapid acquisition of kV-2D images to verify the position of the target volume. Ong et al 21 estimated the dosimetric impact of shifts between 1 and 5 mm simulated during irradiation periods between 5 and 30 s and for 6MV FF (flattened filter) and 10MV FFF (flattening filter-free) beams. The impact of an occasional error of 2 mm during a short time of 30 s can increase the Dmax at the spinal cord by 8% for 6MV FF treatments. The use of the FFF beam increases this dosimetric effect up to 2 times. The coverage of the target volume seems less impacted by sudden movements.
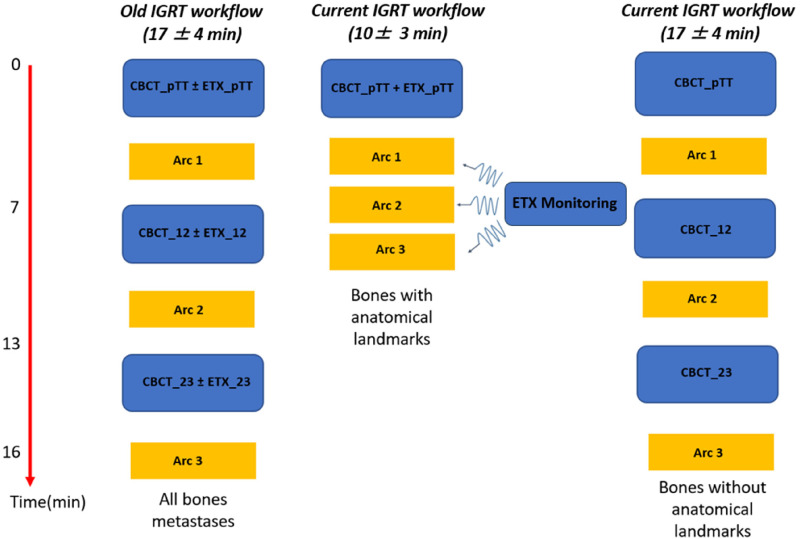
The management of our patients has therefore evolved to take into account these results. Figure 4 summarizes the different protocols of IGRT proposed in our center.
Figure 4.
Summary of our different intrafractional IGRT worflows for spinal and nonspinal stereotactic treatment.(_pTT: pretreatment; _12: between arc1 and arc2; _23: between arc2 and arc3).
IGRT, image-guided radiation therapy.
Our comparison of ETX with kV-CBCT included 10 patients treated for spinal tumors but only 6 patients for nonspinal bone metastases. In comparison, Chang et al included 11 spinal SBRT patients, Park et al 76 spinal patients and Wu et al studied 1019 spinal SBRT treatments.
Planar image acquisition during treatment with the ExacTrac system is only possible when the linac arm, during its rotation in the VMAT technique, is outside the irradiation field of the 2 kV sources. The number of stereoscopic images is therefore limited to an average of 3 to 4 images acquired per-arc. The Truebeam Stx (Varian Medical Systems, Palo Alto, CA, USA) kV-OBI system has the possibility to acquire more frequent images according to different triggers (MU, time or degree). However, this technique does not allow automatic registration but only visual verification by juxtaposing the contour of the target volume on the acquired 2D image. The effective dose delivered to the patient is of the order of 2 mSv per pair of images for the Exactrac system 11 compared with 10 to 20 mSv for a kV-CBCT scan. 22 The benefit of tracking the movement of the target is such that we can accept this extra dose. Nevertheless, the acquisition parameters and the number of images per fraction still need to be optimized to reduce this effective dose. In this article, only the errors due to intrafraction motion were investigated. In a larger study aiming at calculating the adequate margins to use, other sources of uncertainties should be taken into account. The impact of image quality on the results of automatic registration or the accuracy of the calibration of the isocenter of the imaging systems to the radiation isocenter of the accelerator are few examples.
During the intrafraction monitoring by the ExacTrac system, the impact of the choice of a threshold of 1 mm/2°, above which the positioning errors are corrected, on the results was not evaluated. A study is underway to verify the possibility of improving our results by having stricter instructions evaluated from a statistical process control 23 of intrafraction errors during the treatment fractions.
Conclusions
The results obtained validated the use of ETX for 6D IGRT during bone metastasis stereotactic treatments. Real-time tracking of the target volume position with the ExacTrac monitoring module improved the accuracy of the irradiation while minimizing the treatment time. This strategy allowed for faster correction of out-of-tolerance positioning errors. The registration of bone lesions with poor anatomical information is a limitation of this 2D-kV imaging system.
Acknowledgments
All authors in this study and many colleagues helped to collect and analyze data. Thanks for their assistance.
