Abstract
A microbial enrichment culture from marine sediment was able to grow on hexadecan-2-one as the sole source of carbon and energy under sulfate-reducing conditions. Oxidation of the ketone involved carboxylation reactions and was coupled to sulfide production. This enrichment culture also grew on 6,10,14-trimethylpentadecan-2-one.
Degradation of hydrocarbons under anoxic conditions has been demonstrated in microbial communities and pure cultures of sulfate-reducing (1, 2, 7, 15, 18, 26, 31), denitrifying (5, 17, 27), and iron(III)-reducing bacteria (19). Most of these studies involved aromatic hydrocarbons. Indeed, there are only a few reports on the anaerobic degradation of aliphatic hydrocarbons, particularly alkanes (1, 5, 20, 31). Due to the lack of unsaturations in alkanes, their degradation is difficult in the absence of molecular oxygen.
While alkanes cannot be directly photooxidized by solar light, there are several compounds in seawater (10) or petroleum (29) which are capable of introducing oxygen atoms into these hydrocarbon molecules by photochemical mechanisms. The photosensitized oxidation of n-alkanes generates mainly ketones and secondary alcohols with the same number of carbon atoms as the alkanes (11, 14), while oxidation of branched alkanes leads mainly to the formation of tertiary alcohols and ketones (30).
When photooxidation products are not fully degraded under aerobic conditions, they will reach anoxic environments where they might be degraded. Further, in the literature, there are relatively few reports dealing with degradation under anaerobic conditions of ketones and secondary alcohols resulting from hydrocarbon photooxidation. Acetone degradation has been shown to occur in an enrichment culture under methanogenic conditions (23) and also in pure cultures using nitrate (3, 24) or sulfate (25) as the electron acceptor. Higher ketones, such as butanone, pentan-2-one, hexan-2-one, and hexan-3-one, were degraded anaerobically by pure cultures of denitrifying bacteria (24). A pure culture of sulfate-reducing bacteria was also able to degrade butanone (13). A recent study showed the degradation of a branched isoprenoid ketone, 6,10,14-trimethylpentadecan-2-one, by a denitrifying marine bacterium (28). However, no evidence for microbial degradation of ketones higher than butanone or secondary alcohols under sulfate-reducing conditions has been presented. Owing to the abundance of sulfate in anoxic marine environments, experiments were conducted under sulfate-reducing conditions with hexadecan-2-one, nonadecan-10-one, and two pristane photo-oxidation products (6,10,14-trimethylpentadecan-2-one and 2,6,10,14-tetramethylpentadecan-2-ol).
Two of the compounds tested, hexadecan-2-one and 6,10,14-trimethylpentadecan-2-one, were degraded under sulfate-reducing conditions in microcosms and enrichment cultures.
Chemicals.
Hexadecan-2-one and nonadecan-10-one were purchased from Aldrich (Milwaukee, Wis.). 6,10,14-Trimethylpentadecan-2-one was produced by oxidation of phytol (Riedel de Haën) with KMnO4 in acetone (6). 2,6,10,14-Tetramethylpentadecan-2-ol was obtained by condensation of 6,10,14-trimethylpentadecan-2-one and methylmagnesium iodide in anhydrous diethyl ether. Reduction of 6,10,14-trimethylpentadecan-2-one with LiAlH4 in diethyl ether gave the corresponding secondary alcohol. The synthesis of 2-(1-hydroxyethyl)pentadecanoic acid required three steps: (i) condensation of ethyl acetoacetate with 1-bromotridecane in the presence of potassium tert-butoxide (12), (ii) reduction of the resulting β-ketoester with NaBH4 (which did not reduce the carboxyl group of esters) for 15 min in methanol, and (iii) alkaline hydrolysis of the produced β-hydroxyester (5% KOH in 50% CH3OH, reflux for 1 h).
Sediment incubation protocol.
