Abstract
Purpose:
There is considerable interest in very short (ultrahypofractionated) radiation therapy regimens to treat prostate cancer based on potential radiobiological advantages, patient convenience, and resource allocation benefits. Our objective is to demonstrate that detectable changes in health-related quality of life measured by the bowel and urinary domains of the Expanded Prostate Cancer Index Composite (EPIC-50) were not substantially worse than baseline scores.
Methods and Materials:
NRG Oncology’s RTOG 0938 is a nonblinded randomized phase 2 study of National Comprehensive Cancer Network low-risk prostate cancer in which each arm is compared with a historical control. Patients were randomized to 5 fractions (7.25 Gy in 2 week and a day [twice a week]) or 12 fractions (4.3Gy in 2.5 weeks [5 times a week]). Secondary objectives assessed patient-reported toxicity at 5 years using the EPIC. Chi-square tests were used to assess the proportion of patients with a deterioration from baseline of >5 points for bowel, >2 points for urinary, and >11 points for sexual score.
Results:
The study enrolled 127 patients to 5 fractions (121 eligible) and 128 patients to 12 fractions (125 eligible). The median follow-up for all patients at the time of analysis was 5.38 years. The 5-year frequency for >5 point change in bowel score were 38.4% (P = .27) and 23.4% (P = 0.98) for 5 and 12 fractions, respectively. The 5-year frequencies for >2 point change in urinary score were 46.6% (P = .15) and 36.4% (P = .70) for 5 and 12 fractions, respectively. For 5 fractions, 49.3% (P = .007) of patients had a drop in 5-year EPIC-50 sexual score of ≥11 points; for 12 fractions, 54% (P < .001) of patients had a drop in 5-year EPIC-50 sexual score of ≥11 points. Disease-free survival at 5 years is 89.6% (95% CI: 84.0–95.2) in the 5-fraction arm and 92.3 % (95% CI: 87.4–97.1) in the 12-fraction arm. There was no late grade 4 or 5 treatment-related urinary or bowel toxicity.
Conclusions:
This study confirms that, based on long-term changes in bowel and urinary domains and toxicity, the 5- and 12-fraction regimens are well tolerated. These ultrahypofractionated approaches need to be compared with current standard radiation therapy regimens.
Introduction
Patients with localized prostate cancer are often treated with 7.5 to 9 weeks of radiotherapy using high precision radiotherapy techniques. These higher doses of radiotherapy using these techniques result in improved prostate specific antigen (PSA) control.1–3
Radiological modelling suggests that the low α/β ratio of prostate cancer4–10 relative to late normal tissue may confer a therapeutic advantage. This needs to be confirmed by suitably designed RCT’s. The advantages of hypofractionation studies include patient convenience and the ability of patients to be treated with the available resources. There is considerable clinical interest in treating patients with localized disease with hypofractionated prostate radiotherapy regimens (HypoRT).
Moderate hypofractionation regimens(20–28 fractions) using 2.5 to 3Gy fractions have now been shown to result in equivalent PSA control11–13 based on 3 randomised controlled trials(RCTs). One of these studies showed slightly higher late grade2 gastrointestinal and genitourinary toxicity.11
Studies of ultrahypofraction (UHRT) regimens (5–12 fractions) suggest acceptable acute and late toxicity. Long-term efficacy and toxicity results comparing these UHRTs to standard and moderate hypofractionation in the context of RCTs are awaited.14–19 Modern high-precision techniques enable UHRT to be delivered with acceptable acute and late adverse effects.
Bowel and urinary patient-reported outcomes (PROs) using the Expanded Prostate Cancer Index Composite (EPIC) have been reported by several investigators.20–22 The EPIC PRO questionnaire (English version) is a robust 50-item validated prostate cancer questionnaire consisting of 4 domains: bowel, urinary, sexual, and hormonal.22 Scores are transformed to a 0 to 100 scale, with higher scores indicating better outcomes. Although some case series using UHRT23–26 have reported on PROs, this moderately sized prospective multi-institution study collected PRO data as per the study protocol. Typically, rectal and bladder scores decline during and for a few weeks after radiation therapy, returning to pretreatment levels by 1 to 2 years. This study assessing bowel and urinary PROs was undertaken before embarking on an RCT comparing UHRT (5–12 fractions) with SRT. Important outcomes that would be relevant to determining whether UHRT will be used in clinical practice include efficacy, acute and late toxicity, and PROs.
Methods and Materials
Randomization and masking
NRG/RTOG 0938 (ClinicalTrials.gov #NCT01434290) is a nonblinded randomized phase 2 study of National Comprehensive Cancer Network low-risk localized prostate cancer in which each arm was compared with a historical control. Patients were randomized by the NRG Oncology Statistics and Data Management Center using an automated permuted block randomization scheme to receive UHRT to a dose of 36.25 Gy (5 fractions of 7.25 Gy in 2 weeks and a day [twice a week]) or 51.6 Gy (12 fractions of 4.3 Gy in 2.5 weeks [5 times a week]).27 Patients were stratified according to radiation therapy treatment technique (Cyberknife vs intensity modulated radiation therapy [IMRT]/volumetric modulated arc therapy [VMAT] vs protons). The primary results of the trial have previously been published.35
Study patients
Eligibility criteria included patients with prostate adenocarcinoma, Gleason scores of 2 to 6, cT1–2a, and PSA <10 ng/mL. Patients undergoing active surveillance who were rebiopsied and confirmed still to have low-risk disease were eligible for enrollment within 1 year of the repeat biopsy.
