Abstract
Secretory Breast Carcinoma (SBC) is a rare subtype of breast cancer, predominantly affecting young women, and characterized by hormone receptor-negative and HER2-negative tumors with distinctive histological features, including secretory droplets within tumor cells. This article presents 2 unique cases of SBC, Case 1 involving a 42-year-old woman with triple-negative mammary carcinoma later diagnosed with triple-negative secretory carcinoma, and Case 2 featuring a 48-year-old woman with poorly differentiated adenocarcinoma subsequently identified as invasive mammary carcinoma of secretory type. Both cases received diverse treatment regimens, incorporating surgery, chemotherapy, radiotherapy, and hormone therapy. The importance of accurate diagnosis and the need for further research to optimize the management of this rare breast cancer subtype are emphasized. Raising awareness of SBC and reporting additional cases can enhance understanding and improve patient outcomes. Additionally, the integration of clinical, radiological, and histopathological findings, alongside specific molecular markers like S-100 and mammaglobin, is crucial for accurate SBC diagnosis. Given the lack of established guidelines for SBC management, collecting additional cases can aid in defining a more effective strategy for diagnosis, monitoring, and treatment, ultimately contributing to advancements in the field.
Herein, we report 2 cases of this rare disease that were diagnosed and treated in our institution.
Keywords: SBC, carcinoma, breast
Introduction
Secretory breast carcinoma (SBC) is a rare subtype of breast cancer that was first described by McDivitt and Stewart in 1966. 1 The characteristic feature of SBC, namely the presence of abundant intracellular and extracellular secretory material, was later described by Tavassoli and Norris in 1980. 2 Since then, numerous case reports and small case series have been published describing the clinical, histological, and molecular characteristics of SBC. The World Health Organization (WHO) recognized SBC as a distinct histological subtype of breast carcinoma in 2003, 3 and subsequent studies have provided further insights into its epidemiology, management, and prognosis. Until now, fewer than 300 cases have been reported in the literature. It is typically diagnosed in young patients, with a reported age range of 3 to 86 years. 4 However, the age distribution of SBC shows 2 distinct peaks: one in adolescents, with a mean age of 17 years, and another in adults, with a mean age of 56 years.5,6 For instance, SBC in adolescents may present unique challenges due to the hormonal and developmental characteristics of this age group.
Surgical resection remains the cornerstone of therapy, the optimal adjuvant therapy for SBC has yet to be established. Chemotherapy has been utilized as a treatment modality in a variety of breast cancer subtypes, but its effectiveness in SBC has not been extensively studied.7,8 There has been increasing interest in the use of a nanotechnology-based approach to chemotherapy delivery that aims to target cancer cells more selectively, potentially reducing the toxicities associated with conventional chemotherapy. However, limited clinical data exist regarding the use of chemotherapy in SBC. Future studies investigating the efficacy and safety of chemotherapy and chemotherapy mediated by nanotechnology, in SBC are warranted to guide treatment decisions in this rare malignancy.