Microcosms were prepared in 60-ml serum bottles from a volume of sediment and a volume of sterilized site water enriched as described below. Coastal sediment showing little oil contamination (0.06 kg/kg of sediment) was collected at Fos Bay (France). Site water was enriched with 3.7 mM ammonium chloride, 2 mM sodium thiosulfate, 14 mM morpholinepropanesulfonic acid (pH 7.2), and 1 mg of resazurin per liter. The fresh sediment and water were homogenized under an N2-H2-CO2 (85:10:5) atmosphere in an anaerobic chamber (La Calhène). A mixture of four compounds, hexadecan-2-one, nonadecan-10-one, 6,10,14-trimethylpentadecan-2-one, and 2,6,10,14-tetramethylpentadecan-2-ol, had previously been added to the empty bottles; the compounds were dissolved in dichloromethane and distributed so as to provide a final concentration of 200 mg/liter in each microcosm. Dichloromethane was then allowed to evaporate from the serum bottles. Killed controls were prepared with autoclaved sediments. The bottles were sealed with Teflon-coated butyl rubber stoppers (Serflam, Marseille, France) and incubated at 30°C in the dark.
Subculture procedure.
A homogeneous fraction (3 ml) of the microcosm was transferred to serum bottles containing the substrate hexadecan-2-one at a final concentration of 200 mg/liter in artificial seawater medium (27 ml) with 0.4 mM Na2S · 9H2O. Hexadecan-2-one was added as described above and autoclaved under an N2-CO2 (90:10) atmosphere. The seawater medium contained (per liter, unless otherwise noted) 23.5 g of NaCl, 10.6 g of MgCl2 · 6H2O, 3.9 g of Na2SO4, 0.19 g of NaHCO3, 0.66 g of KCl, 0.1 g of KBr, 0.024 g of H3BO3, 0.040 g of SrCl2 · 6H2O, 0.2 g of NH4Cl, 50 mg of yeast extract, 1 mg of resazurin, and 3 g of morpholinepropanesulfonic acid (pH 7.2). After autoclaving, 10 ml of CaCl2 · 2H2O (140 g/liter), 1 ml of trace element solution SL12 (21), 1 ml of selenite-tungstate solution (33), 1 ml of 7-vitamin solution (22), and 4 ml of phosphate buffer (4.4 g of NaH2PO4 · 2H2O and 25.8 g of Na2HPO4 · 12H2O per liter) were added from sterile stock solutions. Prior to inoculation, the medium was incubated overnight in an anaerobic chamber. The bottles were incubated at 30°C in the dark on a reciprocal shaker (100 strokes/min).
Analytical procedures.
Sulfide was quantified with Cu2+ ions yielding CuS (8).
For analyses of the ketones and alcohol, the sediment and the aqueous phase from the microcosm were separated by decantation. The wet sediment was extracted under sonication with isopropanol-hexane (4:1, vol/vol) (9), and the aqueous phase was extracted three times with chloroform. For analysis of the substrate of the subculture without sediment, the entire contents of the vial were extracted with chloroform. The combined hexane and chloroform extracts were dried with anhydrous Na2SO4, filtered, and concentrated by means of rotary evaporation to give extract E1 (containing residual substrates and eventual nonacidic metabolites). The aqueous phase was then acidified with hydrochloric acid (pH 1) and extracted three times with chloroform to give extract E2 (containing acidic compounds). After evaporation of the solvents, extracts E1 and E2 were taken up separately in 250 μl of a mixture of pyridine and N,O-bistrimethylsilyl–trifluoroacetamide (3:1, vol/vol) and allowed to silylate at 50°C for 1 h. Following evaporation to dryness under nitrogen, the residues were dissolved in hexane and analyzed by gas chromatography-mass spectrometry (GC/MS). Compounds were identified (by comparison of their retention times and mass spectra with those of standards) and quantified (after calibration with external standards) by GC/MS. The GC/MS analyses were carried out with an HP 5890 series II plus gas chromatograph connected to an HP 5972 mass spectrometer. The operating conditions included a capillary measuring 15 m by 0.25 mm (inside diameter), a column coated with BPX 35 (SGE), an oven temperature programmed to go from 60 to 150°C at 30°C/min and then from 150 to 320°C at 3°C/min, a carrier gas (He) pressure of 0.48 bar, an injector temperature of 320°C, an electron energy of 70 eV, and a source temperature of 170°C.
The microbial biomass was estimated indirectly by quantifying the cellular protein (4) assuming a 50% protein content.
Depletion experiments in microcosms.
