Abstract
Background:
We previously published a retrospective study of kidney biopsies performed in a tertiary care hospital in London, Ontario from 2012 to 2017. This study resulted in a change of practice in our institution to shorter postbiopsy monitoring for outpatients as well as the development of a risk calculator to predict serious bleeding complications.
Objective:
The primary objective of this study was to determine whether this shorter monitoring time is adequate in the outpatient setting. A secondary objective was to validate the bleeding risk calculator in both inpatients and outpatients.
Design:
This was a retrospective chart review.
Setting:
This study was performed at a tertiary academic hospital in London, Ontario, Canada.
Participants:
This was a retrospective study of 400 adult patients who underwent kidney biopsy between April 30, 2018 and February 25, 2022 at a tertiary academic hospital in London, Canada.
Methods:
We retrospectively assessed frequency and timing of major bleeding complications in patients who underwent kidney biopsy. In secondary analyses, we examined the prediction performance of the risk calculator in discrimination and calibration.
Results:
Major bleeding occurred in 7 patients (1.8%). Five of these patients required blood transfusions (1.3%) and 2 required embolization (0.5%). In the outpatient setting, any major bleeding events were identified immediately (1 patient) or on the routine 2-hour ultrasounds (1 patient). The risk calculator showed good discrimination (C-statistic, 0.91, 95% confidence interval [CI] = [0.84 to 0.95]) and calibration (slope, 1.10, 95% CI = [0.47 to 1.74]; intercept, 95% CI = −0.02 [−0.79 to 0.75]), but with much uncertainty in the estimates.
Limitations:
The occurrence of only a few major bleeding events limits the reliability of our assessment of our risk calculator.
Conclusions:
There appears to be little yield in extending observation beyond 2 hours after an outpatient kidney biopsy with the use of immediate and 2-hour postbiopsy ultrasounds. The bleeding risk calculator (http://perioperativerisk.com/kbrc) warrants further validation.
Keywords: percutaneous kidney biopsy, complications, bleeding, risk prediction, native kidney, transplant kidney
Abrégé
Contexte:
Nous avons publié précédemment une étude rétrospective des biopsies rénales effectuées entre 2012 et 2017 dans un hôpital de soins tertiaires de London, en Ontario. Les résultats de cette précédente étude ont entraîné un changement de pratique dans notre établissement, soit une réduction de la durée de la surveillance post-biopsie pour les patients ambulatoires, et la mise au point d’un calculateur de risque permettant de prédire les complications hémorragiques graves.
Objectifs:
L’objectif principal de l’étude en cours était de vérifier si ce temps de surveillance plus court est adéquat pour les patients ambulatoires. Un deuxième objectif était de valider le calculateur de risque d’hémorragie chez les patients hospitalisés et les patients ambulatoires.
Conception:
Étude rétrospective des dossiers médicaux.
Cadre:
Étude réalisée dans un hôpital de soins tertiaires de London, en Ontario (Canada).
Sujets:
Cette étude rétrospective portait sur 400 patients adultes ayant subi une biopsie rénale entre le 30 avril 2018 et le 25 février 2022 dans un centre hospitalier universitaire de soins tertiaires de London, au Canada.
Méthodes:
Nous avons procédé à un examen rétrospectif de la fréquence des complications hémorragiques graves, et du moment où celles-ci surviennent, chez les patients ayant subi une biopsie rénale. Dans les analyses secondaires, nous avons examiné la puissance prédictive du calculateur de risque en matière de discrimination et d’étalonnage.
Résultats:
Sept patients (1,75 %) ont subi une hémorragie majeure; de ces patients, cinq ont eu besoin de transfusions sanguines (1,3 %) et deux, d’une embolisation (0,5 %). En contexte ambulatoire, tous les événements hémorragiques graves ont été détectés immédiatement (un patient) ou lors de l’échographie de routine à deux heures (un patient). Le calculateur de risque a montré une bonne discrimination (statistique C : 0,91 [IC 95 % : 0,84 à 0,95]) et un bon étalonnage (pente : 1,10 [0,47 à 1,74]; point d’intersection : -0,02 [-0,79 à 0,75]), mais une grande incertitude dans les estimations.
Limitations:
La fiabilité de l’évaluation de notre calculateur de risque est limitée par le très faible échantillon d’événements hémorragiques graves étant survenus.