Patients were only randomized if they were willing and able to complete the EPIC questionnaire. The institutional review board of each participating institution approved the study protocol. All patients were required to read and sign an informed consent document. Ineligibility criteria included prior or concurrent invasive malignancy (except nonmelanomatous skin cancer) or lymphomatous or hematogenous malignancy, unless continually disease-free for a minimum of 5 years. Patients with distant metastases; regional lymph node involvement; previous prostatectomy; cryosurgery; high-intensity focused ultrasound treatment; pelvic irradiation; prostate brachytherapy; bilateral orchiectomy or hormonal therapy, such as luteinizing hormone releasing hormone agonists or antagonists; anti-androgens; estrogens; or previous or concurrent cytotoxic chemotherapy for prostate cancer were ineligible. Patients were also ineligible if they had used finasteride within 30 days or dutasteride within 30 to 90 days before registration. Patients with severe active comorbidities were ineligible.
Study treatment
Patients were treated using stereotactic body radiation therapy (SBRT) in 5 or 12 fractions (UHRT) and could be treated using CyberKnife, IMRT/VMAT techniques, or protons, as long as the protocol-specified dosimetry criteria were met. The dosimetry criteria included planning target volume coverage and normal tissue constraints for the rectum, bladder, urethra, penile bulb, and femoral heads. The urethral dose was <107% of the prescription dose. The dosimetry criteria were identical in each arm regardless of treatment technique except for maximum dose within the planning target volume, which was 120% for CyberKnife technique and 107% for the other techniques (in recognition of achievable dose distributions with CyberKnife). The first 5 patients accrued from each institution were reviewed for quality assurance of protocol-defined dosimetry parameters. The dosimetry constraints were derived from published case series using high-precision techniques and UHRT regimens. Image guidance was required and is detailed in the study protocol.
Patient assessments
In addition to the disease-specific EPIC, EuroQol’s EQ-5D was collected to assess global quality of life. The EQ-5D consists of 2 parts. The first part includes 5 items related to quality of life rated by 3 problem levels.37,38 These 5 items are used to create an index score between 0 (worst health state) and 1 (best health state). The second part is a visual analog scale (VAS) valuing current health state, ranging from 0 for the worst imaginable health state to 100 for the best.
Pretreatment assessments included patient history and physical examination, performance status, PSA measurement, completion of EPIC and EQ-5D questionnaires, and baseline toxicity assessment. Performance status and adverse events were captured weekly during radiation therapy. Performance status, physical examination (including digital rectal examination), PSA measurement, and adverse event evaluation were performed every 3 months for 2 years after randomization and then every 6 months until 5 years. The EPIC and EQ-5D questionnaires were collected at baseline and at 1, 2, and 5 years after the end of radiation therapy.
Endpoints
The coprimary endpoints of this study, the percentage of patients with a >5 or >2 point reduction from baseline in the EPIC bowel and urinary domains, respectively, at 1 year, have previously been published.1
Secondary endpoints reported here include the EPIC bowel, urinary, sexual, and hormonal scores and EQ-5D index and VAS scores at 5 years; an update on late genitourinary and gastrointestinal toxicity as measured by the National Cancer Institute’s Common Terminology Criteria for Adverse Events (version 4.0); PSA failure rates, using the Phoenix definition28 of a PSA increase of >2 ng/mL above nadir; and disease-free survival (DFS), measured from the date of randomization to the date of documentation of recurrence (based on physical examination, PSA, bone scans, computed tomography/magnetic resonance imaging, and biopsies), the date of death, or the patient’s last known follow-up.
Statistical analysis
The EPIC scores were analyzed as dichotomous variables defined using a half SD of NRG/RTOG 0415 data, a prior study in a similar patient population, as a cutoff. Thus, a worsening from baseline in EPIC bowel score of >5 points, urinary score of >2 points, sexual score of ≥ 11, and a drop of ≥ 3 in EPIC hormonal score was thought to be significant. A 1-sided 1-sample binomial test was used to compare the rate of patients with a change greater than the specified value for EPIC domains.30 Mixed effects models were used to assess the effect of time, radiation therapy method (IMRT/VMAT vs CyberKnife vs protons) while adjusting for baseline PSA, age (<65 vs ≥65 years), and race (white vs nonwhite) on each of the continuous EPIC domain scores while adjusting for the baseline score. The EQ-5D index and VAS scores were compared with baseline using a Wilxocon signed rank test. The DFS was analyzed using the Kaplan-Meier method,31 and PSA failure was estimated using cumulative incidence.32 Adverse events were categorized as acute (≤30 days after radiation therapy completion) or late (>30 days after radiation therapy completion). All analyses were conducted using SAS 9.4 (SAS Institute, Cary, NC).