In microcosms fed with the mixture of four compounds, hexadecan-2-one was the first compound to be depleted. Within 2 months, more than 80% was depleted while only 15% of the 6,10,14-trimethylpentadecan-2-one was depleted. After 10 more months, 42% of the 6,10,14-trimethylpentadecan-2-one was depleted with respect to a control incubated just as long. Neither nonadecan-10-one nor 2,6,10,14-tetramethylpentadecan-2-ol was depleted after 1 year of incubation. In the controls, none of the compounds disappeared within 6 months; however, after 1 year of incubation, only 80% of the four substrates remained. Successive subcultures confirmed those depletions. We conclude that of the compounds studied, hexadecan-2-one is the most rapidly depleted.
Evidence that hexadecan-2-one metabolism is linked to sulfate reduction.
Successive transfers to defined medium with hexadecan-2-one were made to establish enrichment cultures without sediment. To link sulfate reduction and hexadecan-2-one oxidation, transfers were made in complete artificial seawater medium and in sulfate-depleted artificial seawater medium. After 1 month of incubation, 80% of the hexadecan-2-one was degraded in the complete artificial seawater medium while only 10% was degraded in the sulfate-depleted artificial seawater medium. Transfer of the enrichment culture to medium with hexadecan-2-one and/or yeast extract showed that yeast extract alone in artificial seawater medium did not support growth, while growth was the same on hexadecan-2-one with or without yeast extract. On the other hand, the weak growth in subcultures without sulfate was detectable only in the presence of both hexadecan-2-one and yeast extract. In conclusion, the experiments show that efficient anaerobic degradation of hexadecan-2-one requires sulfate and the activity of sulfate-reducing bacteria. Nevertheless, some degradation occurred in the absence of sulfate, and hence, other microorganisms are probably involved.
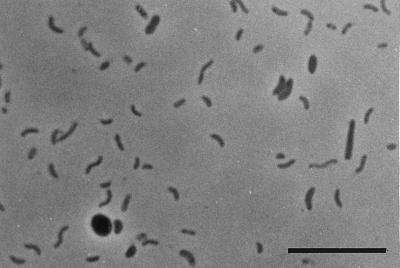
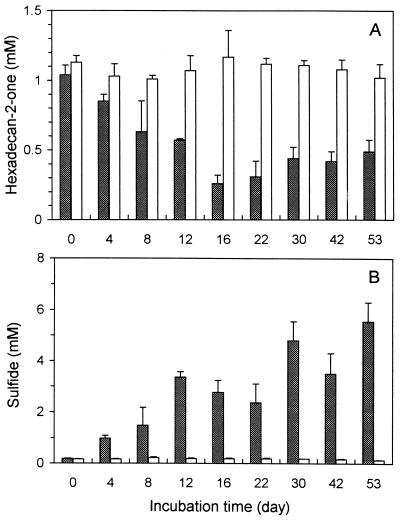
The community growing in yeast extract-free artificial seawater medium with hexadecan-2-one as the sole source of carbon was composed of curved rods and of some oval and rod-shaped cells (Fig. 1). The culture grown on hexadecan-2-one as the sole source of carbon and energy reached its optimum growth within about 20 days with an optical density at 450 nm of about 0.20. The curve is not shown, as the culture often formed aggregates or biofilm on a glass surface. Nevertheless, increased optical density was linked to an increase in microbial biomass, as about 16 mg (dry weight) per liter was produced in a month of growth while 0.6 mM hexadecan-2-one was consumed. At optimum growth, 60% of the hexadecan-2-one was degraded. Although sulfate was not limiting, about 35% of the hexadecan-2-one remained at the end of the experiment (Fig. 2A). The degradation of hexadecan-2-one was linked to sulfide production (Fig. 2A and B). In sterile controls, no depletion occurred and no sulfide was produced. Furthermore, in inoculated controls without substrate, the sulfide produced never exceeded 0.2 mM. At the end of the experiment, 5.42 mM sulfide was produced during the degradation of 0.53 mM hexadecan-2-one. The stoichiometry of complete hexadecan-2-one oxidation through sulfate reduction is as follows: CH3(CH2)13COCH3 + 11.75 SO42−→11.75 S2− + 16 CO2 + 16 H2O. Therefore, the amount of sulfide produced will allow the oxidation of 0.46 mM hexadecan-2-one. The remaining 0.07 mM will be converted to cell carbon, and possibly to energy, by the nonsulfidogenic part of the community. Since it was consistent with the cell mass produced (0.04 mM hexadecanone turned into cell carbon), we concluded that oxidation was complete.
FIG. 1.
Phase-contrast photomicrograph of an enrichment culture grown on hexadecan-2-one and sulfate. Bar, 10 μm.