The ETV6-NTRK3 gene fusion is a rare genetic alteration that has been identified in several cancer types, including secretory breast carcinoma (SBC).8,9 The ETV6-NTRK3 fusion protein can activate the MAPK and PI3K-Akt signaling pathways, which are involved in cell proliferation, survival, and immune evasion, among other processes. 10 Thus, targeting these pathways using ITKs or immune checkpoint inhibitors may be an effective treatment strategy for patients with ETV6-NTRK3 gene fusion-positive cancers. However, further research is needed to validate the clinical utility of this biomarker and to identify the optimal treatment approach for this patient population. 11
SBC is a low-grade tumor that typically has a good prognosis, with a 5-year survival rate of over 95%.12 -14 However, there have been a few reported cases of aggressive behavior, including metastases and death. 13 The evolution and overall survival of patients with SBC were reported in a retrospective study conducted on 83 patients. 15 In this study, the majority of patients underwent surgical treatment, with breast-conserving surgery being the most common approach. Adjuvant radiation therapy was administered in a majority of cases, while chemotherapy was used only in a few instances. The favorable prognosis of SBC, with a 5-year survival rate of 87.2% and 10-year OS was 76.5%, was largely attributed to its indolent nature, as it often presents as small, low-grade tumors with a low risk of metastasis. However, the presence of lymph node involvement, larger tumor size, and higher-grade tumors were associated with a poorer prognosis, highlighting the importance of careful monitoring and prompt treatment for SBC patients with high-risk features. 15
SBC is often misdiagnosed due to its rarity and resemblance to other types of breast cancer, such as invasive ductal carcinoma or lobular carcinoma. SBC is often estrogen receptor (ER) and progesterone receptor (PR), and HER2 negative. 16 The diagnosis of SBC is confirmed by histological examination of the tumor, which shows characteristic features such as intracellular and extracellular eosinophilic secretion and the presence of vacuoles within tumor cells. Due to its rarity and unique histological features, SBC can be difficult to diagnose accurately, and there is currently no standard treatment protocol for this type of cancer. 14 As a result, it is critical to investigate the clinicopathological features of these patients. Reporting and analyzing rare cases of SBC can provide insight into the biology of this disease, including the identification of potential biomarkers and therapeutic targets, and help improve diagnostic and treatment strategies. Collecting more cases of SBC is necessary to better define these strategies and further our understanding of this rare disease.
Herein, we report 2 rare cases of secretory breast carcinoma, emphasizing the clinical and histopathological features of this uncommon subtype. The article highlights the importance of accurate diagnosis, discusses treatment approaches and outcomes, and underscores the need for early detection and long-term surveillance in managing rare breast cancer subtypes like secretory carcinoma.
Presentation of Case 1
A 42-year-old unmarried woman presented with a palpable mass in the left breast’s upper outer quadrant (JQI) along with mastodynia. Notably, skin retraction and nipple discharge were absent. Breast ultrasound identified an ACR4c lesion, leading to the patient undergoing a wide excision procedure. The histopathological analysis revealed an invasive mammary carcinoma of not otherwise specified (NOS) type, Scarff-Bloom-Richardson (SBR) grade II, and displayed a triple-negative profile. Six months later, the patient experienced swelling in the same quadrant, prompting radical treatment. The subsequent histopathological examination revealed a triple-negative secretory carcinoma (Figure 2) with an estimated Ki67 of 5%. The tumor exhibited strong and diffuse expression of PS100 and MUC4, while showing weak expression of CD117 and ACE. Subsequently, the patient received adjuvant treatment, which involved sequential chemotherapy followed by radiotherapy (Table 1).
Figure 2.
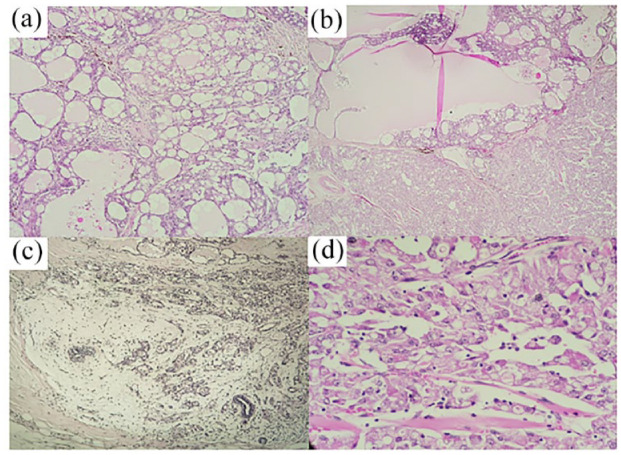
(a) Microscopic analysis displaying tumor proliferation exhibiting a microcystic architecture arranged on a sparse fibrous stroma, (b) papillary architecture observed in certain areas of the tumor proliferation, (c) presence of a mucinous component within the tumor, and (d) histological evaluation depicting medium-sized polygonal cells with vacuolated, granular, and eosinophilic cytoplasm. Minimal pleomorphism and low mitotic activity are observed.