Results
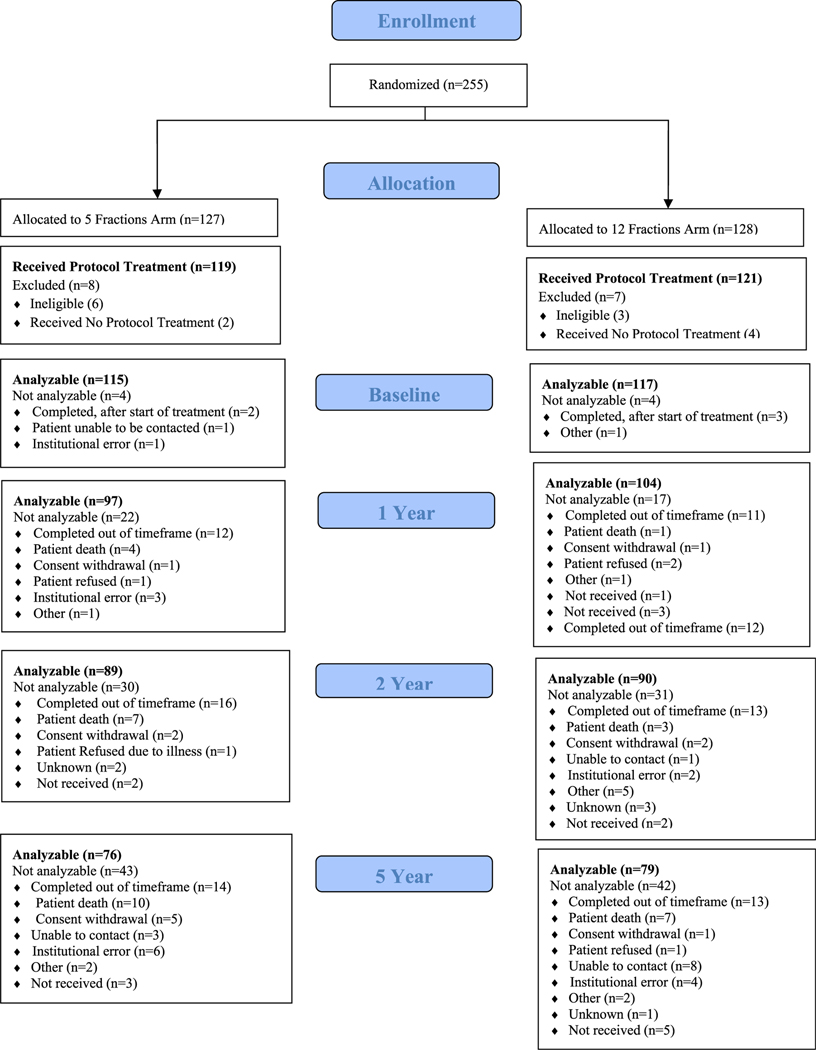
From September 2011 to February 2014, 255 patients were randomized, with 127 patients on the 5-fraction arm of the study and 128 patients on the 12-fraction arm (Fig. 1). Nine patients were found ineligible (7 with baseline PSA and 2 with history and physical examination out of the time window), and 6 patients did not receive protocol treatment. As a result, 119 and 121 patients were analyzable for the 5- and 12-fraction arms, respectively. The median age was 65 years. The majority of patients in the 5-fraction and 12-fraction arms had T1c disease (80.7% and 82.6%) and a median PSA of 5.6 and 5.5, respectively (Table 1). Median follow-up for all patients at the time of analysis was 5.38 years.
Fig. 1.
Consolidated Standards of Reporting Trials diagram.
Table 1.
Pretreatment characteristics
| 5 fractions (n = 119) | 12 fractions (n = 121) | |
|---|---|---|
| Age (y) | ||
| Median | 64 | 66 |
| Q1-Q3 | 59–69 | 60–70 |
| Race | ||
| Asian | 1 (0.8%) | 3 (2.5%) |
| Black or African American | 11 (9.2%) | 10 (8.3%) |
| White | 106 (89.1%) | 105 (86.8%) |
| Unknown | 1 (0.8%) | 3 (2.5%) |
| Ethnicity | ||
| Hispanic or Latino | 4 (3.4%) | 3 (2.5%) |
| Not Hispanic or Latino | 113 (95.0%) | 115 (95.0%) |
| Unknown | 2 (1.7%) | 3 (2.5%) |
| Zubrod performance status | ||
| 0 | 112 (94.1%) | 117 (96.7%) |
| 1 | 7 (5.9%) | 4 (3.3%) |
| Clinical N stage | ||
| N0 | 84 (70.6%) | 92 (76.0%) |
| NX | 35 (29.4%) | 29 (24.0%) |
| Clinical T stage | ||
| T1a | 2 (1.7%) | 0 (0.0%) |
| T1c | 96 (80.7%) | 100 (82.6%) |
| T2 | 1 (0.8%) | 0 (0.0%) |
| T2a | 20 (16.8%) | 21 (17.4%) |
| Treatment techniques/machine* | ||
| All linear accelerator-based treatment (excluding Cyberknife) | 92 (77.3%) | 95 (78.5%) |
| Cyberknife | 27 (22.7%) | 26 (21.5%) |
| PSA | ||
| Median | 5.6 | 5.5 |
| Q1-Q3 | 4.5–7.3 | 4.23–6.93 |
| Serum Testosterone (ng/mL) | (n = 108) | (n = 110) |
| Median | 257.5 | 265.5 |
| Q1-Q3 | 14.6 – 390.5 | 12.9–410 |
Stratification factor.
Abbreviations: Q1 = first quartile; Q3 = third quartile; PSA = prostate specific antigen.
The EPIC questionnaire completion compliance (based on completion of the bowel and urinary domains) was 96.7% before radiation therapy (see Fig. 1) and 85.5% at 1 year, higher than the rates of 79.5% (5 fractions) and 76.3% (12 fractions) at 2 years. At 5 years they were 69.7% (5 fractions) and 69.3% (12 fractions). The 1- and 2-year EPIC results have been previously published.35 This article focuses on the 5-year results.
