Abstract
Background:
Despite the high incidence of acute ischemic stroke (AIS) in cancer patients, there is still no consensus about the safety of recanalization therapies in this cohort.
Objectives:
In this observational study, our aim was to investigate the bleeding risk after acute recanalization therapy in AIS patients with active malignancy.
Methods and Study Design:
We retrospectively analyzed observational data of 1016 AIS patients who received intravenous thrombolysis with rtPA (IVT) and/or endovascular therapy (EVT) between January 2017 and December 2020 with a focus on patients with active malignancy. The primary safety endpoint was the occurrence of stroke treatment-related major bleeding events, that is, symptomatic intracranial hemorrhage (SICH) and/or relevant systemic bleeding. The primary efficacy endpoint was neurological improvement during hospital stay (NI).
Results:
None of the 79 AIS patients with active malignancy suffered from stroke treatment-related systemic bleeding. The increased rate (7.6% versus 4.7%) of SICH after therapy compared to the control group was explained by confounding factors. A total of nine patients with cerebral tumor manifestation received acute stroke therapy, two of them suffered from stroke treatment-related intracranial hemorrhage remote from the tumor, both asymptomatic. The group of patients with active malignancy and the control group showed comparable rates of NI.
Conclusion:
Recanalization therapy in AIS patients with active malignancy was not associated with a higher risk for stroke treatment-related systemic or intracranial bleeding. IVT and/or EVT can be regarded as a safe therapy option for AIS patients with active malignancy.
Keywords: EVT, intracranial bleeding, IVT, malignancy, stroke, systemic bleeding, thrombolysis
Introduction
Cancer patients are at higher risk of ischemic stroke due to tumor-specific risk factors, leading to increased incidence of thromboembolic events in addition to conventional stroke mechanisms.1,2 Up to 15% of patients with malignant tumors suffer from ischemic strokes,3,4 and numerous cerebral ischemia of cryptogenic origin are believed to be associated with underlying or undetected malignancy.5–7 Among others, paraneoplastic hypercoagulability, non-bacterial thrombotic endocarditis, direct tumor mass effect, and posttherapeutic vasculopathy are considered possible pathophysiological mechanisms of tumor- and treatment-associated strokes.1,8–10
In the treatment of acute ischemic stroke (AIS), recent guidelines recommend intravenous thrombolysis (IVT) with recombinant tissue plasminogen activator (rtPA) and/or endovascular therapy (EVT) as standard of care.11,12 While inclusion or exclusion criteria of the approval studies for rtPA and regulatory approval documents did not mention malignancies,13,14 recent guidelines state that systemic malignancy is not an absolute contraindication for IVT, provided that life expectancy is >6 months and contraindications such as systemic bleeding, recent surgery, or coagulopathy do not coexist. 15 To date, especially gastrointestinal and intra-axial primary or metastatic brain tumors are regarded as high risk for bleeding complications after acute stroke therapy.15,16 However, evidence is weak as patients with active malignancy were and still are excluded in most clinical trials on IVT and EVT.13,17,18 While several retrospective studies consistently show higher in-hospital mortality and overall worse outcomes in stroke patients with active malignancy after recanalization therapy, contrary results have been reported regarding the risk of periprocedural hemorrhages.19–22 Higher D-dimer levels were identified as a predictor for early neurologic deterioration in patients with malignancy. 23 Nevertheless, most of these studies focused on IVT or EVT as a single treatment for cerebral ischemia.9,21,22,24 Consequently, a recent comprehensive review on cancer-related stroke emphasized the need to collect further data on these patients. 25 Given the importance of EVT in addition to IVT in case of large vessel occlusion (LVO),26,27 we sought to evaluate the outcome and rate of bleeding complications after IVT and/or EVT in a large cohort of AIS patients with active malignancy, history of cancer or without cancer.
Materials and methods
Data source and study population
This is a retrospective study including data of all patients with AIS treated in the comprehensive stroke center of the University Hospital Essen between January 2017 and December 2020 meeting the following inclusion criteria: AIS, age over 18 years, and recanalization therapy as per standard of care (IVT and/or EVT). The University Hospital Essen has a special focus on the treatment of patients with malignancy and treats approximately 30,000 patients annually at Germany’s largest cancer center, the West German Cancer Center.
Patients were classified into a group with active malignancy (AM) and a control (C) group (history of cancer or without cancer). Active malignancy was defined as cancer diagnosed within 6 months before stroke, recurrent cancer, metastasis of primary cancer, or receiving any cancer treatment within 12 months prior to or post-index stroke event.4,24,28 The selection process is depicted as a flow chart in Figure 1.
Figure 1.
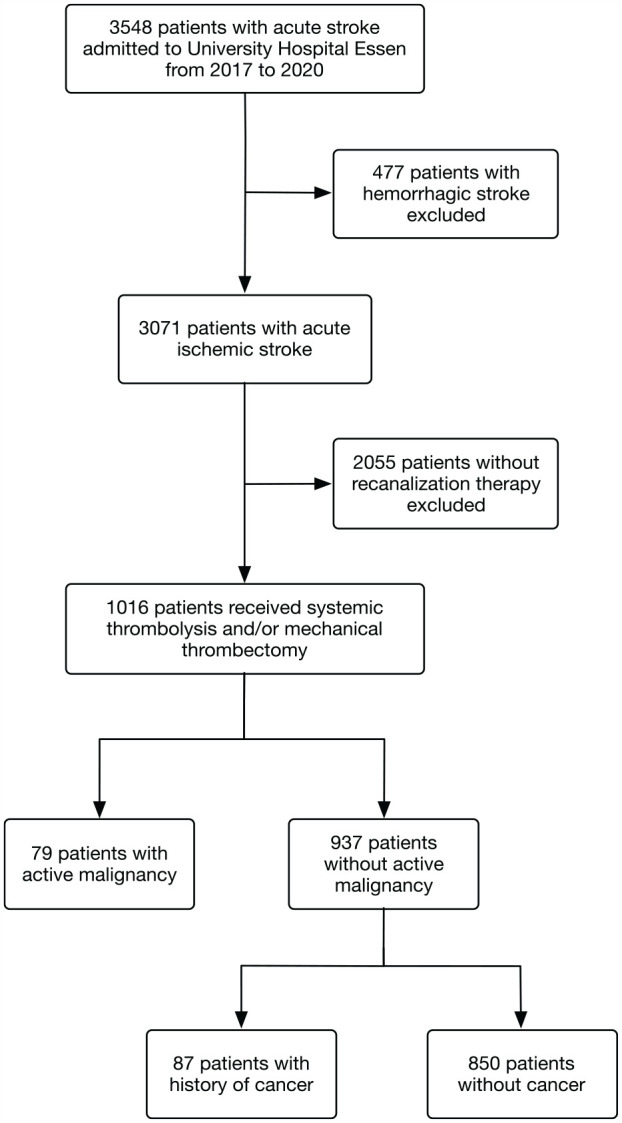
Flow chart with a description of excluded patients and subgroups based on final discharge diagnosis, intervention, and tumor history. Patients with a history of cancer and without a diagnosis of cancer were further referred to as the control group.