Conclusion:
Il semble y avoir peu d’intérêt à prolonger la surveillance au-delà de deux heures après une biopsie rénale chez les patients ambulatoires lorsqu’une échographie est pratiquée immédiatement après la procédure et deux heures plus tard. Le calculateur de risque d’hémorragie (http://perioperativerisk.com/kbrc) nécessite une validation plus approfondie.
Statement of Significance
What was known before
Ultrasound-guided percutaneous kidney biopsy is generally a safe procedure with serious adverse events occurring rarely. When performed in the outpatient setting, optimal monitoring time and protocols are not well established.
What this adds
This follow-up study confirms the safety of a 2-hour monitoring window with repeat ultrasound for outpatient kidney biopsies.
Introduction
In 2020, we published a retrospective study of 617 ultrasound-guided percutaneous kidney biopsies performed between 2012 and 2017 on adult patients at a tertiary care center in London, Ontario. 1 This review demonstrated complications consistent with the literature with major bleeding events requiring intervention occurring in 1.9% of patients. Most bleeding events were detected immediately and because of this finding, our center changed our outpatient kidney biopsy protocol from a 5-hour postprocedure monitoring period to a 2-hour postprocedure monitoring period with repeat ultrasound.
As this change in protocol, another 400 kidney biopsies have been performed at our center. In this second study, our aim was to determine if a 2-hour period was sufficient for observation of outpatients before discharge. Secondarily, we assessed the performance of our previously developed risk calculator with respect to prediction of major bleeding complications (accessible at http://perioperativerisk.com/kbrc). The risk calculator uses age, weight and height (to calculate body mass index), serum platelet concentration, serum hemoglobin concentration, the size of the kidney on ultrasound in greatest dimension, and identification of the kidney as a native kidney or an allograft.
Methods
Reporting and Ethics
We have reported this study according to the Transparent Reporting of a multivariable prediction model for Prognosis or Diagnosis (TRIPOD) statement. 2 This study met criteria for a waiver of review by the Research Ethics Board at Western University, London, Ontario, Canada, as a quality improvement initiative.
Study Design, Source of Data, and Participants
This was a retrospective chart review of all percutaneous kidney biopsies performed on adult patients between 2018 and 2022 at London Health Sciences Centre, University Hospital. As in our previous study, each biopsy (regardless of whether performed in the same patient) was included as a separate event. Demographic data and risk factors including those used for our risk calculator were defined a priori and are summarized in Table 1 (Patient Characteristics). Clinical and laboratory data were collected without blinding to patients’ complication status.
Table 1.
Patient Characteristics.
| N (% of total) or median (IQR) | |
|---|---|
| Age, y, median (IQR) | 56 (42-66) |
| Female | 161 (40.2%) |
| Male | 239 (59.8%) |
| Platelet count before biopsy, ×109/L, median (IQR) | 221 (166-282) a |
| Hemoglobin before biopsy, g/L, median (IQR) | 107 (87-124) b |
| Size of biopsied kidney, cm, median (IQR) | 11.65 (10.8-12.5) |
| Body mass index, kg/m2, median (IQR) | 28.7 (25.2-32.8) c |
| Serum urea, mmol/L, median (IQR) | 12.85 (9.0-21.6) d |
| Serum creatinine, µmol/L, median (IQR) | 213 (147-358) e |
| Native (vs allograft) kidney biopsy | 142 (35.5%) |
| Inpatient (vs outpatient) kidney biopsy | 197 (49.3%) |
Note. Missing data as below. IQR = interquartile range.
Missing data for 3 patients (0.75%).
Missing data for 3 patients (0.75%).
Missing data for 6 patients (1.5%).
Missing data for 42 patients (10.5%).
Missing data for 1 patient (0.25%).
Biopsy Procedures and Peri-Procedure Management
The standard practices for kidney biopsies performed at our center are detailed in our previous paper and are all performed or supervised by experienced nephrologists. 1 In our center, all biopsies are performed for clinical reasons, under ultrasound guidance, and we use the BARD monopty 18-gauge, 16 cm Disposable Core Biopsy Instrument (C.R. Bard, Inc, Tempe, Arizona). This spring-loaded biopsy instrument provides a 22 mm core of tissue. However, it is now standard of care for patients undergoing outpatient biopsies to be monitored for 2 hours and to undergo a repeat postprocedure kidney ultrasound at 2 hours. If this ultrasound indicates an active bleed or expanding hematoma, the patient is admitted to the hospital for further monitoring and management. When the patient is discharged home after the 2-hour repeat scan, they are advised to return to the Emergency Department if they felt unwell, lightheaded, had chest pain, shortness of breath, or a fever, if they had increasing pain at the biopsy site, or difficulty or pain with urination.
