Abstract
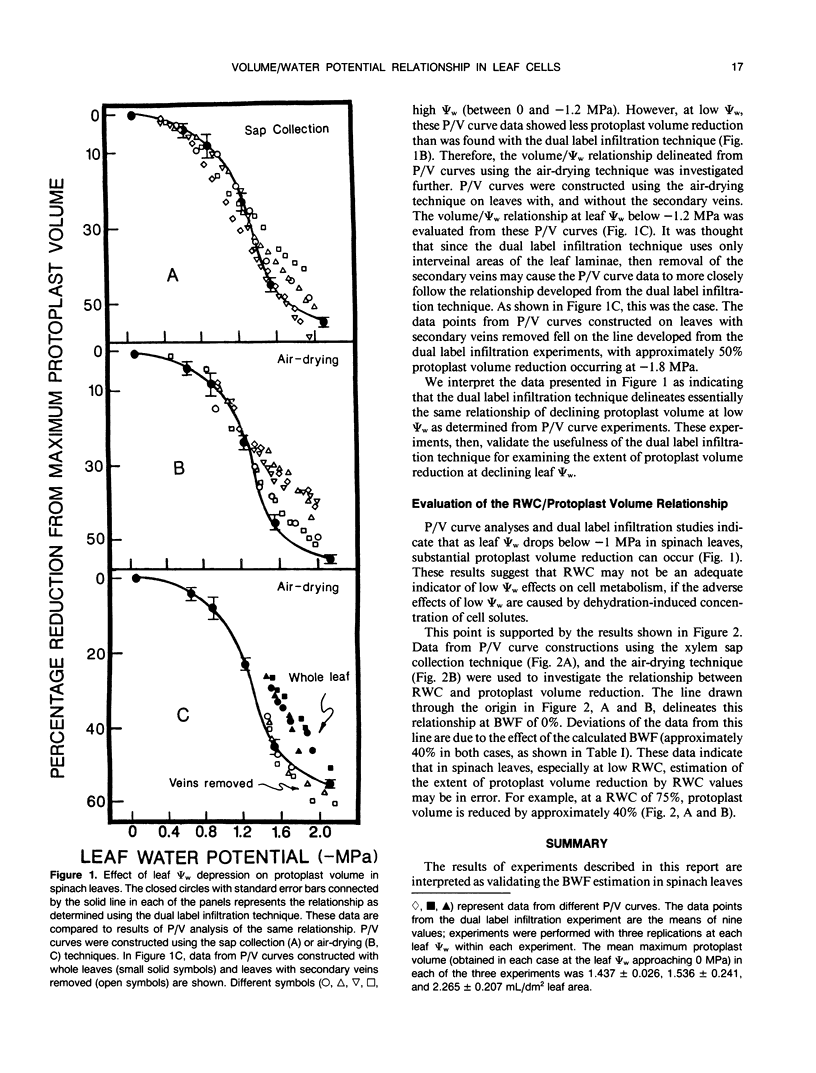
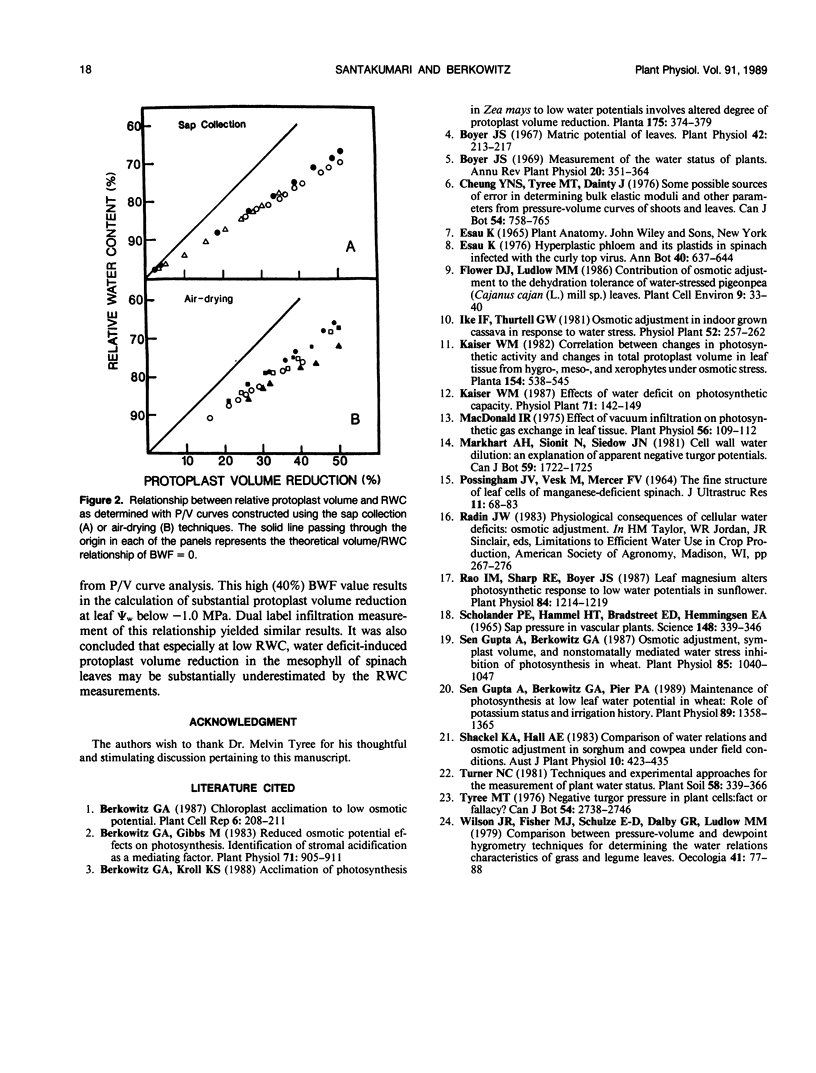
Methods used to estimate the (nonosmotic) bound water fraction (BWF) (i.e. apoplast water) of spinach (Spinacia oleracea L.) leaves were evaluated. Studies using three different methods of pressure/volume (P/V) curve construction all resulted in a similar calculation of BWF; approximately 40%. The theoretically derived BWF, and the water potential (Ψw)/relative water content relationship established from P/V curves were used to establish the relationship between protoplast (i.e. symplast) volume and Ψw. Another method of establishing the protoplast volume/Ψw relationship in spinach leaves was compared with the results from P/V curve experiments. This second technique involved the vacuum infiltration of solutions at a range of osmotic potentials into discs cut from spinach leaves. These solutions contained radioactively labeled H2O and sorbitol. This dual label infiltration technique allowed for simultaneous measurement of the total and apoplast volumes in leaf tissue; the difference yielded the protoplast volume. The dual label infiltration experiments and the P/V curve constructions both showed that below −1 megapascals, protoplast volume decreases sharply with decreasing water potential; with 50% reduction in protoplast volume occurring at −1.8 megapascals leaf water potential.
Full text
PDF



Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Berkowitz G. A., Gibbs M. Reduced osmotic potential effects on photosynthesis : identification of stromal acidification as a mediating factor. Plant Physiol. 1983 Apr;71(4):905–911. doi: 10.1104/pp.71.4.905. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boyer J. S. Matric potentials of leaves. Plant Physiol. 1967 Feb;42(2):213–217. doi: 10.1104/pp.42.2.213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gupta A. S., Berkowitz G. A. Osmotic adjustment, symplast volume, and nonstomatally mediated water stress inhibition of photosynthesis in wheat. Plant Physiol. 1987 Dec;85(4):1040–1047. doi: 10.1104/pp.85.4.1040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gupta A. S., Berkowitz G. A., Pier P. A. Maintenance of photosynthesis at low leaf water potential in wheat : role of potassium status and irrigation history. Plant Physiol. 1989 Apr;89(4):1358–1365. doi: 10.1104/pp.89.4.1358. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Macdonald I. R. Effect of vacuum infiltration on photosynthetic gas exchange in leaf tissue. Plant Physiol. 1975 Jul;56(1):109–112. doi: 10.1104/pp.56.1.109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rao I. M., Sharp R. E., Boyer J. S. Leaf magnesium alters photosynthetic response to low water potentials in sunflower. Plant Physiol. 1987 Aug;84(4):1214–1219. doi: 10.1104/pp.84.4.1214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scholander P. F., Bradstreet E. D., Hemmingsen E. A., Hammel H. T. Sap Pressure in Vascular Plants: Negative hydrostatic pressure can be measured in plants. Science. 1965 Apr 16;148(3668):339–346. doi: 10.1126/science.148.3668.339. [DOI] [PubMed] [Google Scholar]