Table 1.
Patients features.
| Cases | Age | Smoking history | Obesity | Educational level | Occupation | Stage | Treatment |
|---|---|---|---|---|---|---|---|
| Case 1. | 42 | Never | No | Limited | Homemaker | T1N0M0 | Chemotherapy Radiotherapy |
| Case 2. | 48 | Never | No | Limited | Housewife | T4N0M0 | Chemotherapy Radiotherapy |
Presentation of Case 2
A 48-year-old married woman and mother of 4, undergoing treatment for hypertension, reported a gradually enlarging nodule in her right breast 1 year ago. The presentation was accompanied by inflammatory skin signs, and a breast ultrasound revealed a tumoral process involving nearly the entire right breast (Figure 1). Microbiopsy results indicated a widely ulcerated, poorly differentiated adenocarcinoma with 6% estrogen receptor expression, no progesterone receptor expression, and non-amplified HER2. The estimated Ki67 proliferation index was 12%. As part of the treatment plan, the patient underwent neoadjuvant chemotherapy, followed by radical surgical treatment of the right breast. The subsequent histopathological examination identified an invasive mammary carcinoma of secretory type (Figure 2) measuring 6 cm in its largest dimension. The tumor was categorized as SBR grade I and demonstrated no therapeutic response, in situ carcinoma, vascular emboli, or nodal involvement. The dermis infiltration of the tumor with ulceration in its vicinity was classified as ypT4N0. Immunohistochemical analysis revealed the expression of CKAE1/AE3, Muc4, PS100, and vimentin (Figure 3). The patient received adjuvant treatment consisting of 15 sessions of external radiotherapy (42 Gy) and hormone therapy (Table 1). Presently, the patient is under surveillance, with no evidence of local or distant recurrence.
Figure 1.
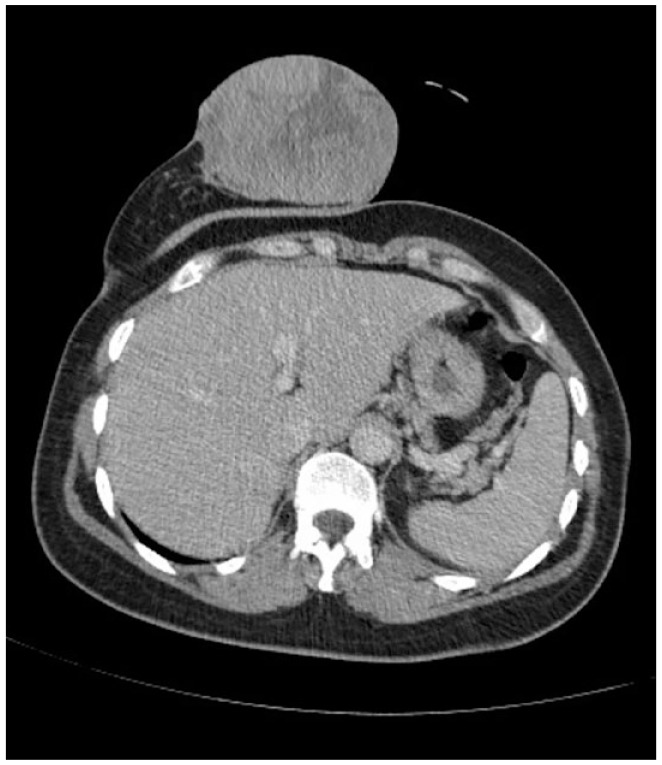
CT image illustrating a right breast mass measuring 10 × 7.6 cm, completely occupying the breast, with invasion of the skin.
Figure 3.