Outcomes
Electronic medical records were reviewed to identify complications occurring up to 1 week postbiopsy procedure. In our previous study, we included data on minor events which we defined as perinephric hematoma, gross hematuria, or bleeding (a drop in hemoglobin <10 g/L from baseline without evidence of ongoing bleeding on repeat imaging) that did not require blood transfusion, embolization, or nephrectomy. In this follow-up study, we have focused only on major complications defined by bleeding events requiring transfusion, surgical intervention, or embolization of the bleeding vessel.
Patient Characteristics
Based on our previous study and risk predictor, as well as prior evidence, we collected data on characteristics that could impact risk of bleeding. In addition, we calculated individual risk for complications using our previously developed risk calculator.
Statistical Analysis
All analyses were performed using R version 4.2.2.
Performance of Risk Calculator
We calculated a measure of discrimination—the C-statistic—which represents the probability that the calculator will assign a higher predicted risk to a patient who ultimately experienced a major bleeding complication than it assigns to a patient who did not have a major bleeding complication. C-statistic exceeding 0.75 is generally considered to represent good discrimination. 3
To assess calibration, we calculated the logistic calibration slope as a measure of the relationship between predicted and observed risk. The calibration slope has a target value of 1, indicating perfect calibration of the sum of the predictor coefficients on average. We also calculated the calibration intercept which is a measure of the discrepancy between the mean predicted risk and the observed risk; it has a target value of 0. 4
Approach to Missing Data
Data were missing on just 2% of patients, and none were missing outcome data. Therefore, all analyses were based on complete cases only.
Results
Patient Characteristics
There were 400 kidney biopsies performed during this 2-year period. Table 1 summarizes the patients’ characteristics. Patients had a median age of 56 years, and 40.2% were female. Half underwent an outpatient biopsy. Nearly two thirds underwent a transplant allograft biopsy.
Types and Timing of Bleeding Events
Major bleeding events occurred in 7 of 400 patients (1.75%, 95% confidence interval [CI] = [0.85% to 3.56%], Table 2). Of these, 5 of 400 (1.25%, 95% CI = [0.54% to 2.89%]) required transfusion and 2 of 400 (0.5%, 95% CI = [0.14% to 1.80%]) required embolization. Inpatient biopsies were complicated by 5 major bleeding events (2.5% of 197 biopsies); outpatient biopsies were complicated by 2 major bleeding events (0.99% of 203 biopsies) (Table 3). Both embolization events were in outpatients—1 recognized immediately and 1 recognized during the routine 2-hour follow-up ultrasound. There were no nephrectomies or deaths associated with kidney biopsies. Compared with outpatients, inpatients had lower hemoglobin and platelets and higher creatinine (Table 4).
Table 2.
Type and Timing of Kidney Biopsy Bleed.
| Bleeding characteristics | Number of events | % of all 400 patients | % of patients with a major bleed |
|---|---|---|---|
| Any major bleed | 7 | 1.75 | 100 |
| Major requiring transfusion | 5 | 1.25 | 71.4 |
| Major requiring embolization | 2 | 0.5 | 28.6 |
| Time to event | |||
| Immediate | 3 | 0.75 | 42.9 |
| 2 hours | 1 | 0.25 | 14.3 |
| 4 hours | 1 | 0.25 | 14.3 |
| 6 hours | 1 | 0.25 | 14.3 |
| 12 hours | 1 | 0.25 | 14.3 |
Table 3.
Major Bleeding Events in Inpatients vs Outpatients.
| Total patients | Major bleeding requiring transfusion | Major bleeding requiring embolization | Major events >2-hour postbiopsy | |
|---|---|---|---|---|
| Inpatient | 197 (49.3%) | 5 (2.5%) | 0 (0%) | 3 (1.5%) |
| Outpatient | 203 (50.7%) | 0 (0%) | 2 (0.99%) | 0 (0%) |
Table 4.