Follow-up brain imaging was performed within 24 h after index stroke and reviewed independently by neurology and neuroradiology staff (WHC and YL). Verified intracranial hemorrhages (ICHs) were categorized according to the European Cooperative Acute Stroke Study (ECASS) III and Heidelberg Bleeding Classification.29,30
All patients with AIS and bleeding events admitted to our stroke center are prospectively collected in a local stroke registry database. The core dataset is entered by the attending physician on admission, completed during hospitalization, and validated by the attending senior stroke neurologist. Structured data of instrument-based examinations are entered by study personnel and laboratory examinations are exported from the local archiving system. Finally, all data are validated by a senior stroke neurologist (BF).
For the present analysis, information on diagnoses and complications has additionally been gained from individual hospital files to achieve maximum accuracy.
Definition of clinical outcome and endpoints
The primary safety endpoint was the occurrence of acute stroke treatment-related major bleeding events. Major bleeding events were defined as symptomatic intracranial hemorrhage (SICH) according to ECASS III Classification and/or major systemic bleeding events. ECASS III defined SICH as any hemorrhage, which was the predominant cause of the neurological deterioration, that is, worsening of greater than or equal to four points of the National Institutes of Health Stroke Scale (NIHSS) compared to NIHSS at baseline or to the lowest NIHSS value in the first 7 days, or which led to death. 29 Systemic bleeding events were considered major bleeding when accompanied by loss of at least two points of hemoglobin and/or the requirement of a transfusion of at least two erythrocyte concentrates.
Secondary safety endpoints were (1) SICH according to ECASS III Classification, (2) SICH according to the Heidelberg Bleeding Classification,29,30 (3) stroke treatment-related systemic bleeding, (4) in-hospital mortality, and (5) prolonged length of hospital stay after stroke treatment. As values of the individual NIHSS categories are only available at admission and discharge for this analysis, the cumulative NIHSS is used for the remaining time points to identify SICH according to the Heidelberg Bleeding Classification. Thus, in the present analysis, SICH according to the Heidelberg Bleeding Classification is defined as worsening of greater than or equal to four points of NIHSS after ICH compared to NIHSS before deterioration or as ICH with the requirement of major medical/surgical interventions (e.g. insert of extraventricular drainage or a hemicraniectomy). 29 Despite its modified use, the Heidelberg Bleeding Classification provides a more thorough classification providing information on intracranial bleeding sites apart from intracerebral hemorrhages. Length of hospital stay was defined as ‘prolonged’ if it was more than 30 days. 31
All bleeding events were assessed for their relatedness to the stroke treatment. Bleeding events were defined as related to stroke treatment if they occurred within 24 h after IVT/EVT or were directly associated with the treatment, such as due to groin puncture. Furthermore, bleeding events were considered cancer related when the cancer was the source of bleeding or when occurred at the cancer site.
The primary efficacy endpoint was neurological improvement during hospital stay (NI), defined as the change in NIHSS at discharge compared to NIHSS at admission in percentage ([admission NIHSS − discharge NIHSS] × 100/admission NIHSS).32–34 The cutoffs to define NI were set at 20% and 30% decrement as well as a decrease of four and eight points in the NIHSS scale. 34 Secondary efficacy endpoint was the absolute change in NIHSS at discharge compared to NIHSS at admission.
Statistical analyses
We performed adjusted and unadjusted logistic regression to compare the occurrence of endpoints in AIS patients with active malignancy with the control group. Results are presented as odds ratio (OR) and 95% confidence interval (95% CI), both for the effect of the active malignancy group and covariates added singularly and multivariate to assess confounding.
Based on the characteristics and distribution of the data, descriptive statistics are depicted as count and percent, mean with standard deviation or median with interquartile range. For continuous variables t-tests or Mann–Whitney U-tests were used according to the distribution of data. Chi-square tests were performed for categorical variables. Statistical analyses were performed with SPSS (IBM SPSS Statistics for Windows, Version 25.0. Armonk, NY: IBM Corp.) and a two-sided p < 0.05 was considered the minimal level of statistical significance.
Results
Baseline and procedural characteristics of the study population
A total of 3548 patients with acute ischemic or hemorrhagic stroke were registered during January 2017 and December 2020. A total of 1016 patients met the predefined inclusion and exclusion criteria of the presented analysis (Figure 1).
Of these, 79 patients had active malignancy at the time of their admission (active malignancy group), while 87 had a history of cancer, and 850 had no previous malignancy. In clinical routine patients with only a history of cancer are considered at similar risk as patients without cancer. In our cohort, both showed comparable risk factors and consequently are summed up in one group for this analysis (control group, compare Supplemental Table S1).
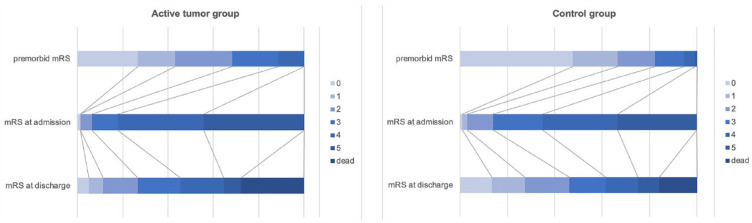
As opposed to the control group, patients with active malignancy showed higher premorbid modified Rankin Scale (mRS) scores (Figure 2) and suffered significantly more often from coexisting coronary artery disease and less often from hypertension. Moreover, significantly higher D-dimer levels, INR, and thrombocytes as well as lower hemoglobin could be found in patients with active malignancy at admission. The prior antithrombotic treatment included more often dual platelet inhibition in patients with active malignancy (Table 1).