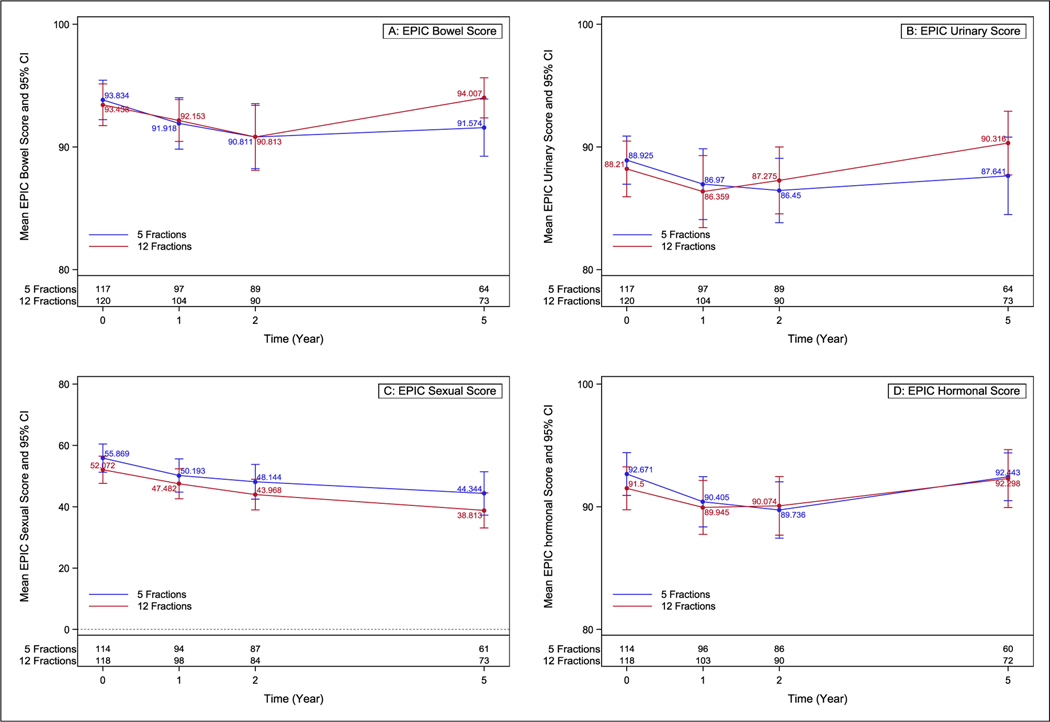
The 5-year EPIC results for >5-point change in bowel and >2 point in urinary score for the 5-fraction arm were 38.4% (P = .27) and 46.6% (P = .15). For the 12-fraction arm, these figures were 23.4% (P = .98) and 36.4% (P = .70). For sexual domains, the changes >11 points in the 5-fraction and 12-fraction arms were 49.3% (P = .007) and 54.0% (P < .001), respectively. There were 35.3% (P = .32) and 34.2% (P = .75) of patients with a >3 point change in hormonal scores in the 5-fraction and 12-fraction arms. One-, 2- and 5-year results are presented in Table 2 and Figure 2. There were no differences in change from baseline for EQ-5D index or VAS scores (Supplemental Table E1).
Table 2.
EPIC domain reductions
| 5 fractions | P value* | 12 fractions | P value* | |
|---|---|---|---|---|
| 1-year reductions | ||||
| Change >5 in EPIC bowel score1 | (n = 94) | (n = 100) | ||
| Yes | 28 (29.8%) | .14 | 29 (28.7%) | .093 |
| No | 66 (70.2%) | 72 (71.3%) | ||
| Change >2 in EPIC urinary score2 | (n = 94) | (n = 100) | ||
| Yes | 43 (45.7%) | .13 | 43 (42.6%) | .29 |
| No | 51 (54.3%)) | 58 (57.4%) | ||
| Change >11 in EPIC sexual score3 | (n = 88) | (n = 94) | ||
| Yes | 29 (32.9%) | .34 | 29 (30.9%) | .20 |
| No | 59 (67.1%) | 65 (69.7%) | ||
| Change >3 in EPIC hormonal score4 | (n = 91) | (n = 99) | ||
| Yes | 34 (37.4%) | .45 | 37 (37.4%) | .45 |
| No | 57 (62.6%) | 62 (62.6%) | ||
| 2-year reductions | ||||
| Change >5 in EPIC bowel score1 | (n = 86) | (n = 88) | ||
| Yes | 26 (30.2%) | .18 | 24 (27.3%) | .06 |
| No | 60 (69.3%) | 64 (72.7%) | ||
| Change >2 in EPIC urinary score2 | (n = 86) | (n = 88) | ||
| Yes | 48 (55.8%) | .001 | 42 (47.7%) | .07 |
| No | 38 (44.2%) | 46 (52.3%) | ||
| Change >11 in EPIC sexual score3 | (n = 83) | (n = 82) | ||
| Yes | 28 (33.7%) | .40 | 41 (50.0%) | .002 |
| No | 55 (66.3%) | 41 (50.0%) | ||
| Change >3 in EPIC hormonal score4 | (n = 80) | (n = 87) | ||
| Yes | 31 (38.7%) | .45 | 38 (43.7%) | .14 |
| No | 49 (61.3%) | 49 (56.3%) | ||
| 5-year reductions | ||||
| Change >5 in EPIC bowel score1 | (n = 73) | (n = 77) | ||
| Yes | 28 (38.4%) | .27 | 18 (23.4%) | .98 |
| No | 45 (61.6%) | 59 (76.6%) | ||
| Change >2 in EPIC urinary score2 | (n = 73) | (n = 77) | ||
| Yes | 34 (46.6%) | .15 | 28 (36.4%) | .70 |
| No | 39 (53.4%) | 49 (63.6%) | ||
| Change >11 in EPIC sexual score3 | (n = 69) | (n = 76) | ||
| Yes | 34 (49.3%) | .007 | 41 (54.0%) | < .001 |
| No | 35 (50.7%) | 35 (46.0%) | ||
| Change >3 in EPIC hormonal score4 | (n = 68) | (n = 76) | ||
| Yes | 24 (35.3%) | .32 | 26 (34.2%) | .75 |
| No | 44 (64.7%) | 50 (65.8%) | ||
Abbreviation: EPIC = Expanded Prostate Cancer Index Composite.
P value from one-sided one-sample-test.
Rate up to 35% is acceptable; a rate 2:55% is unacceptable.
Rate up to 40% is acceptable; a rate 2:60% is unacceptable.
Rate up to 38% is acceptable; a rate 2:58% is unacceptable.
Rate up to 38% is acceptable; a rate 2:58% is unacceptable.
Fig. 2.
Expanded Prostate Cancer Index Composite-50 domain scores across time. Error bars represent the 95% CI at each time point for each arm. (A) bowel, (B) urinary, (C) sexual, (D) hormonal.