Baseline Characteristics in Inpatients vs Outpatients.
| Parameter | Inpatients (N = 197) | Outpatients (N = 203) |
|---|---|---|
| Platelet count before biopsy, ×109/L, median (IQR) | 185 (142-271) a | 248 (191-293) b |
| Hemoglobin before biopsy, g/L, median (IQR) | 89 (79-105) a | 119 (107-134) b |
| Size of biopsied kidney, cm, median (IQR) | 11.9 (11-12.9) | 11.4 (10.7-12.4) |
| Body mass index, kg/m2, median (IQR) | 29.5 (25.6-33.9) c | 28.0 (24.5-32.2) d |
| Serum creatinine, µmol/L, median (IQR) | 342 (214-582) | 155 (120-216) e |
Note. Missing data as below. IQR = interquartile range.
Missing data for 1 patient (0.5%).
Missing data for 2 patients (1%).
Missing data for 3 patients (1.5%).
Missing data for 3 patients (1.5%).
Missing data for 1 patient (0.5%).
Procedure Characteristics
Most biopsies (84.25%) included 2 (68%) or 3 (16.25%) needle passes; 2 biopsies required 6 passes each; however, there were no major bleeds associated with greater than 3 needle passes. Table 5 provides a summary of major bleeding events by number of needle passes.
Table 5.
Number of Biopsy Needle Passes and Major Bleeding Events.
| Number of needle passes | Number of patients | Major bleeding requiring transfusion | Major bleeding requiring embolization |
|---|---|---|---|
| 1 | 36 | 0 (0%) | 0 (0%) |
| 2 | 272 | 3 (1.1%) | 0 (0%) |
| 3 | 65 | 2 (3.1%) | 1 (1.5%) |
| 4 | 21 | 0 (0%) | 1 (4.8%) |
| 5 | 4 | 0 (0%) | 0 (0%) |
| 6 | 2 | 0 (0%) | 0 (0%) |
Transplant Allograft vs Native Kidney Biopsy
Although two thirds of the biopsies performed were transplant allografts, nearly half of the major bleeding complications occurred in these biopsies (3/7). All allograft major complications were in inpatients; both major bleeding events in the outpatient setting were native kidney biopsies.
Risk Calculator
Assessment of the calculator’s prediction performance included the 392 patients (99.8%) with complete data. The C-statistic was estimated at 0.91 (95% CI = [0.84 to 0.95]), calibration slope 1.10 (95% CI [0.47 to 1.74]), and calibration intercept −0.02 (95% CI = [−0.79 to 0.75]). Figure 1 shows the corresponding calibration curve.
Figure 1.
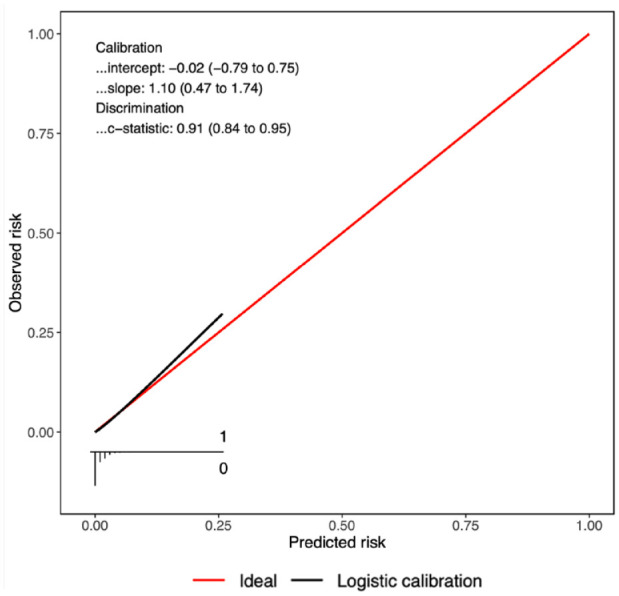
Performance of the risk calculator for predicting major bleeding complications.
Discussion
We performed a retrospective cohort study of 400 patients who had an ultrasound-guided, percutaneous kidney biopsy performed by nephrologists and supervised nephrology trainees at a single academic center in London, Ontario, Canada. In this cohort, 1.75% had major bleeding events, 1.25% requiring transfusion, and 0.5% requiring embolization.
