Abstract
Introduction
The association between extreme birth spacing and adverse outcomes is controversial, and available evidence is fragmented into different classifications of birth spacing.
Material and methods
We conducted a systematic review of observational studies to evaluate the association between birth spacing (i.e., interpregnancy interval and interoutcome interval) and adverse outcomes (i.e., pregnancy complications, adverse birth outcomes). Pooled odds ratios (ORs) with 95% confidence intervals (CI) were calculated using a random‐effects model, and the dose–response relationships were evaluated using generalized least squares trend estimation.
Results
A total of 129 studies involving 46 874 843 pregnancies were included. In the general population, compared with an interpregnancy interval of 18–23 months, extreme intervals (<6 months and ≥ 60 months) were associated with an increased risk of adverse outcomes, including preterm birth, small for gestational age, low birthweight, fetal death, birth defects, early neonatal death, and premature rupture of fetal membranes (pooled OR range: 1.08–1.56; p < 0.05). The dose–response analyses further confirmed these J‐shaped relationships (p non‐linear < 0.001–0.009). Long interpregnancy interval was only associated with an increased risk of preeclampsia and gestational diabetes (p non‐linear < 0.005 and p non‐linear < 0.001, respectively). Similar associations were observed between interoutcome interval and risk of low birthweight and preterm birth (p non‐linear < 0.001). Moreover, interoutcome interval of ≥60 months was associated with an increased risk of cesarean delivery (pooled OR 1.72, 95% CI 1.04–2.83). For pregnancies following preterm births, an interpregnancy interval of 9 months was not associated with an increased risk of preterm birth, according to dose–response analyses (p non‐linear = 0.008). Based on limited evidence, we did not observe significant associations between interpregnancy interval or interoutcome interval after pregnancy losses and risk of small for gestational age, fetal death, miscarriage, or preeclampsia (pooled OR range: 0.76–1.21; p > 0.05).
Conclusions
Extreme birth spacing has extensive adverse effects on maternal and infant health. In the general population, interpregnancy interval of 18–23 months may be associated with potential benefits for both mothers and infants. For women with previous preterm birth, the optimal birth spacing may be 9 months.
Keywords: adverse pregnancy outcome, adverse birth outcome, birth interval, interoutcome interval, interpregnancy interval
In this systematic review and meta‐analysis of 129 studies, extreme short or long birth spacing was associated with increased odds of adverse pregnancy and birth outcomes. In the general population, interpregnancy interval of 18 to 23 months may be associated with potential benefits for both mothers and infants. For women with previous preterm birth, the optimal birth spacing may be 9 months.

Abbreviations
- CI
confidence interval
- GDM
gestational diabetes mellitus
- IOI
interoutcome interval
- IPI
interpregnancy interval
- LBW
low birthweight
- OR
odds ratio
- PIH
pregnancy‐induced hypertension
- PROM
premature rupture of fetal membranes
- PTB
preterm birth
- SGA
small for gestational age
- WHO
World Health Organization
Key message.
Interpregnancy interval of 18 to 23 months could have benefits in the general population, and women with previous preterm birth should wait 9 months before conceiving again. To further explore this association and underlying unmeasured confounders, higher quality cohorts are needed.
1. INTRODUCTION
Optimal birth spacing is an important component of postpartum family planning criteria that can yield short‐ and long‐term benefits for mothers children. 1 , 2 To reduce adverse events in subsequent pregnancies, the 2005 WHO guidelines recommend that women wait at least 2 years after a live birth and 6 months after a miscarriage or induced abortion before conceiving again. 3 When evaluating the evidence on birth spacing, WHO identified four intervals, including interpregnancy interval (IPI, the period between the previous live birth or pregnancy loss and the conception of the index pregnancy), interoutcome interval (IOI, the period between the outcome of the previous pregnancy and the outcome of the index pregnancy), birth‐to‐conception interval (the period from the previous live birth to the conception of the index pregnancy), and birth‐to‐birth interval (the period from the delivery of the previous livebirth to the subsequent live birth). 3 Compared with IPI, the use of birth intervals overestimates the risk of adverse outcomes for very short intervals between pregnancies. 4 Substantial differences were observed in risk estimates related to birth spacing according to the measurement of birth spacing used. However, differences in risk estimates related to birth spacing have not been well addressed in published systematic reviews, with a lack of stratification for the start of birth spacing or blurred distinction between birth and pregnancy intervals. 4 , 5 , 6 , 7 It is therefore important to quantify the differences by pooling current evidence.
To date, IPI has been among the most studied birth spacing intervals. Previous studies have reported on J‐shaped dose–response relationships between IPI and the risk of adverse outcomes (eg gestational diabetes mellitus [GDM], low birthweight [LBW], preterm birth [PTB], and small for gestational age [SGA]). 8 , 9 , 10 Moreover, subsequent pregnancies after live birth or pregnancy loss have been variably associated with adverse pregnancy and birth outcomes. 11 , 12 Short intervals after a pregnancy loss might prevent adverse outcomes, including PTB, LBW, SGA, and recurrent preeclampsia. 12 , 13 , 14 However, risk factors associated with short intervals, such as poor socioeconomic status, are independently associated with increased risk of adverse pregnancy outcomes. 15 , 16 Given the increase of relevant large‐scale population studies in recent years, 17 , 18 , 19 there is a need to incorporate empirical data and obtain robust estimates through meta‐analytic approaches.