A total of 78.5% of patients were treated with IMRT/VMAT technique and 21.5% with Cyberknife. No patients were treated with protons. For both the 5-fraction and 12-fraction arms, there was no significant effect of radiation therapy technique on the EPIC bowel, urinary, sexual, or hormonal scores (Supplemental Table E2).
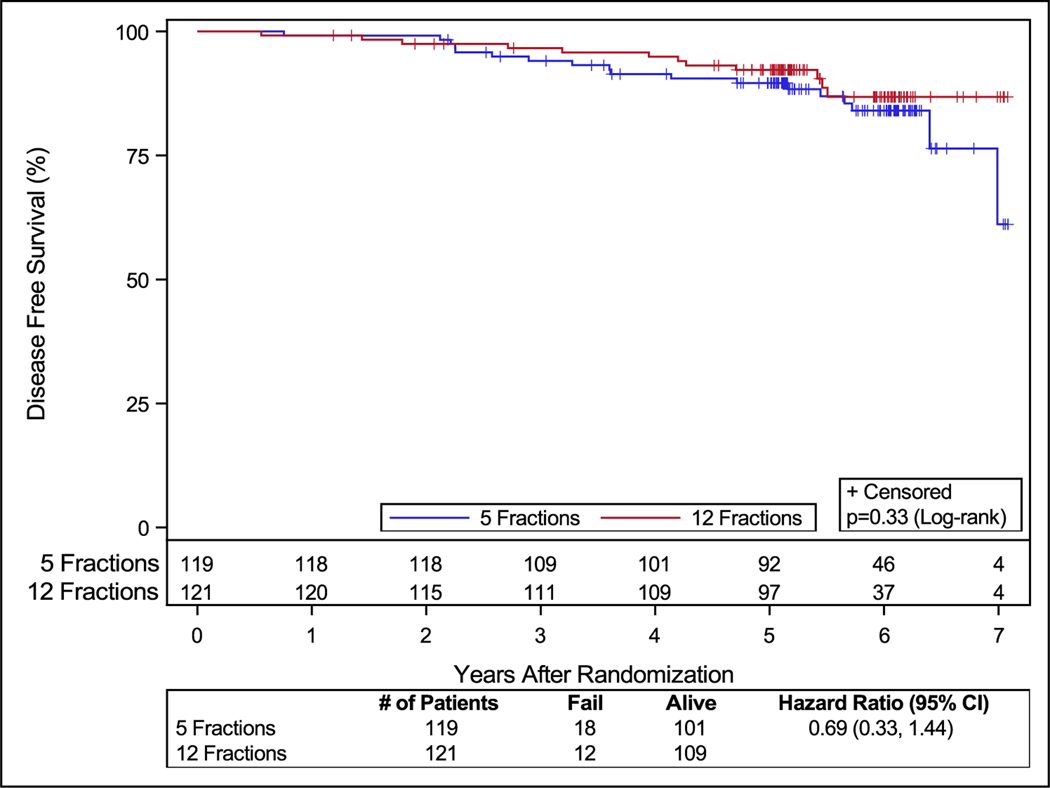
In the 5-fractions arm, 2 patients (1.6%) had late grade 3 treatment-related bladder or bowel toxicity (1 patient with proctitis and another with cystitis noninfective, renal, and urinary disorders [other, urinary incontinence, and urinary tract obstruction]). There were no grade 4 or 5 treatment-related late urinary or bowel toxicities reported. Two patients (1.6%) reported late grade 3 treatment-related toxicities (proctitis, urinary retention, and colonic fistula) in the 12-fraction arm. One of these 3 patients reported a colonic fistula, which was considered a grade 3 late toxicity; further details of the patient’s course and management are not available. No patient reported grade 4 or 5 late treatment-related urinary or bowel toxicity (Table 3). The rate of PSA failures and DFS events was low. The PSA failure rates at the time of analysis were 5.04% (6 patients) and 4.1 % (5 patients), respectively, in the 5- and 12-fraction arms. The DFS rates were 84.9% (18 patients) and 90.1% (12 patients), respectively, in the 5-fraction and 12-fraction arms of the study (Fig. 3).
Table 3.
Number of patients with an adverse event by system organ class, term, and grade late gastrointestinal/genitourinary grade 3 + treatment-related toxicity
| System organ class term | 5 Fractions (n = 119) Grade |
12 Fractions (n = 121) Grade |
||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| 1 | 2 | 3 | 4 | 5 | 1 | 2 | 3 | 4 | 5 | |
| Gastrointestinal disorders | 0 | 0 | 1 | 0 | 0 | 0 | 0 | 1 | 0 | 0 |
| Colonic fistula | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 1 | 0 | 0 |
| Proctitis | 0 | 0 | 1 | 0 | 0 | 0 | 0 | 1 | 0 | 0 |
| Renal and urinary disorders | 0 | 0 | 1 | 0 | 0 | 0 | 0 | 2 | 0 | 0 |
| Cystitis noninfective | 0 | 0 | 1 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| Hematuria | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 1 | 0 | 0 |
| Renal and urinary disorders (other) | 0 | 0 | 1 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| Urinary incontinence | 0 | 0 | 1 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| Urinary retention | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 1 | 0 | 0 |
| Urinary tract obstruction | 0 | 0 | 1 | 0 | 0 | 0 | 0 | 1 | 0 | 0 |
Includes adverse events where relationship to protocol treatment is missing. Adverse events were graded with CTCAE version 4.
Fig. 3.
Disease-free survival.