FIG. 2.
Concentrations of hexadecan-2-one (A) and sulfide (B) in an enrichment culture growing in the presence of hexadecan-2-one. Open bars correspond to sterile controls. The data are mean values from three cultures. At each time, the entire contents of three vials were analyzed.
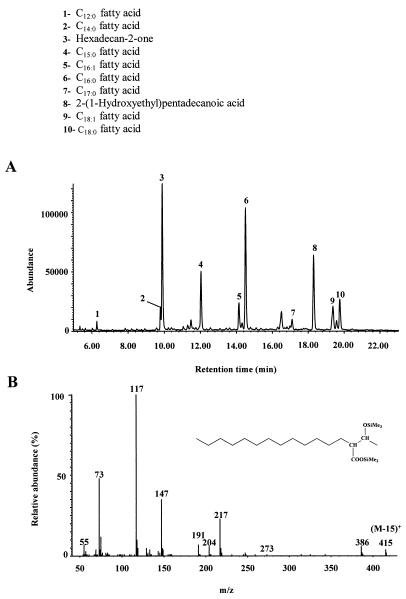
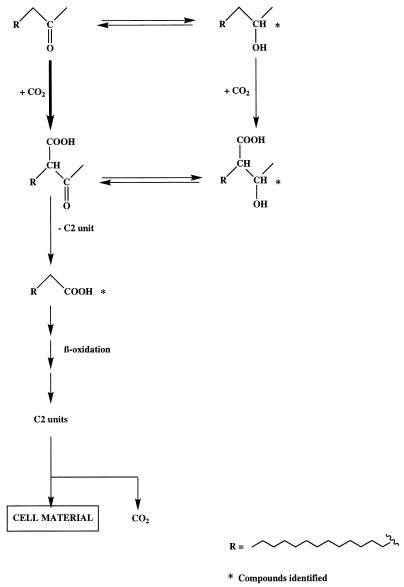
After growth of the enrichment culture on hexadecan-2-one, we detected 2-(1-hydroxyethyl)pentadecanoic acid in extract E2 (Fig. 3A). This metabolite, which was undetectable in controls with a sterilized inoculum, was identified unambiguously by comparison of its retention time and mass spectrum (Fig. 3B) with those of a synthesized standard. The presence of this compound clearly shows that the metabolism of hexadecan-2-one by the enrichment culture involves carboxylation reactions. Such a mechanism has already been described for the anaerobic oxidation of acetone (3, 23, 24). Partial reduction of the carbonyl group to the corresponding secondary alcohol was also observed. The involvement of this “blind-alley” pathway probably results from nonspecific enzyme activity not related to hexadecan-2-one degradation (16). On the basis of these results, we propose the pathway described in the Fig. 4 for the metabolism of hexadecan-2-one by the enrichment culture. The detection of a relatively strong proportion of pentadecanoic acid in extract E2 (Fig. 3A) is in good agreement with such a pathway. If carboxylation of ketones is easier than that of the corresponding alcohols, due to activation of the carbon α relative to the carbonyl group by keto-enol tautomerism, the possibility of hexadecan-2-ol carboxylation cannot be completely excluded (3, 32).
FIG. 3.
(A) Total ion chromatogram of (silylated) extract E2 obtained after the growth of an enrichment culture on hexadecan-2-one. (B) Electron impact mass spectrum of metabolite 8, identified as (silylated) 2-(1-hydroxyethyl)pentadecanoic acid.
FIG. 4.
Proposed pathway for the metabolism of hexadecan-2-one by an enrichment culture.
Although the carboxylation takes place on the secondary carbon in the α position relative to the carbonyl group of hexadecan-2-one, the enrichment culture was unable to degrade nonadecan-10-one. An attempt at hexadecan-3-one biodegradation confirmed that only ketones with the keto group at position 2 could be degraded by the enrichment culture.
These results confirm once more the importance of the community of sulfate-reducing bacteria in anoxic environments and, in particular, in the degradation of hydrocarbons. Furthermore, it is the first demonstration of the degradation of a typical hydrocarbon photooxidation product under sulfate-reducing conditions.
Acknowledgments
We are indebted to F. Widdel for helpful discussions and to R. Guyoneaud for microphotograph preparation.
This work was supported by a grant from the Centre National de la Recherche Scientifique and Elf Aquitaine through Research Groupe HYCAR 1123.
