Abstract
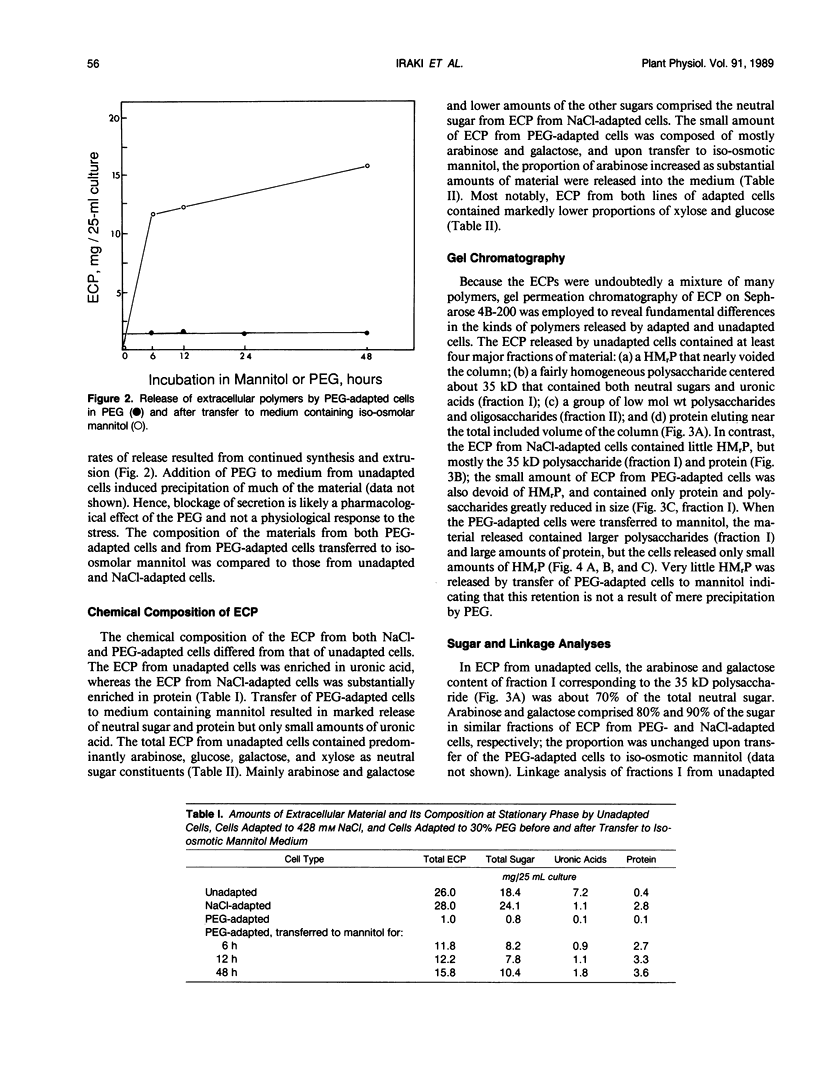
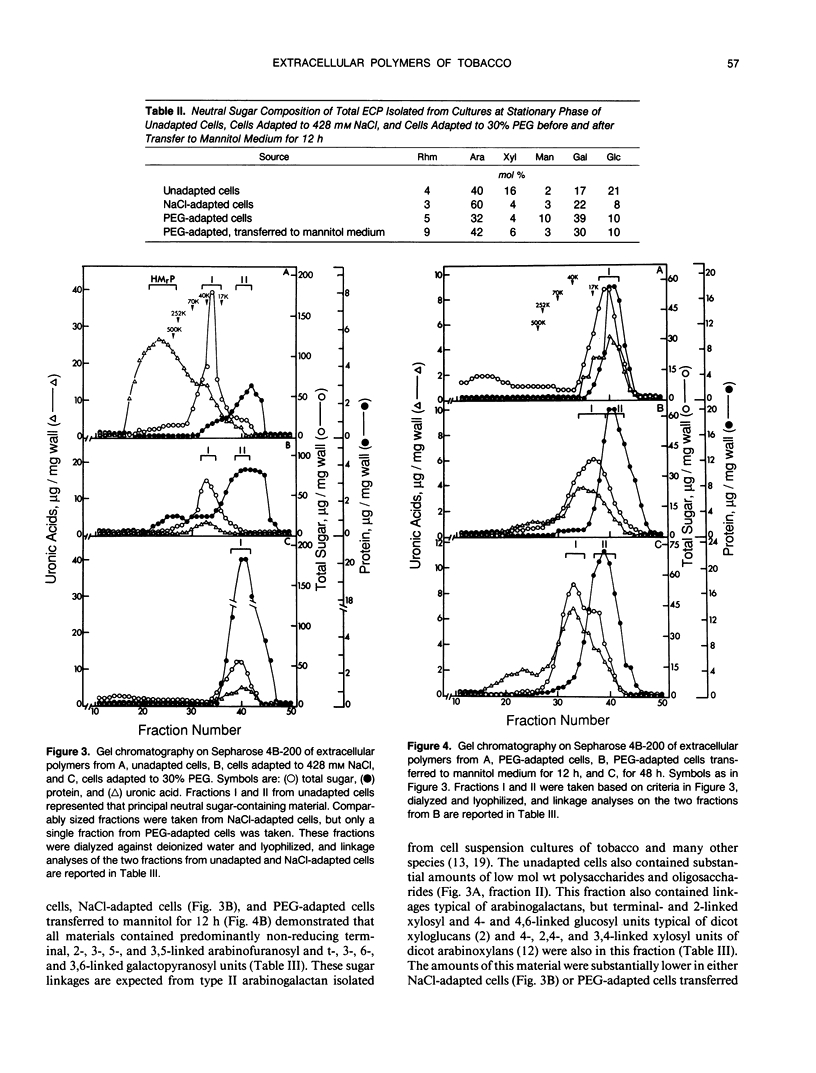
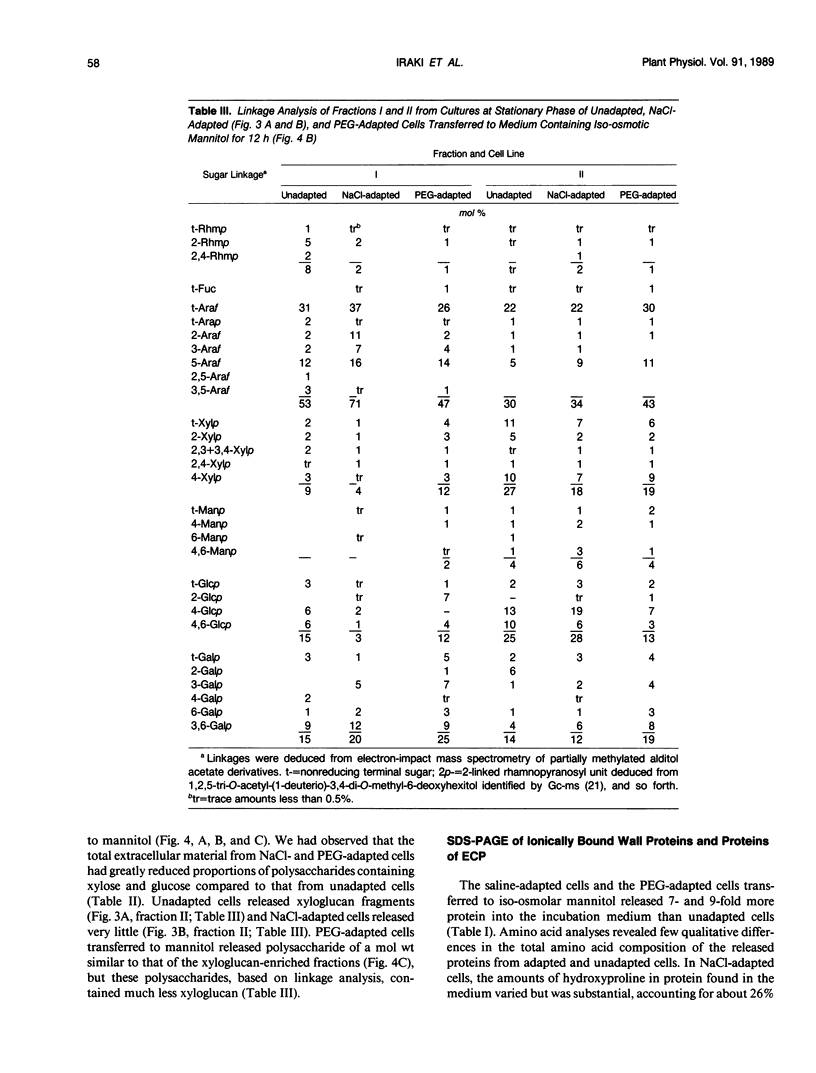
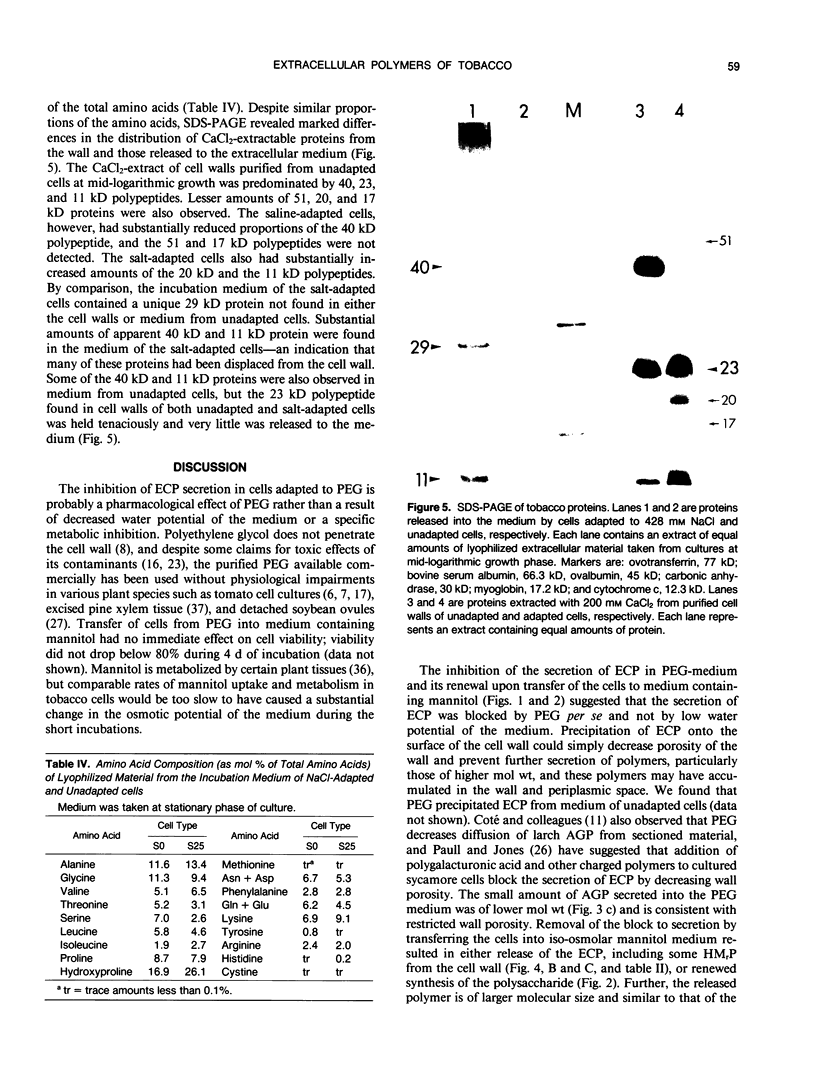
The chemical composition of extracellular polymers released by cells of tobacco (Nicotiana tabacum L. cv W38) adapted to a medium containing 30% polyethylene glycol 8000 (−28 bar) or 428 millimolar NaCl (−23 bar) was compared to the composition of those released by unadapted cells. Unadapted cells released uronic acid-rich material of high molecular weight, arabinogalactan-proteins, low molecular weight fragments of hemicellulosic polysaccharides, and a small amount of protein. Cells adapted to grow in medium containing NaCl released arabinogalactan and large amounts of protein but not the uronic acid-rich material, and cells adapted to grow in polyethylene glycol released only small amounts of an arabinogalactan of much lower molecular weight and some protein. Secretion of all material was nearly blocked by polyethylene glycol, but when cells were transferred to a medium containing iso-osmolar mannitol, they again released extracellular polymers at rates similar to those of unadapted cells. Like cells adapted to NaCl, however, these cells released arabinogalactan and large amounts of protein but only small amounts of the uronic acid-rich material. Media of NaCl-adapted cells were enriched in 40, 29, and 11 kilodalton polypeptides. CaCl2 extracted the 40 and 11 kilodalton polypeptides from walls of unadapted cells, but the 29 kilodalton