Discussion
Recently reported RCT data using moderate HypoRT have shown bNED (no evidence of biochemical failure) rates and toxicity outcomes comparable to those with SRT (7.5–8 weeks).11–13 Early published results of one ultra hypofractionated radiation therapy regimen (42.7 Gy in 7 fractions) compared with conventional fractionated radiation therapy found noninferiority.36 Longer term results from these studies are awaited. The benefits of HypoRT include patient convenience and the ability for more patients to be treated with available resources. Based on radiobiological data suggesting a low α/β ratio of prostate cancer may confer a therapeutic gain, UHRT using high-precision radiation therapy techniques has been shown in case series to result in low incidence of acute and late toxicity.14–19 This multi-institutional study evaluating 2 UHRTs in localized prostate cancer is one of the first studies to use bowel and urinary PROs in a hypofractionated trial to inform future RCTs comparing UHRT with SRT. In general, a change in these functions exceeding half of a standard deviation is a minimal important difference when assessing PROs.29 Although other investigators have suggested using other measures, such as twice the minimal important difference,33,34 in this study, as per the National Cancer Institute−approved protocol, we have reported on the results based on a change in PROs of more than half of a standard deviation.
The BED and EQD2 using alpha/beta of 10 for the 2 regimens were 62.53 and 52.11 (36.25 Gy/5 fractions) and 73.79 and 61.49 (51.6 Gy/12 fractions), respectively. For alpha/beta of 3 for the 2 regimens, they were 123.85 and 74.31 (36.25 Gy/5 fractions) and 125.56 and 75.34 (51.6 Gy/12 fractions), respectively.
In this study, the percentage of patients with more than a half SD change in EPIC domains scores was compared with baseline. In the standard arm of the NRG/RTOG 0415, 39% of patients experienced a change in urinary score >2 at 1 year, whereas in NRG/RTOG 0938 45.7% at 5 years and 42.6% at 1 year experienced a >2 point change in urinary score. Although this is higher than the 1-year rate in NRG/RTOG 0415, it is well below the unacceptable 60% rate specified in the protocol. In addition, a change of urinary function of 2 points is small and is felt not to be clinically meaningful. The urinary score change at >2 is low compared with the bowel and sexual scores. Thus, from a clinical perspective, this small level is not as clinically meaningful. It is a limitation of the concept that quality of life clinical trialists have put forward that the half SD is meaningful, but when the half SD value is low it may not be as clinically meaningful. It is also the general experience of clinicians treating these patients with this regimen that it is not clinically meaningful.
Overall, the NRG/RTOG 0938 1- and 5-year (47.5%) urinary results are felt to be acceptable. In comparison to the standard SRT arm of the NRG/RTOG 0415 study, the PROs for urinary, bowel, and sexual function for the 5- and 12-fraction radiation therapy regimens of this study are comparable and acceptable. The results of this study are also in keeping with case series reporting on PROs using prostate SBRT.23–26 The late toxicity rate of these regimens is low and comparable with those with SRT, although longer follow-up is required. Given the variability in sexual functioning with age and other clinical situations, comparisons between SBRT and current standard radiation therapy with regard to sexual function as a secondary endpoint are best made in the context of an RCT comparing the 2 treatments.
In this study, as long as the dosimetry parameters (especially normal tissue constraints) could be met, patients could be treated by any radiation therapy technique (CyberKnife, VMAT, IMRT, or protons). A strength of this study is that real-time radiation therapy and plan review were undertaken as part of the quality assurance of protocol-defined dosimetry parameters. A limitation of this study is the high degree of missing PRO data at 5 years—about 30% in both arms. Collecting long-term PRO data is an ongoing challenge for many studies, and NRG Oncology has a task force focused on improving compliance. The PROs for patients treated with IMRT/VMAT technique and CyberKnife were comparable.
The PSA failure rate in this study is low, although longer follow-up is required. The DFS rate is higher because it includes death from all causes.
Conclusions
The 5- and 12-fraction UHRTs in this study are well tolerated. The late toxicity rates for the 5-fraction and 12-fraction regimens were low. The bowel, urinary, sexual, and hormonal PROs are comparable to those reported for SRT. Ongoing and maturing randomized trials, such as PACE and NRG-GU005, are comparing UHRT with moderate HypoRT and conventional fractionation regimens, and we await their mature results.
Supplementary Material
Disclosures:
J.A.E. receives consulting fees from Blue Earth Diagnostics, Boston Scientific, AstraZeneca, and Genentech and participation on data safety monitoring or advisory boards for Progenics, Merck, Roviant Pharma, Myovant Sciences, Janssen, and Bayer Health Care. H.M.S. is a member of the clinical trial steering committee at Janssen and a member of the board of directors at ASTRO. He holds stock from inactive medical advisory board Radiogel. F.Y.F. has consulted for Janssen, Bayer, PFS Genomics, Myovant Sciences, Roivant, Astellas, Foundation Medicine, Varian, Bristol Meyers Squibb, Exact Sciences, BlueStar Genomics, Novartis, and Tempus. He also received stock options from SerImmune for serving on its scientific advisory board. He is cofounder and advisor of Artera, a company focused on digital pathology biomarkers in prostate cancer and serves as chairman of the Genitourinary Cancer Committee for NRG Oncology. H.R.L. receives study support from RTOG/NRG Oncology per patient funding to the institution and advisory board consulting fees from Abbvie, Tersera,Tolmar, Sanofi, Jansen. D.D.G. received honoria from Abbvie and serves on the data safety or advisory board for Ferring and Ter-Sera. L.A.K. reports NCI NRG Oncology NCORP Associate PI 2.0% effort to oversee all NCORP trial development, enrollment, and analysis/publication. She is also an unpaid board member for the RTOG Foundation. D.W. B. received consulting fees from Flatiron Health Inc and served as an unpaid member of American Society Clinical Oncology (ASCO) Fatigue Guideline Update Panel and as a member of the Society for Integrative Oncology (SIO) and American Society of Clinical Oncology (ASCO) joint guideline committee “An integrative approach to cancer-related pain management” and the NRG Oncology Executive Advisory Board and Georgia advisory committee of the U.S. Global Leadership Coalition and Board of Directors, Center for Global Health Innovation, Atlanta, GA and Evaluating Physical Functioning Using Patient-reported Outcome Measures: How does the question form and recall period influence patients’ interpretation? She is a member of the Stakeholder Advisory Panel, Duke University, Bryce Reeve, PhD, (PI). She receives funding from the FDA Broad Agency Announcement (BAA) and Building Interdisciplinary Research Careers in Women’s Health (BIRCWH) program. She is a member of the Executive Committee, Emory University, Claire Sterk & Igho Ofotokun (co-PIs), funded through NIH. She serves on the Electronic Patient Reporting of Symptoms During Cancer Treatment (PRO-TECT) Member Advisory Board. University North Carolina, Chapel Hill, Ethan Basch, PhD (PI). Funded through PCORI and Alliance. Her meeting travel expenses were paid by the PROTEUS Consortium (Patient- Reported Outcomes Tools: Engaging Users & Stakeholders). J.-P. B. received payment for expert testimony and serves as the unpaid vice chairman for Canadian Affairs.
