Abstract
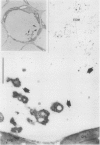
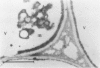
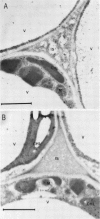
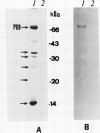
Citrus exocortis viroid induces in tomato plants (Lycopersicon esculentum) synthesis and accumulation of a pathogenesis-related protein (P69) previously reported to be a proteinase (Vera P, Conejero V [1988] Plant Physiol 87: 58-63). By immunogold/transmission electron microscopy, we have studied the distribution of this protein in thin sections of parenchymatous leaf tissue. The enzyme was present intra- and extracellularly. The intracellular location was limited to the vacuole and was always associated with engulfed cell material. When extracellularly located, the enzyme was associated with a dispersed, electron-dense material in the intercellular spaces. This latter location was confirmed after analysis of intercellular washing fluids obtained by vacuum infiltration of leaves. These observations provide new data for the understanding of viroid pathogenesis and the biological role of the pathogenesis-related proteinase P69.
Full text
PDF


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Boller T., Vögeli U. Vacuolar localization of ethylene-induced chitinase in bean leaves. Plant Physiol. 1984 Feb;74(2):442–444. doi: 10.1104/pp.74.2.442. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carr J. P., Dixon D. C., Nikolau B. J., Voelkerding K. V., Klessig D. F. Synthesis and localization of pathogenesis-related proteins in tobacco. Mol Cell Biol. 1987 Apr;7(4):1580–1583. doi: 10.1128/mcb.7.4.1580. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gianinazzi S., Martin C., Vallée J. C. Hypersensibilité aux virus, température et protéines soubles chez le Nicotiana Xanthi n.c. Apparition de nouvelles macromolécules lors de la répression de la synthèse virale. C R Acad Sci Hebd Seances Acad Sci D. 1970 May 11;270(19):2383–2386. [PubMed] [Google Scholar]
- Kauffmann S., Legrand M., Geoffroy P., Fritig B. Biological function of ;pathogenesis-related' proteins: four PR proteins of tobacco have 1,3-beta-glucanase activity. EMBO J. 1987 Nov;6(11):3209–3212. doi: 10.1002/j.1460-2075.1987.tb02637.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kombrink E., Schröder M., Hahlbrock K. Several "pathogenesis-related" proteins in potato are 1,3-beta-glucanases and chitinases. Proc Natl Acad Sci U S A. 1988 Feb;85(3):782–786. doi: 10.1073/pnas.85.3.782. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Legrand M., Kauffmann S., Geoffroy P., Fritig B. Biological function of pathogenesis-related proteins: Four tobacco pathogenesis-related proteins are chitinases. Proc Natl Acad Sci U S A. 1987 Oct;84(19):6750–6754. doi: 10.1073/pnas.84.19.6750. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Singh N. K., Bracker C. A., Hasegawa P. M., Handa A. K., Buckel S., Hermodson M. A., Pfankoch E., Regnier F. E., Bressan R. A. Characterization of osmotin : a thaumatin-like protein associated with osmotic adaptation in plant cells. Plant Physiol. 1987 Oct;85(2):529–536. doi: 10.1104/pp.85.2.529. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thayer S. S., Huffaker R. C. Vacuolar Localization of Endoproteinases EP(1) and EP(2) in Barley Mesophyll Cells. Plant Physiol. 1984 May;75(1):70–73. doi: 10.1104/pp.75.1.70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van der Wilden W., Herman E. M., Chrispeels M. J. Protein bodies of mung bean cotyledons as autophagic organelles. Proc Natl Acad Sci U S A. 1980 Jan;77(1):428–432. doi: 10.1073/pnas.77.1.428. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vera P., Conejero V. Pathogenesis-related proteins of tomato : p-69 as an alkaline endoproteinase. Plant Physiol. 1988 May;87(1):58–63. doi: 10.1104/pp.87.1.58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walker-Simmons M., Ryan C. A. Immunological Identification of Proteinase Inhibitors I and II in Isolated Tomato Leaf Vacuoles. Plant Physiol. 1977 Jul;60(1):61–63. doi: 10.1104/pp.60.1.61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wittenbach V. A., Lin W., Hebert R. R. Vacuolar localization of proteases and degradation of chloroplasts in mesophyll protoplasts from senescing primary wheat leaves. Plant Physiol. 1982 Jan;69(1):98–102. doi: 10.1104/pp.69.1.98. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Loon L. C., van Kammen A. Polyacrylamide disc electrophoresis of the soluble leaf proteins from Nicotiana tabacum var. "Samsun" and "Samsun NN". II. Changes in protein constitution after infection with tobacco mosaic virus. Virology. 1970 Feb;40(2):190–211. doi: 10.1016/0042-6822(70)90395-8. [DOI] [PubMed] [Google Scholar]