Figure 2.
Stacked horizontal bar graphs depicting premorbid mRSs (top row), mRS at admission (middle row), and at discharge (bottom row) of patients in the active malignancy group [(a), n = 79] and control group [(b), n = 937]. Each box of the horizontal bar graph corresponds to the mRS score defined by the color code.
mRS, modified Rankin Scale.
Table 1.
Baseline characteristics of patients with AM (n = 79) and in the control group (C, n = 937).
| Baseline characteristics | AM | C | p-Value |
|---|---|---|---|
| Age (years), mean (SD) | 73.7 (13.5) | 71.2 (14.0) | 0.130 |
| Sex (male) | 31 (39.2) | 460 (49.1) | 0.092 |
| History | |||
| Hypertension | 51 (64.6) | 713 (76.1) | 0.023 |
| Diabetes | 25 (31.6) | 251 (26.8) | 0.351 |
| Atrial fibrillation | 33 (41.8) | 297 (31.7) | 0.066 |
| Coronary artery disease | 22 (27.8) | 167 (17.8) | 0.028 |
| Peripheral artery disease | 9 (11.4) | 67 (7.2) | 0.169 |
| Prior stroke | 20 (25.3) | 197 (21.0) | 0.371 |
| Prior TIA | 2 (2.5) | 41 (4.4) | 0.434 |
| Admission | |||
| Large vessel occlusion | 45 (57.0) | 444 (47.4) | 0.102 |
| NIHSS | 11 (5–18) | 9 (4–16) | 0.078 |
| D-dimer (mg/l) | 3.5 (1.5–7.3) a | 1.2 (0.6–3.2) c | <0.001 |
| International normalized ratio | 1.07 (1.01–1.20) b | 1.03 (0.99–1.10) d | 0.001 |
| Activated partial thromboplastin time (s) | 24,5 (22.9–27.5) | 24,8 (23.1–26.8) | 0.668 |
| Hemoglobin (g/dl) | 11,3 (9.8–12.8) | 13,3 (12.1–14.5) | <0.001 |
| Thrombocytes (/nl) | 249.0 (189.0–311.0) | 237.0 (192.0–287.0) | 0.002 |
| Prior antithrombotic treatment | |||
| Single platelet inhibition | 23 (29.1) | 296 (31.6) | 0.706 |
| Dual platelet inhibition | 4 (5.1) | 13 (1.4) | 0.037 |
| Oral anticoagulant | 10 (12.7) | 104 (11.1) | 0.855 |
Data are n (%) or median (interquartile range) if not indicated otherwise.
Data available in an = 64; bn = 77 (AM group); cn = 869; dn = 932 (control group).
AM, active malignancy; C, control group; NIHSS, National Institute of Health Stroke Scale; TIA, transient ischemic attack.
Comparisons of procedural characteristics revealed that, despite comparable door-to-needle times, the time from symptom onset to IVT was significantly longer in patients with active malignancy (Table 2).
Table 2.
Procedural characteristics of patients with AM (n = 79) and in the control group (C, n = 937).
| Procedural characteristics | AM | C | p-Value |
|---|---|---|---|
| Time (min) from symptom onset to IVT | 135.0 (79–185) a | 97.0 (69.5–144.0) d | 0.021 |
| Time (min) from admission to IVT | 31.5 (22.0–49.5) a | 30.0 (21.0–46.0) e | 0.537 |
| Time (min) from symptom onset to EVT | 160.0 (120–285) b | 156.5 (115.8–220.0) f | 0.457 |
| Time (min) from admission to EVT | 76.5 (36.5–99.2) c | 58.0 (31.0–79.7) g | 0.045 |
Data are median (IQR).
Data available in an = 42; bn = 27; cn = 38 (active tumor group); dn = 561; en = 611; fn = 238; gn = 368 (control group).
AM, active malignancy; EVT, endovascular therapy; IVT, intravenous thrombolysis.
Among patients with active malignancy, the three most common tumor entities were tumors of the respiratory tract (n = 22; 27.8%), gastrointestinal tract (n = 19; 24.1%), and urogenital tract (n = 13; 16.5%). The most common tumors of patients with a history of cancer were urogenital tumors (n = 24; 27.6%), gastrointestinal tumors (n = 23; 26.4%), and breast tumors (n = 14; 16.1%). In the active malignancy group, 10 patients had hematological tumors, while 13 of the group of patients with a history of cancer had hematological tumors.
Detailed profiles of patients’ tumor entities in our cohort are presented in Supplemental Table S2.