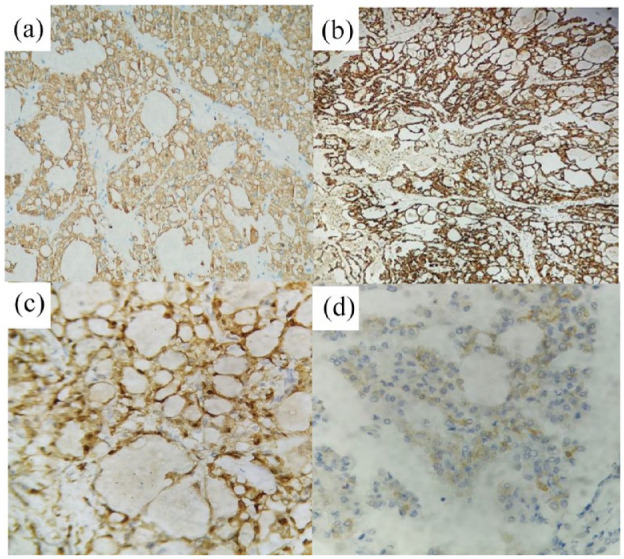
Immunohistochemical analysis demonstrating: (a) positive cytokeratin 8/18 staining, (b) positive Muc 4 staining, (c) positive PS100 and vimentin staining, and (d) positive CD17 staining.
The subjects remain under ongoing observation, and to date, there have been no indications of either local or distant recurrence. The most recent follow-up assessments were conducted 3 months ago for the 48-year-old female and 2 months ago for the 42-year-old female.
Discussion
The etiology of SBC is not well understood, and no specific risk factors have been identified. However, prior studies have explored associations between certain risk factors and breast cancer, including SBC. Obesity and smoking, known risk factors for breast cancer in general, have been studied, but their specific role in SBC requires further investigation due to its rarity and unique characteristics. Some cases of SBC have been reported to occur in patients with a history of radiation exposure or previous breast surgery, but these associations have not been consistently demonstrated.
The majority of patients typically present at an early stage with a relatively slow-moving clinical course. Lymph node metastasis is observed in approximately 30% of cases, while distant metastasis is not common.15,17 The histomorphological features of secretory breast carcinomas (SBCs) are quite distinct and can be easily distinguished from invasive ductal carcinomas. The characteristic features include a lobulated growth pattern, abundant eosinophilic secretory material, and uniform small cells with round nuclei and inconspicuous nucleoli. There are mainly 3 histological patterns observed, including solid, microcystic, and tubular. Tumor cells show generally minimal cellular atypia.8,16 The hormone receptor status of SBCs is also characteristic, with frequent negativity for ERBB2 (HER2/neu), progesterone receptor (PR), and estrogen receptor (ER). Therefore, SBCs often exhibit similar characteristics to triple-negative breast carcinoma (TNBC), such as the expression of c-Kit (CD117) and, cytokeratin 5/6, 14, 17, which are also present in basal-like breast carcinomas (BLBC). 12 TNBCs are typically classified as belonging to the basal-like breast cancer group, with around 75-80% of these tumors behaving more aggressively and leading to rapid vascular invasion. 18
Breast cancer transcriptomic analysis has identified unique genetic signatures for intrinsic molecular subtyping, with BLBC being one of these subtypes. BLBC is defined by the expression of genes found in the basally located epithelial layer of the mammary gland and uniformly lacks molecular targets for highly effective targeted therapy.19,20
Recent studies highlight the heterogeneity among the TNBC group, which encompasses tumors with varying prognoses. 21 Additionally, a rare SBC tumor has been noted to exhibit a characteristic genetic translocation t (12;15), resulting in the creation of a fusion protein ETV6-NTRK3. 22 This fusion product has been shown to activate the mitogen-activated protein kinase (MAPK) pathway and promote cell proliferation, contributing to the development of SBC. 13
According to a study, the 5- and 10-year disease-free survival rates for SBC were 94% and 91%, respectively. 23 However, cases of recurrence and metastasis have been reported, and while double primary tumors have been observed, they are exceedingly rare.24 -26 Currently, the molecular characteristics that underlie the biological behavior of SBC are not well understood, and no driver gene has been identified, except for the ETV6-NTRK3 fusion.8,25,27 Furthermore, due to a lack of clinical and genomic data, particularly for cases of double primary SBC, there is no established standard treatment regimen, and no relevant genome studies have been conducted. However, surgery, including lumpectomy or mastectomy, is typically the mainstay of treatment, and adjuvant therapy with chemotherapy or radiation may be considered based on the stage and grade of the tumor.14,24 Hormonal therapy has also been reported to be effective in some cases, particularly those that express estrogen or progesterone receptors.8,28
Despite the low-grade nature of SBC, some cases have been reported to have a more aggressive clinical course, with distant metastasis and poor outcomes. The role of adjuvant therapy in preventing these outcomes is not well established, and more research is needed to better understand the natural history of SBC and optimal treatment strategies.