Abbreviations
- SBRT
stereotactic body radiation therapy
- CT
computed tomography
- CBCT
cone-beam computed tomography
- ETX
ExacTrac X-ray
- VMAT
volumetric modulated arc therapy
- AAA
analytical anisotropic algorithm
- RMS
root mean square
- SD
standard deviation
- AP
anterior–posterior
- SI
superior–inferior
- LR
left–right
- Pitch
rotation around LR direction
- Roll
rotation around SI direction
- Yaw
rotation around AP direction
Footnotes
Author Responsible for Statistical Analyses: David Gensanne (PhD), CENTRE HENRI BECQUEREL 1 rue d’Amiens 76000 ROUEN (FRANCE), +33(0)2 32 08 25 85, david.gensanne@chb.unicancer.fr.
Authors’ Contributions: All authors have substantial contributions to the conception and design of the work or the acquisition, analysis or interpretation of data. All authors read and approved the final manuscript.
Availability of Data and Materials: All data and materials have been presented in the manuscript.
Competing Interests: This work was supported in part by a grant from Brainlab. The sponsor was not involved in the study design, data collection, analysis, and interpretation, the writing of this article or the decision to submit it for publication.
Consent for Publication: Not applicable.
The authors declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Funding: The authors received no financial support for the research, authorship, and/or publication of this article.
Ethics Approval and Consent to Participate: Our retrospective monocenter study received the institutional consent of Centre Henri Becquerel necessary for its realization (reference number: 2018-155, May 3, 2018). Patients received a letter of consent in accordance with the French recommendations of the MR004 regulations and the study was registered institutionally under the name “SPINE SRS.”
ORCID iD: Ahmed Hadj Henni https://orcid.org/0000-0002-5521-1484
References
- 1.Gong Y, Xu L, Zhuang H, et al. Efficacy and safety of different fractions in stereotactic body radiotherapy for spinal metastases: a systematic review. Cancer Med. 2019;8(14):6176-6184. doi: 10.1002/cam4.2546 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Benedict SH, Yenice KM, Followill D, et al. Stereotactic body radiation therapy: the report of AAPM task group 101. Med Phys. 2010;37(8):4078. [DOI] [PubMed] [Google Scholar]
- 3.Wang H, Shiu A, Wang C, et al. Dosimetric effect of translational and rotational errors for patients undergoing image-guided stereotactic body radiotherapy for spinal metastases. Int J Radiat Oncol Biol Phys. 2008;71(4):1261-1271. [DOI] [PubMed] [Google Scholar]
- 4.Agazaryan N, Tenn SE, Desalles AA, Selch MT. Image-guided radiosurgery for spinal tumors: methods, accuracy and patient intrafraction motion. Phys Med Biol. 2008;53(6):1715-1727. doi: 10.1088/0031-9155/53/6/015 [DOI] [PubMed] [Google Scholar]
- 5.Li W, Sahgal A, Foote M, et al. Impact of immobilization on intrafraction motion for spine stereotactic body radiotherapy using cone beam computed tomography. Int J Radiat Oncol Biol Phys. 2012;84(2):520-526. [DOI] [PubMed] [Google Scholar]
- 6.Hyde D, Lochray F, Korol R, et al. Spine stereotactic body radiotherapy utilizing cone beam CT image-guidance with a robotic couch: intrafraction motion analysis accounting for all six degrees of freedom. Int J Radiat Oncol Biol Phys. 2012;82(3):e555-e562. [DOI] [PubMed] [Google Scholar]
- 7.Lewis BC, Snyder WJ, Kim S, Kim T. Monitoring frequency of intra-fraction patient motion using the ExacTrac system for LINAC-based SRS treatments. J Appl Clin Med Phys. 2018;19(3):58-63. doi: 10.1002/acm2.12279. Epub 2018 Mar 25. PMID: 29577592; PMCID: PMC5978384. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Gallo JJ, Kaufman I, Powell Ret al. Single-fraction spine SBRT end-to-end testing on TomoTherapy, Vero, TrueBeam, and CyberKnife treatment platforms using a novel anthropomorphic phantom. J Appl Clin Med Phys. 2015;16(1):5120. doi: 10.1120/jacmp.v16i1.5120 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Ma L, Sahgal A, Hossain S, et al. Non-random intrafraction target motions and general strategy for correction of spine stereotactic body radiotherapy. Int J Radiat Oncol Biol Phys. 2009;75(4):1261-1265. [DOI] [PubMed] [Google Scholar]