Safety outcomes in the active malignancy group and the control group
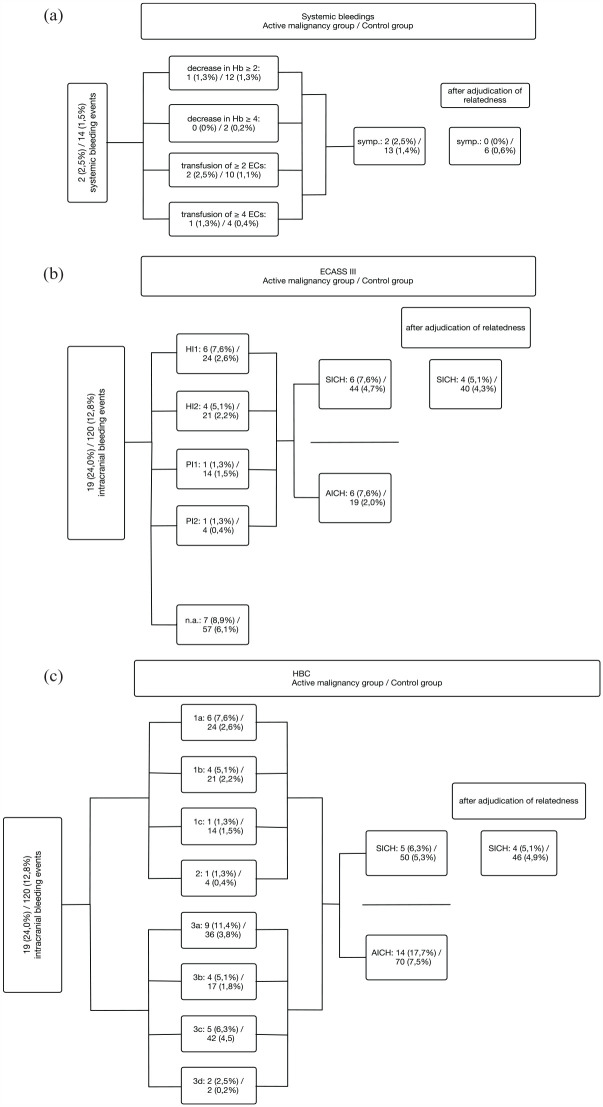
In the active malignancy group, stroke treatment-related major bleeding events occurred in four (5.1%) patients, of which all were SICHs and not tumor related. Systemic bleeding events occurred in two (2.6%) patients and were neither related to recanalization therapy [Figure 3(a) and Table 3] nor tumor related. One of them had active rectal cancer and suffered from gastral bleeding 3 days after endovascular stroke treatment under platelet inhibition and simultaneous anticoagulation with heparin. The indication for dual platelet inhibition in this patient was due to stenting for peripheral artery disease prior to stroke and a mechanical heart valve. Rectal cancer was resected 4 months prior to stroke and no source of bleeding was detected in the coloscopy. The other patient had an atrial myxoma which was diagnosed after an ischemic stroke with occlusion of the middle cerebral artery. 35 She also suffered from an acute Leriche syndrome and thus endovascular treatment was performed not only for the intracranial artery but also for the abdominal aorta. After restoration of the blood flow in the abdominal aorta, an acute compartment syndrome occurred and a fasciotomy of both lower legs and right upper leg was performed. Perioperative bleeding required a transfusion of 14 erythrocyte concentrates within 2 days.
Figure 3.
Systemic bleeding events (a) and classification of intracranial hemorrhages (b and c) according to ECASS III (b) and HBC (c) according to Neuberger et al. 29 First number refers to bleeding events in patients with active malignancy, while the latter indicates bleeding events in patients in the control group. The percentages in brackets indicate numbers in relation to the total number of the respective group (n = 79 for the active malignancy group and n = 937 for the control group). All bleeding events were adjudicated for their relatedness to stroke treatment. Type 1 (HBC): Hemorrhagic transformation of infarcted tissue; Type 1a (HBC) HI1 (hemorrhagic infarction) (ECASS III): Scattered small petechia, no mass effect; Type 1b (HBC)/HI2 (ECASS III): Confluent petechia, no mass effect; Type 1c (HBC)/PH1 (parenchymal hemorrhage) (ECASS III): Hematoma within infarcted tissue, occupying <30%, no substantive mass effect; Type 2 (HBC)/PH2 (ECASS III): Hematoma occupying >30% or more of the infarcted tissue, with obvious mass effect; Type 3 (HBC): Intracranial hemorrhage outside the infarcted brain tissue or intracranial-extracerebral hemorrhage; Type 3a (HBC): Parenchymal hematoma remote from infarcted brain tissue; Type 3b (HBC): Intraventricular hemorrhage; Type 3c (HBC): Subarachnoid hemorrhage; Type 3d (HBC): Subdural hemorrhage.
AICH, asymptomatic intracranial hemorrhage; ECASS, European Cooperative Acute Stroke Study; HBC, Heidelberg Bleeding Classification; SICH, symptomatic intracranial hemorrhage.
Table 3.
Outcome.
| Outcome | AM | C | OR (95% CI) |
|---|---|---|---|
| Primary safety endpoint | |||
| Major bleeding event* | 4 (5.0) | 46 (4.9) | 1.03 (0.36–2.94) |
| Secondary safety endpoint | |||
| SICH_ECASS* | 4 (5.1) | 40 (4.3) | 1.67 (0.69–4.04) |
| SICH_HBC* | 4 (5.1) | 46 (4.9) | 1.20 (0.46–3.10) |
| Systemic bleeding* | 0 (0) | 6 (0.6) | n.a. |
| Mortality | 25 (31.6) | 153 (16.3) | 2.37 (1.43–3.93) |
| Prolonged length of hospital stay | 8 (10.1) | 33 (3.5) | 3.09 (1.37–6.93) |
| Primary efficacy endpoint | |||
| NI 20% or more | 42 (53.2) | 582 (62.1) | 0.69 (0.44–1.10) |
| NI 30% or more | 40 (50.6) | 539 (57.5) | 0.76 (0.48–1.20) |
| Secondary efficacy endpoint | |||
| NIHSS decrease 4 or more | 30 (38.0) | 375 (40.0) | 0.92 (0.57–1.47) |
| NIHSS decrease 8 or more | 17 (21.5) | 192 (20.5) | 1.06 (0.61–1.86) |
Data are n (%). AM: n = 79; C: n = 937. Logistic regression was performed to compare the occurrence of each endpoint in the active malignancy group with the control group. Results are presented as OR and 95% CI.
Stroke treatment related.
AM, active malignancy group; C, control group; CI, confidence interval; n.a., not applicable; NI, neurological improvement during hospital stay; NIHSS, National Institute of Health Stroke Scale; OR, odds ratio; SICH_ECASS, symptomatic intracranial hemorrhage according to ECASS III Classification; SICH_HBC, symptomatic intracranial hemorrhage according to Heidelberg Bleeding Classification.
In the control group, six (0.6%) patients suffered from stroke treatment-related systemic bleeding, of which four (0.4%) had groin bleeding after endovascular treatment. Three of these six patients had a history of cancer (hematological, gastrointestinal, and breast tumor, respectively).
ICHs were detected in 139 (13.7%) of 1016 patients, of which 75 (7.4%) were categorized into different hemorrhage types according to ECASS III Classification [Figure 3(b)]. The remaining 64 (6.3%) ICHs were remote, intraventricular, subarachnoid, or subdural hemorrhages and were further differentiated by applying Heidelberg Bleeding Classification [Figure 3(c)]. In a detailed analysis of the bleeding events in relation to performed recanalization therapy, patients with active malignancy who underwent IVT only or IVT in combination with EVT suffered significantly more often from stroke treatment-related SICHs than the control group (both p < 0.001), while no stroke treatment-related SICH was observed in active malignancy patients after EVT only. Stroke treatment-related systemic bleeding events occurred significantly more often in the control group after combined stroke treatment compared to the active malignancy group (p = 0.003), whereas this was not significant in patients after IVT or EVT only (p = 0.637 and p = 0.48 for IVT and EVT, respectively) (Table 4).