Funding:
This project was supported by grants UG1CA189867 (NCORP) from the National Cancer Institute (NCI)
Footnotes
All data will be made available per the NCTN Data Archive rules. The link for the archive is: https://nctn-data-archive.nci.nih.gov/.
Supplementary material associated with this article can be found, in the online version, at doi:10.1016/j.ijrobp.2022.12.022.
This protocol (NRG/RTOG 0938 NCT01434290) is registered with ClinicalTrials.gov.
References
- 1.Kuban DA, Tucker SL, Dong L, et al. Long-term results of the M. D. Anderson randomized dose-escalation trial for prostate cancer. Int J Radiat Oncol Biol Phys 2008;70:67–74. [DOI] [PubMed] [Google Scholar]
- 2.Zietman AL, DeSilvio ML, Slater JD, et al. Comparison of conventional-dose vs high-dose conformal radiation therapy in clinically localized adenocarcinoma of the prostate: A randomized controlled trial. JAMA 2005;294:1233–1239. [DOI] [PubMed] [Google Scholar]
- 3.Bruner DW, Hunt D, Michalski JM, et al. Preliminary patient-reported outcomes analysis of 3-dimensional radiation therapy versus intensity-modulated radiation therapy on the high-dose arm of the Radiation Therapy Oncology Group (RTOG) 0126 prostate cancer trial. Cancer 2015;121:2422–2430. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Brenner D, Armour E, Corry P, Hall E. Sublethal damage repair times for a late-responding tissue relevant to brachytherapy (and external-beam radiotherapy): Implications for new brachytherapy protocols. Int J Radiat Oncol Biol Phys 1998;41:135–138. [DOI] [PubMed] [Google Scholar]
- 5.Brenner DJ, Hall EJ. Fractionation and protraction for radiotherapy of prostate carcinoma. Int J Radiat Oncol Biol Phys 1999;43:1095–1101. [DOI] [PubMed] [Google Scholar]
- 6.Fowler JF, Ritter MA, Chappell RJ, Brenner DJ. What hypofractionated protocols should be tested for prostate cancer? Int J Radiat Oncol Biol Phys 2003;56:1093–1104. [DOI] [PubMed] [Google Scholar]
- 7.Thames HD, Suit HD. Tumor radioresponsiveness versus fractionation sensitivity. Int J Radiat Oncol Biol Phys 1986;12:687–691. [DOI] [PubMed] [Google Scholar]
- 8.Wang JZ, Guerrero M, Li XA. How low is the alpha/beta ratio for prostate cancer? Int J Radiat Oncol Biol Phys 2003;55:194–203. [DOI] [PubMed] [Google Scholar]
- 9.Wang JZ, Li XA, Yu CX, DiBiase SJ. The low alpha/beta ratio for prostate cancer: What does the clinical outcome of HDR brachytherapy tell us? Int J Radiat Oncol Biol Phys 2003;57:1101–1108. [DOI] [PubMed] [Google Scholar]
- 10.Brenner DJ, Martinez AA, Edmundson GK, Mitchell C, Thames HD, Armour EP. Direct evidence that prostate tumors show high sensitivity to fractionation (low alpha/beta ratio), similar to late-responding normal tissue. Int J Radiat Oncol Biol Phys 2002;52:6–13. [DOI] [PubMed] [Google Scholar]
- 11.Lee WR, Dignam JJ, Amin MB, et al. Randomized phase III non-inferiority study comparing two radiotherapy fractionation scheduled in patients with low-risk prostate cancer. J Clin Oncol 2016;34:2325–2332. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Dearnaley D, Syndikus I, Mossop H, et al. Conventional versus hypofractionated high dose intensity modulated radiotherapy for prostate cancer: 5-year outcomes of the randomised, non-inferiority phase 3 CHHiP trial. Lancet Oncol 2006;17:1047–1060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Catton CN, Lukka H, Gu CS, et al. Randomised trial of a hypofractionated radiation regimen for the treatment of localised prostate cancer. J Clin Oncology 2017;35:1884–1890. [DOI] [PubMed] [Google Scholar]
- 14.King CR, Brooks JD, Gill H, Pawlicki T, Cotrutz C, Presti Jr JC. Stereotactic body radiotherapy for localized prostate cancer: Interim results of a prospective phase II clinical trial. Int J Radiat Oncol Biol Phys 2009;73:1043–1048. [DOI] [PubMed] [Google Scholar]
- 15.King CR, Freeman D, Kaplan I, et al. Stereotactic Body radiotherapy for localised prostate cancer: pooled analysis from a multi-institutional consortium of prospective phase II trials. Radiother Oncol 2013;109:217–221. [DOI] [PubMed] [Google Scholar]
- 16.Madsen BL, Hsi RA, Pham HT, Fowler JF, Esagui L, Corman J. Stereotactic hypofractionated accurate radiotherapy of the prostate (SHARP), 33.5 Gy in five fractions for localized disease: First clinical trial results. Int J Radiat Oncol Biol Phys 2007. Mar 15;67:1099–1105. [DOI] [PubMed] [Google Scholar]
- 17.Menkarios C, Nguyen DHA, Vigneault E, et al. Hypofractionated radiotherapy for low risk prostate cancer: Preliminary results of a phase I/II trials. Radiother Oncol 2009;92(Suppl 2):S43. [Google Scholar]