- 10.Hadj Henni A, Gensanne D, Roge M, et al. Evaluation of inter- and intra-fraction 6D motion for stereotactic body radiation therapy of spinal metastases: influence of treatment time. Radiat Oncol. 2021;16(1):168. doi: 10.1186/s13014-021-01892-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Montgomery C, Collins M. An evaluation of the BrainLAB 6D ExacTrac/Novalis Tx system for image-guided intracranial radiotherapy. J Radiother Pract. 2017;16(3):326-333. doi: 10.1017/S1460396917000139 [DOI] [Google Scholar]
- 12.Murphy MJ, Balter J, Balter S, et al. The management of imaging dose during image-guided radiotherapy: report of the AAPM task group 75. Med Phys. 2007;34(10):4041-4063. [DOI] [PubMed] [Google Scholar]
- 13.Hirai R, Ohkubo YU, Igari Met al. Time dependence of intra-fractional motion in spinal stereotactic body radiotherapy. In Vivo. 2021;35(4):2433-2437. doi: 10.21873/invivo.12521 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Hazelaar C, Dahele M, Mostafavi H, Van der Weide L, Slotman BJ, Verbakel W. Subsecond and submillimeter resolution positional verification for stereotactic irradiation of spinal lesions. Int J Radiat Oncol Biol Phys. 2016;94(5):1154-1162. [DOI] [PubMed] [Google Scholar]
- 15.Chang Z, Wang Z, Ma J, O’Daniel JC, Kirkpatrick J, Yin FF. 6D Image guidance for spinal non invasive stereotactic body radiation therapy: comparison between ExacTrac X-ray 6D with kilo-voltage cone beam CT. Radiother Oncol. 2010;95(1):116-121. [DOI] [PubMed] [Google Scholar]
- 16.Li J, Shi W, Andrews Det al. et al. Comparison of online 6 degree-of-freedom image registration of Varian Truebeam cone-beam CT and BrainLab ExacTrac X-ray for intracranial radiosurgery. Technol Cancer Res Treat. 2017;16(3):339-343. doi: 10.1177/1533034616683069 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Oh SA, Park JW, Yea JW, Kim SK. Evaluations of the setup discrepancy between BrainLAB 6D ExacTrac and cone-beam computed tomography used with the imaging guidance system Novalis-Tx for intracranial stereotactic radiosurgery. PLoS ONE. 2017;12(5):e0177798. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Ma J, Chang Z, Wang Z, Jackie Wu Q, Kirkpatrick JP, Yin FF. Exactrac X-ray 6 degree-of-freedom image-guidance for intracranial non-invasive stereotactic radiotherapy: comparison with kilo-voltage cone-beam CT. Radiother Oncol. 2009;93(3):602-608. doi: 10.1016/j.radonc.2009.09.009 [DOI] [PubMed] [Google Scholar]
- 19.Park J, Yea JW, Park JW, Oh SA. Evaluation of the setup discrepancy between 6D ExacTrac and cone beam computed tomography in spine stereotactic body radiation therapy. PLoS One. 2021;16(5):e0252234. doi: 10.1371/journal.pone.0252234 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Wu J, Wu J, Ballangrud Å, Mechalakos J, Yamada J, Lovelock DM. Frequency of large intrafractional target motions during spine stereotactic body radiation therapy. Pract Radiat Oncol. 2020;10(1):e45-e49. doi: 10.1016/j.prro.2019.08.006 [DOI] [PubMed] [Google Scholar]
- 21.Ong CL, Dahele M, Cuijpers JP, Senan S, Slotman BJ, Verbakel WFAR. Dosimetric impact of intrafraction motion during RapidArc stereotactic vertebral radiation therapy using flattened and flattening filter-free beams. Int J Radiat Oncol Biol Phys. 2013;86(3):420-425. doi: 10.1016/j.ijrobp.2012.12.028. Epub 2013 Mar 20. PMID: 23523183. [DOI] [PubMed] [Google Scholar]
- 22.Kan MW, Leung LH, Wong W, Lam N. Radiation dose from cone beam computed tomography for image-guided radiation therapy. Int J Radiat Oncol Biol Phys. 2008;70(1):272-279. doi: 10.1016/j.ijrobp.2007.08.062. Epub 2007 Nov 5. PMID: 17980510. [DOI] [PubMed] [Google Scholar]
- 23.Moore SJ, Herst PM, Louwe RJW. Review of the patient positioning reproducibility in head-and-neck radiotherapy using Statistical Process Control. Radiother Oncol. 2018;127(2):183-189. doi: 10.1016/j.radonc.2018.01.006. Epub 2018 Jan 31. PMID: 29395288. [DOI] [PubMed]