Table 4.
Subgroup analysis of different intervention strategies in n = 1016 patients.
| Treatment | IVT | IVT + EVT | EVT | |||
|---|---|---|---|---|---|---|
| Group | AM | C | AM | C | AM | C |
| Patients (n) | 38 | 534 | 22 | 259 | 19 | 144 |
| Systemic bleeding | 0 (0.0) | 4 (0.7) | 1 (4.5) | 6 (2.3) | 1 (5.3) | 4 (2.8) |
| STR systemic bleeding | 0 (0.0) | 1 (0.2) | 0 (0.0) | 4 (1.5) | 0 (0.0) | 1 (0.7) |
| SICH | 2 (5.3) | 15 (2.8) | 3 (13.6) | 18 (6.9) | 1 (5.3) | 11 (7.6) |
| STR SICH | 2 (5.3) | 15 (2.8) | 2 (9.0) | 16 (6.2) | 0 (0.0) | 9 (6.3) |
| ICH | 7 (18.4) | 52 (9.7) | 8 (36.4) | 45 (17.4) | 4 (21.0) | 23 (16.0) |
| STR ICH | 7 (18.4) | 50 (9.3) | 7 (31.8) | 41 (15.8) | 1 (5.3) | 17 (11.8) |
Data are presented in n and n (%) in relation to each subgroup, respectively.
AM, active malignancy group; C, control group; EVT, endovascular therapy; ICH, intracranial hemorrhage; IVT, intravenous thrombolysis; SICH, symptomatic intracranial hemorrhage; STR, stroke treatment related.
Overall, stroke treatment-related SICHs occurred slightly more often in patients with active malignancy compared to the control group (5.1% versus 4.3%; unadjusted OR: 1.67; 95% CI: 0.69–4.04). However, this effect was not significant, and decreased after adjustment for established risk factors associated with tumor diagnosis, as presented in Supplemental Table 3. Logistic regression analysis of secondary safety outcomes further revealed a slightly increased probability for mortality and length of hospital stay of 30 days or more for patients with active malignancy compared to the control group (Table 3).
Safety outcome analysis according to tumor entity
Of all cancer patients, only two patients with inactive hematological tumors suffered from a bleeding event related to stroke treatment. One had groin bleeding after EVT, and one had SICH after thrombolysis.
Two patients with brain metastases received acute treatment for their AIS, both from an active tumor of the respiratory tract. One of them received thrombolysis and the other one was EVT. Neither of them suffered from bleeding after acute stroke treatment. Furthermore, 23 stroke patients with hematological tumors underwent acute stroke therapy, of which only 2 patients with inactive hematological tumors suffered from bleeding events related to stroke treatment.
Predictors of intracranial bleeding complications after recanalization therapy
Adjustment of logistic regression for single covariates identified atrial fibrillation, LVO, NIHSS, and D-dimers at admission as confounding factors for SICH according to ECASS III Classification when added to the group effect for active malignancy (Supplemental Table 3). Point estimates of the effect of active malignancy were attenuated considerably after adjustment for these four factors. The same was observed for SICH according to the Heidelberger Bleeding Classification (Supplemental Table 4).
Efficacy outcomes in the active malignancy group and the control group
In the logistic regression analysis of efficacy outcomes, the absolute NIHSS decrease of four or eight points was similar between groups. In addition, NI of 20% or more as well as 30% or more, respectively, were not influenced by the presence of active malignancy (Table 3).
Discussion
We investigated the safety and efficacy of recanalization therapy for AIS in a large cohort of patients with active malignancy. In the analysis of major bleeding events, tumor- or stroke treatment-related systemic bleeding did not occur in patients with active malignancy after recanalization therapy, and only 0.4% of patients in the control group suffered mainly from groin bleeding after puncture for EVT, especially after prior administration of IVT. Commonly, access-site hematoma occurs in 2–10% and ranges from small hematoma to life-threatening bleeding. 36 Access through the femoral artery was chosen for the endovascular treatment in all patients of our cohort and other potential complications such as arterial perforation did not occur. Most importantly, after acute stroke treatment, tumor-related bleeding was not observed. The rate of SICHs was numerically higher among AIS patients with active malignancy, but this difference was not significant in logistic regression and the risk estimates diminished after adjustment for the major confounding factors. Certain established risk factors for SICHs, that is, atrial fibrillation, LVO, increased NIHSS, and D-dimers at admission were more prevalent in patients with active malignancy. Furthermore, patients with active malignancy had more often dual platelet inhibition as prior antithrombotic treatment which was also associated with a higher rate of coronary artery disease in this group. To date, there are controversial results on the risk of SICHs after acute stroke treatment in patients with active malignancy. Some report an increased risk of intracranial bleeding after recanalization therapy due to mechanisms such as disseminated intravascular coagulation or compromised synthesis of coagulation factors in the liver in case of primary liver tumor or metastases.22,37–39 Especially hematological tumors are believed to be associated with a higher risk of SICHs due to low platelet count or platelet dysfunction. 40 However, this has been questioned by several other research groups, and the relatedness of the SICHs to stroke treatment in AIS patients with tumors is still not clear.19,24,41,42 One of the advantages of our study is the implementation of both, SICH according to ECASS III Classification and Heidelberg Bleeding Classification for the endpoints, of which the latter is a modern classification including extra-axial hemorrhages and thus allows a more sensitive detection of SICHs.
In our study, patients with active malignancy were more likely to have a longer hospital stay as well as higher mortality compared to the control group. This can be attributed to higher stroke severity, represented by higher NIHSS at admission.19,20,28,43 Moreover, the initial condition of cancer patients was worse compared to the control group due to the tumor itself or for example, an aggressive chemotherapy. This is depicted by higher premorbid mRS in the group of patients with active malignancy, which inevitably leads to higher mRS at admission and discharge (Figure 2). Lower mRS at admission is regarded as an independent prognostic factor for the outcome after a stroke. 44 Further predictors for poor outcome due to disturbance of recanalization and increase of reperfusion injury are higher D-dimer levels indicating tumor-mediated hypercoagulability in advanced tumor stages, larger tumors, and/or metastases.22,23,45 Also a postponed anti-tumor therapy due to functional impairment after AIS, resulting in further tumor progression and thus increased mortality could lead to unfavorable outcomes in patients with active malignancy. 46 However, the comparison of NI after therapy did not show any significant differences between the active malignancy group and the control group.