REFERENCES
- 1.Aeckersberg F, Bak F, Widdel F. Anaerobic oxidation of saturated hydrocarbons to CO2 by a new type of sulfate-reducing bacterium. Arch Microbiol. 1991;156:5–14. [Google Scholar]
- 2.Beller H R, Spormann A M, Sharma P K, Cole J R, Reinhard M. Isolation and characterization of a novel toluene-degrading, sulfate-reducing bacterium. Appl Environ Microbiol. 1996;62:1188–1196. doi: 10.1128/aem.62.4.1188-1196.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Bonnet-Smits E M, Robertson L A, Van Dijken J P, Senior E, Kuenen J G. Carbon dioxide fixation as the initial step in the metabolism of acetone by Thiosphaera pantotropha. J Gen Microbiol. 1988;134:2281–2289. [Google Scholar]
- 4.Bradford M M. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem. 1976;72:248–254. doi: 10.1006/abio.1976.9999. [DOI] [PubMed] [Google Scholar]
- 5.Bregnard T P A, Höhener P, Häner A, Zeyer J. Degradation of weathered diesel fuel by microorganisms from a contaminated aquifer in aerobic and anaerobic microcosms. Environ Toxicol Chem. 1996;15:299–307. [Google Scholar]
- 6.Cason J, Graham D W. Isolation of isoprenoid acids from a California petroleum. Tetrahedron. 1965;21:471–483. [Google Scholar]
- 7.Coates J D, Anderson R T, Lovley D R. Oxidation of polycyclic aromatic hydrocarbons under sulfate-reducing conditions. Appl Environ Microbiol. 1996;62:1099–1101. doi: 10.1128/aem.62.3.1099-1101.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Cord-Ruwisch R. A quick method for the determination of dissolved and precipitated sulfides in cultures of sulfate-reducing bacteria. J Microbiol Methods. 1985;4:33–36. [Google Scholar]
- 9.de Leeuw J W, Simoneit B R T, Boon J J, Rijpstra W I C, de Lange F, van der Linden J C W, Correia V A, Burlingame A L, Schenck P A. Phytol derived compounds in the geosphere. In: Campos R, Goni J, editors. Advances in organic geochemistry 1975. Madrid, Spain: Enadimsa; 1977. pp. 61–79. [Google Scholar]
- 10.Ehrhardt M, Bouchertall F, Hopf P. Aromatic ketones concentrated from Baltic Sea water. Mar Chem. 1982;11:449–461. [Google Scholar]
- 11.Ehrhardt M, Petrick G. The sensitized photo-oxidation of n-pentadecane as a model for abiotic decomposition of aliphatic hydrocarbons in seawater. Mar Chem. 1985;16:227–238. [Google Scholar]
- 12.Etheredge S J. Bicyclic ketones by intramolecular alkylation: a reinvestigation. J Am Chem Soc. 1966;12:1990–1994. [Google Scholar]
- 13.Galushko A S, Rozanova E P. Desulfobacterium cetonicum sp. nov.: a sulfate-reducing bacterium which oxidizes fatty acids and ketones. Microbiology. 1992;60:742–746. . (English translation from Mikrobiologiya 60:102–107, 1991.) [Google Scholar]
- 14.Guiliano M, El Anba-Lurot F, Doumenq P, Mille G, Rontani J F. Photo-oxidation of n-alkanes in simulated marine environmental conditions. J Photochem Photobiol Ser A. 1997;102:127–132. [Google Scholar]
- 15.Haag F, Reinhard M, McCarty P L. Degradation of toluene and p-xylene in anaerobic microcosms: evidence for sulfate as a terminal electron acceptor. Environ Toxicol Chem. 1991;10:1379–1389. [Google Scholar]
- 16.Klug M J, Markovetz A J. Utilization of aliphatic hydrocarbons by microorganisms. Adv Microb Physiol. 1971;5:1–43. doi: 10.1016/s0065-2911(08)60404-x. [DOI] [PubMed] [Google Scholar]