polypeptide was found only in the medium of the NaCl-adapted cells. Accumulation of low molecular weight polysaccharide fragments in the medium was also substantially reduced in both NaCl- and polyethylene glycol-adapted cells, and specifically, the material was composed of lower proportions of xyloglucan fragments. Our results indicate that adaptation to saline or water stress results in inhibition of both the hydrolysis of hemicellulosic xyloglucan and release of uronic acid-rich material into the culture medium.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Aspinall G. O., Molloy J. A., Craig J. W. Extracellular polysaccharides from suspension-cultured sycamore cells. Can J Biochem. 1969 Nov;47(11):1063–1070. doi: 10.1139/o69-170. [DOI] [PubMed] [Google Scholar]
- Binzel M. L., Hasegawa P. M., Handa A. K., Bressan R. A. Adaptation of Tobacco Cells to NaCl. Plant Physiol. 1985 Sep;79(1):118–125. doi: 10.1104/pp.79.1.118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Binzel M. L., Hasegawa P. M., Rhodes D., Handa S., Handa A. K., Bressan R. A. Solute Accumulation in Tobacco Cells Adapted to NaCl. Plant Physiol. 1987 Aug;84(4):1408–1415. doi: 10.1104/pp.84.4.1408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bozarth C. S., Mullet J. E., Boyer J. S. Cell wall proteins at low water potentials. Plant Physiol. 1987 Sep;85(1):261–267. doi: 10.1104/pp.85.1.261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bressan R. A., Handa A. K., Handa S., Hasegawa P. M. Growth and water relations of cultured tomato cells after adjustment to low external water potentials. Plant Physiol. 