Due to its non-randomized retrospective character, our study has inevitable limitations. Underlying selection bias might affect study quality, and except for CT scans 24 h after intervention, further images were not routinely obtained. As subscore values of the NIHSS were only available at admission and discharge for this analysis, the cumulative NIHSS was used for the time points during a hospital stay. Thus, the definition of SICH according to Heidelberg Bleeding Classification could not be fully adopted in our study. Moreover, longitudinal follow-ups such as 90-day mRS are missing since our study is limited to the period of hospital stay. However, to our knowledge, previous retrospective studies included only a small number of patients and prospectively conducted randomized controlled trials lack on this topic and no explicit statement exists so far. The proportion of patients receiving acute stroke treatment in our study is higher than in primary stroke centers since our analysis is from a comprehensive stroke center with approximately 30% of patients referred from other hospitals for acute stroke treatment. Although active malignancy is not an established contraindication for systemic thrombolysis and/or endovascular treatment in acute stroke patients, many physicians still hesitate deciding on acute stroke treatment. This is mirrored in longer door-to-needle- and door-to-groin-time in the active malignancy group in our study.
Thus, the analysis of our large cohort from a comprehensive stroke center located in the university hospital of Germany’s largest cancer center is an important contribution to the improvement of clinical decision-making in the treatment of AISs in cancer patients.
Conclusion
In conclusion, recanalization therapy, that is, IVT and EVT, is not associated with a higher risk of systemic bleeding and SICHs in AIS patients with active malignancy and can be regarded as a safe therapy option. The unfavorable outcome of these patients can rather be explained by comorbidities, prior dependency as well as tumor activity itself. The rate of neurological improvement after therapy was comparable between groups.
Supplemental Material
Supplemental material, sj-docx-1-tan-10.1177_17562864231207508 for Treatment of acute ischemic stroke in patients with active malignancy: insight from a comprehensive stroke center by Woon Hyung Chae, Annika Vössing, Yan Li, Cornelius Deuschl, Lennart Steffen Milles, Jordi Kühne Escolà, Anika Hüsing, Marvin Darkwah Oppong, Philipp Dammann, Martin Glas, Michael Forsting, Christoph Kleinschnitz, Martin Köhrmann and Benedikt Frank in Therapeutic Advances in Neurological Disorders
Acknowledgments
None.
Footnotes
ORCID iDs: Woon Hyung Chae  https://orcid.org/0000-0003-1338-7278
https://orcid.org/0000-0003-1338-7278
Jordi Kühne Escolà  https://orcid.org/0000-0002-8742-0694
https://orcid.org/0000-0002-8742-0694
Marvin Darkwah Oppong  https://orcid.org/0000-0003-1021-5024
https://orcid.org/0000-0003-1021-5024
Benedikt Frank  https://orcid.org/0000-0001-8837-9489
https://orcid.org/0000-0001-8837-9489
Supplemental material: Supplemental material for this article is available online.
Contributor Information
Woon Hyung Chae, Department of Neurology and Center for Translational Neuro- and Behavioral Sciences, University Hospital Essen, Essen, Germany.
Annika Vössing, Department of Neurology and Center for Translational Neuro- and Behavioral Sciences, University Hospital Essen, Essen, Germany.
Yan Li, Institute for Diagnostic and Interventional Radiology and Neuroradiology, University Hospital Essen, Essen, Germany.
Cornelius Deuschl, Institute for Diagnostic and Interventional Radiology and Neuroradiology, University Hospital Essen, Essen, Germany.
Lennart Steffen Milles, Department of Neurology and Center for Translational Neuro- and Behavioral Sciences, University Hospital Essen, Essen, Germany.
Jordi Kühne Escolà, Department of Neurology and Center for Translational Neuro- and Behavioral Sciences, University Hospital Essen, Essen, Germany.
Anika Hüsing, Institute of Medical Informatics, Biometry and Epidemiology, University Hospital Essen, Essen, Germany.
Marvin Darkwah Oppong, Department of Neurosurgery and Spine Surgery and Center for Translational Neuro- and Behavioral Sciences, University Hospital Essen, Essen, Germany.
Philipp Dammann, Department of Neurosurgery and Spine Surgery and Center for Translational Neuro- and Behavioral Sciences, University Hospital Essen, Essen, Germany.
Martin Glas, Department of Neurology and Center for Translational Neuro- and Behavioral Sciences, University Hospital Essen, Essen, Germany.
Michael Forsting, Institute for Diagnostic and Interventional Radiology and Neuroradiology, University Hospital Essen, Essen, Germany.
Christoph Kleinschnitz, Department of Neurology and Center for Translational Neuro- and Behavioral Sciences, University Hospital Essen, Essen, Germany.
Martin Köhrmann, Department of Neurology and Center for Translational Neuro- and Behavioral Sciences, University Hospital Essen, Essen, Germany.
Benedikt Frank, Department of Neurology, University Hospital Essen, Hufelandstraße 55, Essen 45147, Germany.
Declarations
Ethics approval and consent to participate: The study protocol was reviewed and approved by the ethics committee of the medical faculty of the University Duisburg-Essen (approval number 20-9517-BO) and the data protection authority of the University Hospital Essen. The study was performed in accordance with the principles of the Helsinki Declaration and its later amendments or comparable ethical standards. The need for written informed consent for participation was waived by the ethics committee given its retrospective nature.
Consent for publication: The need for written informed patient consent for publication of these anonymized data was waived by the ethics committee.
Author contributions: Woon Hyung Chae: Conceptualization; Data curation; Formal analysis; Investigation; Methodology; Software; Validation; Visualization; Writing – original draft.
Annika Vössing: Formal analysis; Validation; Writing – review & editing.
Yan Li: Formal analysis; Validation; Writing – review & editing.
Cornelius Deuschl: Investigation; Validation; Writing – review & editing.