There appears to be little yield in extending observation beyond 2 hours. In the outpatient setting, all major complications were detected either immediately or during the scheduled, repeat ultrasound at 2 hours postprocedure. In the inpatient setting, it took up to 12 hours to identify one of the 5 major bleeding events. A higher risk of bleeding in the inpatient setting has also been noted in the literature,5,6 and it is likely of less clinical importance as patients are already being monitored closely with easily accessible interventions if needed. Concordant with observations made in our previous cohort study, there was a similar risk of major bleeding (1.75% vs 1.9%), risk of transfusion (1.25% vs 1.6%), and risk of embolization (0.5% vs 0.3%). 1 By nature, inpatients are generally more sick than outpatients, and this was certainly evident in our cohort in which inpatients had lower hemoglobin, lower platelets, and higher creatinine compared with outpatients.
In our cohort, two thirds of biopsies were transplant allografts (258) in which there was a lower risk of a major bleeding event compared with native kidney biopsies (1.16% [2/258] vs 3.5%, [5/142]). This is consistent with the available literature and may be owing to the procedure being technically easier given the location and accessibility of transplant allografts compared with native kidneys.7,8
The risk of serious events found in our studies remains consistent with those seen in other recent studies. In a systematic review and meta-analysis published in 2020 that included over 100 000 native kidney biopsies, 1.6% required transfusions, and 0.3% required embolization—similar to the results seen in both of our studies. 5 In this study, death occurred in 0.06% of patients; however, this was driven primarily by 1 study in which the cause of death could not be directly attributed to the biopsy. Another retrospective review of 2204 percutaneous native kidney biopsies at a single center in the United States showed a 1.64% frequency of serious bleeding events. 9
Regarding optimal observation for outpatient biopsies, there remains variability in the literature. In an Australian study published in 2022, in 225 outpatient, native kidney biopsies that were considered low risk (normal international normalized ratio [INR], activated partial thromboplastin time [aPTT], platelets, preprocedure blood pressure <160 mm Hg and lived within 1 hour from the hospital), a 4-hour observation period was assessed as an alternative to the usual 6- to 8-hour window used at that center. 10 Seven percent of this cohort had bleeding events (16 patients) which included minor bleeding; no patient had major bleeding according to our definitions, although 2 patients had hematuria with an obstructing clot requiring catheterization and 1 patient had hemodynamic instability requiring fluid resuscitation which was recognized within the 4-hour period. Of the complications, 14/16 (87.5%) were detected within the 4-hour period. This study used only observation, blood pressure monitoring, and patient symptoms rather than repeat imaging. In another study from a center that performs primarily outpatient biopsies of transplant and native kidneys, a 4- to 6-hour observation period is used postprocedure. In over 800 biopsies, their rate of major complications (those requiring intervention) was 0.5% and all of these emerged during the 4- to 6-hour window. 11 Another study of over 2000 native and transplant biopsies, 87% (13/15) outpatient major bleeding events were identified in a 4-hour observation period.
In this study, 2 of 203 outpatient biopsies resulted in a major complication. In our previous study, 2 of 260 biopsies resulted in a major complication. 1 All of these complications were identified either immediately (3 of 4 complications) or within 2 hours after biopsy (1 complication).
Most risk calculators are never tested in new patients, and most that are tested perform poorly on that test. We made extensive use of shrinkage techniques and resampling methods when we developed the risk calculator in our previous study, and these were intended to improve performance on future patients. Although the risk calculator appeared to perform well in this new study, there were very few major complication events which greatly limits the reliability of our assessments. Confidence intervals around performance estimates were wide and consistent with significant over-and-under-prediction of risk. The findings warrant further validation in large independent samples.
Conclusions
In this retrospective study of 400 ultrasound-guided, percutaneous kidney biopsies at a tertiary care center in Canada, major bleeding requiring intervention was rare and consistent with our previous study at the same institution. In the outpatient setting, all major bleeding was identified immediately or on the 2-hour repeat ultrasound. These data support the safety of a 2-hour monitoring window for patients undergoing outpatient biopsy with the use of a repeat scan to determine whether a patient is safe for discharge or should be admitted for further monitoring or intervention. Our risk calculator (http://perioperativerisk.com/kbrc) performed well and warrants further validation.