1982 Nov;70(5):1303–1309. doi: 10.1104/pp.70.5.1303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carpita N., Sabularse D., Montezinos D., Delmer D. P. Determination of the pore size of cell walls of living plant cells. Science. 1979 Sep 14;205(4411):1144–1147. doi: 10.1126/science.205.4411.1144. [DOI] [PubMed] [Google Scholar]
- Darvill J. E., McNeil M., Darvill A. G., Albersheim P. Structure of Plant Cell Walls: XI. GLUCURONOARABINOXYLAN, A SECOND HEMICELLULOSE IN THE PRIMARY CELL WALLS OF SUSPENSION-CULTURED SYCAMORE CELLS. Plant Physiol. 1980 Dec;66(6):1135–1139. doi: 10.1104/pp.66.6.1135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Greenway H., Hiller R. G., Flowers T. Respiratory inhibition in Chlorella produced by "purified" polyethylene glycol 1540. Science. 1968 Mar 1;159(3818):984–985. doi: 10.1126/science.159.3818.984. [DOI] [PubMed] [Google Scholar]
- Handa S., Bressan R. A., Handa A. K., Carpita N. C., Hasegawa P. M. Solutes contributing to osmotic adjustment in cultured plant cells adapted to water stress. Plant Physiol. 1983 Nov;73(3):834–843. doi: 10.1104/pp.73.3.834. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hayashi T., Yoshida K. Cell expansion and single-cell separation induced by colchicine in suspension-cultured soybean cells. Proc Natl Acad Sci U S A. 1988 Apr;85(8):2618–2622. doi: 10.1073/pnas.85.8.2618. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Iraki N. M., Bressan R. A., Hasegawa P. M., Carpita N. C. Alteration of the physical and chemical structure of the primary cell wall of growth-limited plant cells adapted to osmotic stress. Plant Physiol. 1989 Sep;91(1):39–47. doi: 10.1104/pp.91.1.39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Iraki N. M., Singh N., Bressan R. A., Carpita N. C. Cell Walls of Tobacco Cells and Changes in Composition Associated with Reduced Growth upon Adaptation to Water and Saline Stress. Plant Physiol. 1989 Sep;91(1):48–53. doi: 10.1104/pp.91.1.48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LAGERWERFF J. V., OGATA G., EAGLE H. E. Control of osmotic pressure of culture solutions with polyethylene glycol. Science. 1961 May 12;133(3463):1486–1487. doi: 10.1126/science.133.3463.1486. [DOI] [PubMed] [Google Scholar]
- Labavitch J. M., Ray P. M. Relationship between Promotion of Xyloglucan Metabolism and Induction of Elongation by Indoleacetic Acid. Plant Physiol. 1974 Oct;54(4):499–502. doi: 10.1104/pp.54.4.499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pikaard C. S., Cherry J. H. Maintenance of Normal or Supranormal Protein Accumulation in Developing Ovules of Glycine max L. Merr. during PEG-Induced Water Stress. Plant Physiol. 1984 May;75(1):176–180. doi: 10.1104/pp.75.1.176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Singh N. K., Bracker C. A., Hasegawa P. M., Handa A. K., Buckel S., Hermodson M. A., Pfankoch E., Regnier F. E., Bressan R. A. Characterization of osmotin : a thaumatin-like protein associated with osmotic adaptation in plant cells. Plant Physiol. 