Lennart Steffen Milles: Formal analysis; Validation; Writing – review & editing.
Jordi Kühne Escolà: Formal analysis; Validation; Writing – review & editing.
Anika Hüsing: Formal analysis; Writing – review & editing.
Marvin Darkwah Oppong: Investigation; Writing – review & editing.
Philipp Dammann: Investigation; Writing – review & editing.
Martin Glas: Investigation; Writing – review & editing.
Michael Forsting: Investigation; Validation; Writing – review & editing.
Christoph Kleinschnitz: Investigation; Writing – review & editing.
Martin Köhrmann: Conceptualization; Supervision; Validation; Writing – review & editing.
Benedikt Frank: Conceptualization; Data curation; Formal analysis; Investigation; Methodology; Project administration; Supervision; Validation; Visualization; Writing – original draft; Writing – review & editing.
Funding: The authors disclosed receipt of the following financial support for the research, authorship, and/or publication of this article: Author WHC was supported as a Junior Clinician Scientist within the University Medicine Essen Academy (UMEA) program, funded by the IFORES program (Interne Forschungsförderung Essen) and the Faculty of Medicine, University of Duisburg-Essen.
The authors declare that there is no conflict of interest.
Availability of data and materials: Not applicable.
References
- 1. Dearborn JL, Urrutia VC, Zeiler SR. Stroke and cancer: a complicated relationship. J Neurol Transl Neurosci 2014; 2: 1039. [PMC free article] [PubMed] [Google Scholar]
- 2. Sorigue M, Miljkovic MD. Atrial fibrillation and stroke risk in patients with cancer: a primer for oncologists. J Oncol Pract 2019; 15: 641–650. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Grisold W, Oberndorfer S, Struhal W. Stroke and cancer: a review. Acta Neurol Scand 2009; 119: 1–16. [DOI] [PubMed] [Google Scholar]
- 4. Selvik HA, Bjerkreim AT, Thomassen L, et al. When to screen ischaemic stroke patients for cancer. Cerebrovasc Dis 2018; 45: 42–47. [DOI] [PubMed] [Google Scholar]
- 5. Salazar-Camelo RA, Moreno-Vargas EA, Cardona AF, et al. Ischemic stroke: a paradoxical manifestation of cancer. Crit Rev Oncol Hematol 2021; 157:103181. [DOI] [PubMed] [Google Scholar]
- 6. Uemura J, Kimura K, Sibazaki K, et al. Acute stroke patients have occult malignancy more often than expected. Eur Neurol 2010; 64: 140–144. [DOI] [PubMed] [Google Scholar]
- 7. Kim SJ, Park JH, Lee MJ, et al. Clues to occult cancer in patients with ischemic stroke. PLoS One 2012; 7(9): e44959. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Dardiotis E, Aloizou AM, Markoula S, et al. Cancer-associated stroke: Pathophysiology, detection and management (Review). Int J Oncol 2019; 54: 779–796. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Nam KW, Kim CK, Kim TJ, et al. Intravenous thrombolysis in acute ischemic stroke with active cancer. Biomed Res Int 2017; 2017: 4635829. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Zaorsky NG, Zhang Y, Tchelebi LT, et al. Stroke among cancer patients. Nat Commun 2019; 10: 5172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Berge E, Whiteley W, Audebert H, et al. European Stroke Organisation (ESO) guidelines on intravenous thrombolysis for acute ischaemic stroke. Eur Stroke J 2021; 6: I–LXII. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Powers WJ, Rabinstein AA, Ackerson T, et al. 2018 guidelines for the early management of patients with acute ischemic stroke: a guideline for healthcare professionals from the American Heart Association/American Stroke Association. Stroke 2018; 49: e46–e110. [DOI] [PubMed] [Google Scholar]
- 13. National Institute of Neurological Disorders and Stroke rt-PA Stroke Study Group. Tissue plasminogen activator for acute ischemic stroke. New Engl J Med 1995; 333: 1581–1588. [DOI] [PubMed] [Google Scholar]
- 14. Hacke W, Kaste M, Bluhmki E, et al. ; ECASS Investigators. Thrombolysis with alteplase 3 to 4.5 hours after acute ischemic stroke. New Engl J Med 2008; 359: 1317–1329. [DOI] [PubMed] [Google Scholar]
- 15. Powers WJ, Rabinstein AA, Ackerson TT, et al. Guidelines for the early management of patients with acute ischemic stroke: 2019 update to the 2018 guidelines for the early management of acute ischemic stroke: a guideline for healthcare professionals from the American Heart Association/American Stroke Association. Stroke 2019; 50: e344–e418. [DOI] [PubMed] [Google Scholar]
- 16. Etgen T, Steinich I, Gsottschneider L. Thrombolysis for ischemic stroke in patients with brain tumors. J Stroke Cerebrovasc Dis 2014; 23: 361–366. [DOI] [PubMed] [Google Scholar]
- 17. Campbell BC, Mitchell PJ, Kleinig TJ, et al. Endovascular therapy for ischemic stroke with perfusion-imaging selection. New Engl J Med 2015; 372: 1009–1018. [DOI] [PubMed] [Google Scholar]
- 18. Saver JL, Goyal M, Bonafe A, et al. SWIFT PRIME Investigators. Stent-retriever thrombectomy after intravenous t-PA vs. t-PA alone in stroke. New Engl J Med 2015; 372: 2285–2295. [DOI] [PubMed] [Google Scholar]
- 19. Masrur S, Abdullah AR, Smith EE, et al. Risk of thrombolytic therapy for acute ischemic stroke in patients with current malignancy. J Stroke Cerebrovasc Dis 2011; 20: 124–130. [DOI] [PubMed] [Google Scholar]
- 20. Ciolli L, Bigliardi G, Ferraro D, et al. Efficacy of mechanical thrombectomy in patients with ischemic stroke and cancer. J Clin Neurosci 2021; 91: 20–22. [DOI] [PubMed] [Google Scholar]
- 21. Lee D, Lee DH, Suh DC, et al. Intra-arterial thrombectomy for acute ischaemic stroke patients with active cancer. J Neurol 2019; 266: 2286–2293. [DOI] [PubMed] [Google Scholar]