One notable limitation of this study is the rarity of secretory breast carcinoma (SBC), which inherently leads to a paucity of reported cases. This scarcity of SBC cases poses challenges in achieving substantial sample sizes for comprehensive analyses and may impede statistical robustness in observed findings. Consequently, the limited availability of data hampers the ability to conduct large-scale clinical trials, leading to potential difficulties in establishing standardized treatment guidelines for this rare malignancy. Given the scarcity of cases and the lack of established therapeutic protocols, there is a need for further research and comprehensive reporting of additional cases to enhance our understanding of SBC’s clinical characteristics and refine optimal management strategies for affected patients.
In conclusion, SBC is a rare form of breast cancer that is characterized by unique histological features and a generally indolent clinical behavior. Despite its rarity, accurate diagnosis is important for appropriate management, and molecular studies have shed light on the underlying pathogenesis of this disease. Further research is needed to better understand the optimal treatment approach for SBC.
Footnotes
Funding: The author(s) received no financial support for the research, authorship, and/or publication of this article.
The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
References
- 1. McDivitt RW. Breast carcinoma in children. JAMA. 1966;195:388-390. [PubMed] [Google Scholar]
- 2. Tavassoli FA, Norris HJ. Secretory carcinoma of the breast. Cancer. 1980;45:2404-2413. [DOI] [PubMed] [Google Scholar]
- 3. Tavassoli FA, Devilee P. Pathology and Genetics of Tumours of the Breast and Female Genital Organs. Breast Cancer Research; 2003;6:3. [Google Scholar]
- 4. Lee SG, Jung SP, Lee HY, et al. Secretory breast carcinoma: A report of three cases and a review of the literature. Oncol Lett. 2014;8:683-686. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Kavalakat AJ, Covilakam RK, Culas TB. Secretory carcinoma of breast in a 17-year-old male. World J Surg Oncol. 2004;2:17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Jenkins S, Kachur ME, Rechache K, Wells JM, Lipkowitz S. Rare breast cancer subtypes. Curr Oncol Rep. 2021;23:54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Mittendorf EA, Buchholz TA, Tucker SL, et al. Impact of chemotherapy sequencing on local-regional failure risk in breast cancer patients undergoing breast-conserving therapy. Ann Surg. 2013;257:173-179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Laé M, Fréneaux P, Sastre-Garau X, et al. Secretory breast carcinomas with ETV6-NTRK3 fusion gene belong to the basal-like carcinoma spectrum. Mod Pathol. 2009;22:291-298. [DOI] [PubMed] [Google Scholar]
- 9. Manea CA, Badiu DC, Ploscaru IC, et al. A review of NTRK fusions in cancer. Ann Med Surg. 2022;79:103893. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Cocco E, Scaltriti M, Drilon A. NTRK fusion-positive cancers and TRK inhibitor therapy. Nat Rev Clin Oncol. 2018;15:731-747. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Drilon A. Efficacy of larotrectinib in TRK fusion-positive cancers in adults and children. New Engl J Med. 2018;378:731-739. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Vasudev P, Onuma K. Secretory breast carcinoma: unique, triple-negative carcinoma with a favorable prognosis and characteristic molecular expression. Arch Pathol Lab Med. 2011;135:1606-1610. [DOI] [PubMed] [Google Scholar]