- 18.Ritter MA, Forman JD, Kupelian PA, et al. A phase I/II trial of increasingly hypofractionated radiation therapy for prostate cancer. Int J Radiat Oncol Biol Phys 2009;75(Suppl):S80–S81. [Google Scholar]
- 19.Tang CI, Loblaw DA, Cheung P, et al. Phase I/II study of a five fraction hypofractionated accelerated radiotherapy treatment for low-risk localized prostate cancer: Early results of pHART3. Clin Oncol 2008;20:729–737. [DOI] [PubMed] [Google Scholar]
- 20.Wu AW, Jacobson KD, Frick DL, et al. Validity and responsiveness of the EQ5D as a measure of health-related quality of life in people enrolled in an AIDS clinical trial. Qual Life Res 2002;11:273–282. [DOI] [PubMed] [Google Scholar]
- 21.Sanda MG, Dunn RL, Michalski J, et al. Quality of life and satisfaction with outcome among prostate cancer survivors. N Engl J Med 2008;358:1250–1261. [DOI] [PubMed] [Google Scholar]
- 22.WEi JT, Dunn RL, Sandler HM. Comprehensive comparison of health related quality of Life after contemporary therapies for localised prostate cancer. J Clin Oncol 2000;20:557–566. [DOI] [PubMed] [Google Scholar]
- 23.Kat AJ, Santoro M, Diblasio F, Ashley R. Stereotactic body radiotherapy for localized prostate cancer: Disease control and quality of life at 6 years. Radiat Oncol 2013;8:118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Boyer MJ, Papagikos MA, Kiteley R, et al. Toxicity and quality of life report of phase II study of stereotactic body radiotherapy (SBRT) for low and intermediate risk prostate cancer. Radiat Oncol 2017;12:14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Chen LN, Suy S, Wang H, et al. Patient-reported urinary incontinence following stereotactic body radiation therapy (SBRT) for clinically localized prostate cancer. Radiat Oncol 2014;9:148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Jackson WC, Dess RT, Litenberg DW, et al. A multi-institutional phase 2 trial of prostate stereotactic body radiation therapy (SBRT) using continuous real-time evaluation of prostate motion with patient-reported quality of life. Pract Radiat Oncol 2018;8:40–47. [DOI] [PubMed] [Google Scholar]
- 27.Zelen M. The randomization and stratification of patients to clinical trials. J Chronic Dis 1974. Sep;27:365–375. [DOI] [PubMed] [Google Scholar]
- 28.Roach 3rd M, Hanks G, Thames Jr H, et al. Defining biochemical failure following radiotherapy with or without hormonal therapy in men with clinically localised prostate cancer: recommendations of the RTOG-ASTRO Phoenix Consensus Conference. Int J Radiat Oncol Biol Phys 2006;65:965–974. [DOI] [PubMed] [Google Scholar]
- 29.Brundage M, Osoba D, Bezjak A, Tu D, Palmer M, Pater J. National Cancer Institute of Canada Clinical Trials Group. Lessons learned in the assessment of health-related quality of life: Selected examples from the National Cancer Institute of Canada Clinical Trials Group. J Clin Oncol 2007;25:5078–5081. [DOI] [PubMed] [Google Scholar]
- 30.Hosmer DW, Lemeshow S. Applied Logistic Regression. 2nd ed. New York, NY: John Wiley & Sons, Inc; 2000. [Google Scholar]
- 31.Kaplan EL, Meier P. Non-parametric estimation from incomplete observations. J Am Stat Assoc 1958;53:457–481. [Google Scholar]
- 32.Kalbfleisch JD, Prentice RL. The Statistical Analysis of Failure Time Data. New York, NY: John Wiley and Sons; 1980. [Google Scholar]
- 33.Evans JR, Zhao S, Daignault S , et al. Patient-reported quality of life after stereotactic body radiotherapy (SBRT), intensity modulated radiotherapy (IMRT), and brachytherapy. Radiother Oncol 2015; 116:179–184. [DOI] [PubMed] [Google Scholar]
- 34.Dess RT, Jackson WC, Suy S, et al. Predictors of multidomain decline in health-related quality of life after stereotactic body radiation therapy (SBRT) for prostate cancer. Cancer 2017;123:1635–1642. [DOI] [PubMed] [Google Scholar]
- 35.Lukka HR, Pugh SL, Bruner DW, et al. Patient reported Outcomes in NRG Oncology RTOG 0938, Evaluating Two ultrahypofractionated Regimens for Prostate Cancer. Int J Radiat Oncol Biol Phys 2018;102:287–295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Widmark A, Gunnlaugsson A, Beckman L, et al. Ultra-hypofractionated versus conventionally fractionated radiotherapy for prostate cancer: 5-year outcomes of the HYPO-RT-PC randomised, non-inferiority, phase 3 trial. Lancet 2019. Aug 3;394:385–395. [DOI] [PubMed] [Google Scholar]
- 37.The EuroQol Group. EuroQol-a new facility for the measurement of health-related quality of life. Health Policy 1990;16:199–208. [DOI] [PubMed] [Google Scholar]
- 38.Brooks R. EuroQol: The current state of play. Health Policy 1996;37:53–72. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.