- 22. Lee EJ, Bae J, Jeong HB, et al. Effectiveness of mechanical thrombectomy in cancer-related stroke and associated factors with unfavorable outcome. BMC Neurol 2021; 21: 57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Nam KW, Kim CK, Kim TJ, et al. D-dimer as a predictor of early neurologic deterioration in cryptogenic stroke with active cancer. Eur J Neurol 2017; 24: 205–211. [DOI] [PubMed] [Google Scholar]
- 24. Selvik HA, Naess H, Kvistad CE. Intravenous thrombolysis in ischemic stroke patients with active cancer. Front Neurol 2018; 9: 811. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Aloizou AM, Palaiodimou L, Aloizou D, et al. Acute reperfusion treatment and secondary prevention of cancer-related stroke: comprehensive overview and proposal of clinical algorithm. Ther Adv Neurol Disord 2023; 16: 17562864231180717. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Tsivgoulis G, Katsanos AH, Schellinger PD, et al. Successful reperfusion with intravenous thrombolysis preceding mechanical thrombectomy in large-vessel occlusions. Stroke 2018; 49: 232–235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Katsanos AH, Malhotra K, Goyal N, et al. Intravenous thrombolysis prior to mechanical thrombectomy in large vessel occlusions. Ann Neurol 2019; 86: 395–406. [DOI] [PubMed] [Google Scholar]
- 28. Guo YJ, Chang MH, Chen PL, et al. Predictive value of plasma (D)-dimer levels for cancer-related stroke: a 3-year retrospective study. J Stroke Cerebrovasc Dis 2014; 23: e249–e254. [DOI] [PubMed] [Google Scholar]
- 29. Neuberger U, Möhlenbruch MA, Herweh C, et al. Classification of bleeding events: comparison of ECASS III (European Cooperative Acute Stroke Study) and the New Heidelberg Bleeding Classification. Stroke 2017; 48: 1983–1985. [DOI] [PubMed] [Google Scholar]
- 30. von Kummer R, Broderick JP, Campbell BC, et al. The Heidelberg Bleeding Classification: Classification of bleeding events after ischemic stroke and reperfusion therapy. Stroke 2015; 46: 2981–2986. [DOI] [PubMed] [Google Scholar]
- 31. Lin KH, Lin HJ, Yeh PS. Determinants of prolonged length of hospital stay in patients with severe acute ischemic stroke. J Clin Med 2022; 2022: 11(12): 3457. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Toth G, Ortega-Gutierrez S, Tsai JP, et al. The safety and feasibility of mechanical thrombectomy for mild acute ischemic stroke with large vessel occlusion. Neurosurgery 2020; 86: 802–807. [DOI] [PubMed] [Google Scholar]
- 33. Xirasagar S, Wu Y, Heidari K, et al. Does emergency medical services transportation mitigate post-stroke discharge disability? A prospective observational study. J Gen Intern Med 2020; 35: 3173–3180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Agarwal S, Scher E, Lord A, et al. Redefined measure of early neurological improvement shows treatment benefit of alteplase over placebo. Stroke 2020; 51: 1226–1230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Li Y, Nensa F, Theysohn J, et al. From acute cerebrovascular occlusion to critical limb ischemia: A multidisciplinary challenge in a patient with ruptured atrial papillary myxoma. J Vasc Interv Radiol 2021; 32: 771–773. [DOI] [PubMed] [Google Scholar]
- 36. Balami JS, White PM, McMeekin PJ, et al. Complications of endovascular treatment for acute ischemic stroke: prevention and management. Int J Stroke 2018; 13: 348–361. [DOI] [PubMed] [Google Scholar]
- 37. Weeda ER, Bohm N. Association between comorbid cancer and outcomes among admissions for acute ischemic stroke receiving systemic thrombolysis. Int J Stroke 2019; 14: 48–52. [DOI] [PubMed] [Google Scholar]
- 38. Levi M. Disseminated intravascular coagulation in cancer patients. Best Pract Res Clin Haematol 2009; 22: 129–136. [DOI] [PubMed] [Google Scholar]
- 39. Velander AJ, DeAngelis LM, Navi BB. Intracranial hemorrhage in patients with cancer. Curr Atheroscler Rep 2012; 14: 373–381. [DOI] [PubMed] [Google Scholar]
- 40. Chen CY, Tai CH, Cheng A, et al. Intracranial hemorrhage in adult patients with hematological malignancies. BMC Med 2012; 10: 97. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Murthy SB, Karanth S, Shah S, et al. Thrombolysis for acute ischemic stroke in patients with cancer: a population study. Stroke 2013; 44: 3573–3576. [DOI] [PubMed] [Google Scholar]
- 42. Casado-Naranjo I, Calle ML, Falcon A, et al. Intravenous thrombolysis for acute stroke in patients with cancer. J Neurol Neurosurg Psychiatry 2011; 82: 1404–1405. [DOI] [PubMed] [Google Scholar]
- 43. Schwarzbach CJ, Schaefer A, Ebert A, et al. Stroke and cancer: the importance of cancer-associated hypercoagulation as a possible stroke etiology. Stroke 2012; 43: 3029–3034. [DOI] [PubMed] [Google Scholar]
- 44. Seystahl K, Hug A, Weber SJ, et al. Cancer is associated with inferior outcome in patients with ischemic stroke. J Neurol 2021; 268: 4190–4202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Yao T, Tian BL, Li G, et al. Elevated plasma D-dimer levels are associated with short-term poor outcome in patients with acute ischemic stroke: a prospective, observational study. BMC Neurol 2019; 19: 175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Eun MY, Jeon ET, Seo KD, et al. Reperfusion therapy in acute ischemic stroke with active cancer: a meta-analysis aided by machine learning. J Stroke Cerebrovasc Dis 2021; 30: 105742. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplemental material, sj-docx-1-tan-10.1177_17562864231207508 for Treatment of acute ischemic stroke in patients with active malignancy: insight from a comprehensive stroke center by Woon Hyung Chae, Annika Vössing, Yan Li, Cornelius Deuschl, Lennart Steffen Milles, Jordi Kühne Escolà, Anika Hüsing, Marvin Darkwah Oppong, Philipp Dammann, Martin Glas, Michael Forsting, Christoph Kleinschnitz, Martin Köhrmann and Benedikt Frank in Therapeutic Advances in Neurological Disorders