- 17.Kuhn E P, Zeyer J, Eicher P, Schwarzenbach R P. Anaerobic degradation of alkylated benzenes in denitrifying laboratory aquifer columns. Appl Environ Microbiol. 1988;54:490–496. doi: 10.1128/aem.54.2.490-496.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Lovley D R, Coates J D, Woodward J C, Phillips E J P. Benzene oxidation coupled to sulfate reduction. Appl Environ Microbiol. 1995;61:953–958. doi: 10.1128/aem.61.3.953-958.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Lovley D R, Lonergan D J. Anaerobic oxidation of toluene, phenol, and p-cresol by the dissimilatory iron-reducing organism, GS-15. Appl Environ Microbiol. 1990;56:1858–1864. doi: 10.1128/aem.56.6.1858-1864.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Morikawa M, Kanemoto M, Imanaka T. Biological oxidation of alkane to alkene under anaerobic conditions. J Ferment Bioeng. 1996;82:309–311. [Google Scholar]
- 21.Pfennig N, Trüper H G. The family Chromatiaceae. In: Balows A, Trüper H G, Dworkin M, Harder W, Schleifer K H, editors. The prokaryotes. 2nd ed. New York, N.Y: Springer-Verlag; 1992. pp. 3200–3221. [Google Scholar]
- 22.Pfennig N, Widdel F, Trüper H G. The dissimilatory sulfate-reducing bacteria. In: Starr M P, Stolp H, Trüper H G, Balows A, Schlegel H G, editors. The prokaryotes. 1st ed. New York, N.Y: Springer-Verlag; 1981. pp. 926–940. [Google Scholar]
- 23.Platen H, Schink B. Methanogenic degradation of acetone by an enrichment culture. Arch Microbiol. 1987;149:136–141. doi: 10.1007/BF00425079. [DOI] [PubMed] [Google Scholar]
- 24.Platen H, Schink B. Anaerobic degradation of acetone and higher ketones via carboxylation by newly isolated denitrifying bacteria. J Gen Microbiol. 1989;135:883–891. doi: 10.1099/00221287-135-4-883. [DOI] [PubMed] [Google Scholar]
- 25.Platen H, Temmes A, Schink B. Anaerobic degradation of acetone by Desulfococcus biacutus spec. nov. Arch Microbiol. 1990;154:355–361. doi: 10.1007/BF00276531. [DOI] [PubMed] [Google Scholar]
- 26.Rabus R, Fukui M, Wilkes H, Widdel F. Degradative capacities and 16S rRNA-targeted whole-cell hybridization of sulfate-reducing bacteria in an anaerobic enrichment culture utilizing alkylbenzenes from crude oil. Appl Environ Microbiol. 1996;62:3605–3613. doi: 10.1128/aem.62.10.3605-3613.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Rabus R, Widdel F. Utilization of alkylbenzenes during anaerobic growth of pure cultures of denitrifying bacteria on crude oil. Appl Environ Microbiol. 1996;62:1238–1241. doi: 10.1128/aem.62.4.1238-1241.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Rontani J-F, Gilewicz M J, Michotey V D, Zheng T L, Bonin P C, Bertrand J-C. Aerobic and anaerobic metabolism of 6,10,14-trimethylpentadecan-2-one by a denitrifying bacterium isolated from marine sediments. Appl Environ Microbiol. 1997;63:636–643. doi: 10.1128/aem.63.2.636-643.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Rontani J-F, Giral P J-P. Significance of photochemical degradation of petroleum hydrocarbon fractions in seawater. Int J Environ Anal Chem. 1990;42:61–68. [Google Scholar]
- 30.Rontani J-F, Giusti G. Photosensitized oxidation of pristane in sea water: effect of photochemical reactions on tertiary carbons. J Photochem Photobiol Ser A. 1987;40:107–120. [Google Scholar]
- 31.Rueter P, Rabus R, Wilkes H, Aeckersberg F, Rainey F A, Jannasch H W, Widdel F. Anaerobic oxidation of hydrocarbons in crude oil by new types of sulphate-reducing bacteria. Nature (London) 1994;372:455–458. doi: 10.1038/372455a0. [DOI] [PubMed] [Google Scholar]
- 32.Schink B. Principles and limits of anaerobic degradation: environmental and technological aspects. In: Zehnder A J B, editor. Biology of anaerobic microorganisms. New York, N.Y: Wiley; 1988. pp. 771–846. [Google Scholar]
- 33.Widdel F, Bak F. Gram-negative mesophilic sulfate-reducing bacteria. In: Balows A, Trüper H G, Dworkin M, Harder W, Schleifer K H, editors. The prokaryotes. 2nd ed. New York, N.Y: Springer-Verlag; 1992. pp. 3353–3378. [Google Scholar]