Resource Utilization
By reducing the observation period needed for outpatient renal biopsies, we increase the availability of the postanesthesia care unit, potentially making more procedures possible in a particular unit of time (day or week). The time needed for an ultrasound technologist and radiologist to reassess a biopsy site is certainly much less than the additional 4 or more hours usually set aside for monitoring these patients. In costs, a formal cost analysis would have to be done to compare the cost of the technician and radiologist compared with the cost of a postanesthesia care unit (PACU) bed. Although point-of-care ultrasound is increasingly being used in hospitals across Canada, it has not yet become routine in postbiopsy assessments; this would perhaps be an even more cost-effective method to use in the future.
Footnotes
Ethics Approval and Consent to Participate: As a quality improvement project, this study met criteria for a waiver of review by the Research Ethics Board at Western University, London, Ontario, Canada.
Consent for Publication: All authors provided consent for publication.
Availability of Data and Materials: Data and materials will be available upon request.
Author Contributions: M.S., P.S.R., and A.A.H. contributed to study concept and design. J.V. and A.A.H. performed data collection; A.A.H., P.S.R., and M.S. analyzed and interpreted the data. Drafting of the manuscript was done by M.S. with revisions of the manuscript done by M.S., P.S.R., and A.A.H. for important intellectual content.
The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Funding: The author(s) received no financial support for the research, authorship, and/or publication of this article.
ORCID iDs: Melissa Schorr  https://orcid.org/0000-0003-1076-770X
https://orcid.org/0000-0003-1076-770X
Andrew A. House  https://orcid.org/0000-0001-7152-6163
https://orcid.org/0000-0001-7152-6163
References
- 1. Schorr M, Roshanov PS, Weir MA, House AA. Frequency, timing, and prediction of major bleeding complications from percutaneous renal biopsy. Can J Kidney Health Dis. 2020;7. doi: 10.1177/2054358120923527 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Collins GS, Reitsma JB, Altman DG, Moons KG. Transparent Reporting of a multivariable prediction model for Individual Prognosis or Diagnosis (TRIPOD): the TRIPOD statement. Ann Intern Med. 2015;162:55-63. doi: 10.7326/m14-0697 [DOI] [PubMed] [Google Scholar]
- 3. Hosmer DW, Lemeshow S, Sturdivant RX. Applied Logistic Regression. 3rd ed. John Wiley; 2013:1632. [Google Scholar]
- 4. Steyerberg EW, Vergouwe Y. Towards better clinical prediction models: seven steps for development and an ABCD for validation. Eur Heart J. 2014;35:1925-1931. doi: 10.1093/eurheartj/ehu207 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Poggio ED, McClelland RL, Blank KN, et al. Systematic review and meta-analysis of native kidney biopsy complications. Clin J Am Soc Nephrol. 2020;15:1595-1602. doi: 10.2215/cjn.04710420 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Bonani M, Seeger H, Weber N, Lorenzen JM, Wüthrich RP, Kistler AD. Safety of kidney biopsy when performed as an outpatient procedure. Kidney Blood Press Res. 2021;46:310-322. doi: 10.1159/000515439 [DOI] [PubMed] [Google Scholar]
- 7. Whittier WL, Gashti C, Saltzberg S, Korbet S. Comparison of native and transplant kidney biopsies: diagnostic yield and complications. Clin Kidney J. 2018;11:616-622. doi: 10.1093/ckj/sfy051 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Preda A, Van Dijk LC, Van Oostaijen JA, Pattynama PMT. Complication rate and diagnostic yield of 515 consecutive ultrasound-guided biopsies of renal allografts and native kidneys using a 14-gauge Biopty gun. Eur Radiol. 2003;13:527-530. doi: 10.1007/s00330-002-1482-3 [DOI] [PubMed] [Google Scholar]
- 9. Monahan H, Gunderson T, Greene E, Schmit G, Atwell T, Schmitz J. Risk factors associated with significant bleeding events after ultrasound-guided percutaneous native renal biopsies: a review of 2204 cases. Abdom Radiol. 2019;44:2316-2322. doi: 10.1007/s00261-019-01962-z [DOI] [PubMed] [Google Scholar]
- 10. Trinh K, Bency R, Kairaitis L. Native renal biopsy: outcomes with a 4-h observation period in low-risk outpatients. Intern Med J. 2022;52:130-133. doi: 10.1111/imj.15645 [DOI] [PubMed] [Google Scholar]
- 11. Aaltonen S, Finne P, Honkanen E. Outpatient kidney biopsy: a single center experience and review of literature. Nephron. 2020;144:14-20. doi: 10.1159/000503255 [DOI] [PubMed] [Google Scholar]