1987 Oct;85(2):529–536. doi: 10.1104/pp.85.2.529. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Singh N. K., Larosa P. C., Handa A. K., Hasegawa P. M., Bressan R. A. Hormonal regulation of protein synthesis associated with salt tolerance in plant cells. Proc Natl Acad Sci U S A. 1987 Feb;84(3):739–743. doi: 10.1073/pnas.84.3.739. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stevenson T. T., McNeil M., Darvill A. G., Albersheim P. Structure of Plant Cell Walls : XVIII. An Analysis of the Extracellular Polysaccharides of Suspension-Cultured Sycamore Cells. Plant Physiol. 1986 Apr;80(4):1012–1019. doi: 10.1104/pp.80.4.1012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stuart D. A., Varner J. E. Purification and Characterization of a Salt-extractable Hydroxyproline-rich Glycoprotein from Aerated Carrot Discs. Plant Physiol. 1980 Nov;66(5):787–792. doi: 10.1104/pp.66.5.787. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taylor R. L., Conrad H. E. Stoichiometric depolymerization of polyuronides and glycosaminoglycuronans to monosaccharides following reduction of their carbodiimide-activated carboxyl groups. Biochemistry. 1972 Apr 11;11(8):1383–1388. doi: 10.1021/bi00758a009. [DOI] [PubMed] [Google Scholar]
- Terry M. E., Jones R. L. Soluble Cell Wall Polysaccharides Released from Pea Stems by Centrifugation : I. EFFECT OF AUXIN. Plant Physiol. 1981 Sep;68(3):531–537. doi: 10.1104/pp.68.3.531. [DOI] [PMC free article] [PubMed] [Google Scholar]