- 13. Min N, Zhu J, Liu M, Li X. Advancement of secretory breast carcinoma: a narrative review. Ann Transl Med. 2022;10:1178-1178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Wu IK, Lai YC, Chiou HJ, Hsu CY. Secretory carcinoma of the breast: A case report and literature review. J Med Ultrasound. 2021;29:57-59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Horowitz DP, Sharma CS, Connolly E, Gidea-Addeo D, Deutsch I. Secretory carcinoma of the breast: results from the survival, epidemiology and end results database. Breast. 2012;21:350-353. [DOI] [PubMed] [Google Scholar]
- 16. Banerjee N, Banerjee D, Choudhary N. Secretory carcinoma of the breast, commonly exhibits the features of low grade, triple negative breast carcinoma- A case report with updated review of literature. Autops Case Rep. 2021;11:e2020227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Li D, Xiao X, Yang W, et al. Secretory breast carcinoma: a clinicopathological and immunophenotypic study of 15 cases with a review of the literature. Mod Pathol. 2012;25:567-575. [DOI] [PubMed] [Google Scholar]
- 18. Magdalena Koziol MPAZ. PARP inhibitors and their role in the therapy of triple-negative metastatic breast cancer. Prz Lek. 2012;69:265-270. [PubMed] [Google Scholar]
- 19. Yehiely F, Moyano JV, Evans JR, Nielsen TO, Cryns VL. Deconstructing the molecular portrait of basal-like breast cancer. Trends Mol Med. 2006;12:537-544. [DOI] [PubMed] [Google Scholar]
- 20. Perou CM, Sørlie T, Eisen MB, et al. Molecular portraits of human breast tumours. Nature. 2000;406:747-752. [DOI] [PubMed] [Google Scholar]
- 21. Pareja F, Geyer FC, Marchiò C, et al. Triple-negative breast cancer: the importance of molecular and histologic subtyping, and recognition of low-grade variants. NPJ Breast Cancer. 2016;2:16036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Tognon C, Knezevich SR, Huntsman D, et al. Expression of the ETV6-NTRK3 gene fusion as a primary event in human secretory breast carcinoma. Cancer Cell. 2002;2:367-376. [DOI] [PubMed] [Google Scholar]
- 23. Lei T, Deng X, Peng Y, Chen T. The genomic profile of double primary secretory breast carcinoma in one patient provides evidence for the treatment of such carcinoma: A case report. Pathol Res Pract. 2022;236:154006. [DOI] [PubMed] [Google Scholar]
- 24. Tang H, Zhong L, Jiang H, et al. Secretory carcinoma of the breast with multiple distant metastases in the brain and unfavorable prognosis: a case report and literature review. Diagn Pathol. 2021;16:56-57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Del Castillo M, Chibon F, Arnould L, et al. Secretory breast carcinoma: A histopathologic and genomic spectrum characterized by a joint specific ETV6-NTRK3 gene fusion. Am J Surg Pathol. 2015;39:1458-1467. [DOI] [PubMed] [Google Scholar]
- 26. Hoda RS, Brogi E, Pareja F, et al. Secretory carcinoma of the breast: clinicopathologic profile of 14 cases emphasising distant metastatic potential. Histopathology. 2019;75:213-224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Krings G, Joseph NM, Bean GR, et al. Genomic profiling of breast secretory carcinomas reveals distinct genetics from other breast cancers and similarity to mammary analog secretory carcinomas. Mod Pathol. 2017;30:1086-1099. [DOI] [PubMed] [Google Scholar]
- 28. Jacob JD, Hodge C, Franko J, et al. Rare breast cancer: 246 invasive secretory carcinomas from the National Cancer Data Base. J Surg Oncol. 2016;113:721-725. [DOI] [PubMed] [Google Scholar]