To inform the strategies for postpartum family planning, we systematically reviewed the evidence on the associations between birth spacing and adverse pregnancy and birth outcomes and evaluated these associations using meta‐analytical synthesis with dose–response analysis.
2. MATERIAL AND METHODS
The present study was conducted and reported following the Preferred Reporting Items for Systematic Reviews and Meta‐Analyses (PRISMA) guideline (Appendix S1). 20 This review was not prospectively registered.
2.1. Identification of studies
PubMed, Embase, and Web of Science Core Collection were searched from database inception until March 20, 2022, to identify observational studies that measured the association between birth spacing and risk of adverse pregnancy and birth outcomes. In accordance with the Peer Review of Electronic Search Strategies (PRESS) guideline, 21 two experienced medical information specialists (GXP, ZFF) developed the search strategy and performed the literature search without language restrictions. The full list of outcomes of interest and details of search terms are listed in the Supporting information (Appendix S2). To identify additional items not retrieved by database searches, we analyzed the reference list of included articles. We additionally manually searched the related articles generated by PubMed and Google Scholar (https://scholar.google.com/).
The selection of potentially eligible studies was made by reviewing titles and abstracts by two independent reviewers (NWZ and GXP). In case of discrepancies, a third review author (ZFF) was involved, and consensus was reached by discussion. If the inclusion and exclusion criteria could not be determined from the titles and abstracts, the full articles were obtained to verify eligibility. The full text of potentially included studies was carefully reviewed by at least two reviewers.
2.2. Selection criteria
To systematically review the current evidence on this topic, we included studies that met the following criteria: (1) Observational studies (cohort, cross‐sectional, or case–control design) or experimental studies (analyzed as cohort design) evaluated the association between birth spacing and any adverse pregnancy or birth outcome. (2) The classification of birth spacing was defined in accordance with the standard proposed by WHO. 3 (3) Studies evaluated the risk of adverse outcomes of interest and reported effect estimates (i.e., odds ratios [ORs], risk ratios, or hazard ratios). Studies were excluded from systematic review if they were conference abstracts, letters, or review articles; if they had particularly short birth spacing that did not contain previous identified safe interval (i.e., 18–23 months); if they provided data for two or fewer birth spacing strata. Studies were further included in meta‐analytical synthesis if they met the following additional criteria: (1) used multivariate analysis and adjusted for at least maternal age and any socioeconomic variable; (2) reported either the number of cases and participants in each birth spacing stratum or the data necessary to calculate these; and (3) reported 95% confidence intervals (CIs) of risk estimates. For associations that involved overlapping data or populations, only the most recent study with the largest data set was included in meta‐analytical synthesis.
2.3. Data extraction and quality assessment
Two investigators (NWZ and GXP) independently extracted information on: first author, year of publication, setting (country and detailed geographical location if possible), specific data source (the name of cohort or database), sample size, birth spacing characteristics (definition of birth spacing, strata, details of previous birth or pregnancy loss, outcome of index pregnancy with corresponding definition or diagnostic criteria), maximally adjusted risk estimates with 95% CIs and confounders.
Newcastle–Ottawa Quality Assessment Scale (NOS) was used to evaluate the methodological quality of included studies on the basis of selection, comparability, exposure (for case–control or cross‐sectional studies) or outcome (for cohort studies). 22 Studies could be awarded a maximum of nine stars, and a study with eight or more stars was of methodological high‐quality. The Grading of Recommendations, Assessment, Development and Evaluations (GRADE) guidance was used to rate the certainty of evidence 23 and is presented in Table S1.
2.4. Statistical analyses
For studies using different units to measure birth spacing (i.e., weeks, months, or years), we converted these different units of exposure to months. Based on the incidence of adverse pregnancy and birth outcomes, OR were considered as the common approximations of all risk estimates. The DerSimonian & Laird method 24 random effects model was used to conduct all meta‐analyses, because it accounted for both within‐ and between‐study heterogeneity. 25 Different designs and measures of birth spacing could pose a threat to the internal validity of quantitative synthesis 4 ; therefore, we conducted four separate meta‐analytical syntheses (Figure 1).
FIGURE 1.
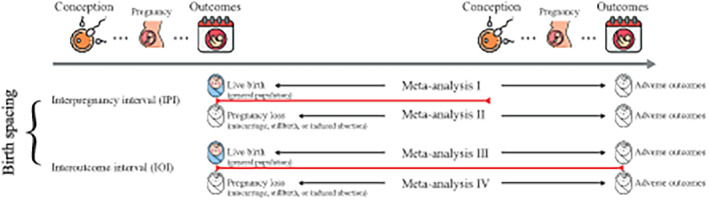
The classification of birth spacing and analysis strategy. Four separate meta‐analytical syntheses were conducted in accordance with the WHO definition of birth spacing as follows: (1) Meta I, the association between interpregnancy interval (IPI) and risk of adverse outcomes after a live birth (general population); (2) Meta II, the association between IPI and risk of adverse outcomes subsequent to pregnancy loss (miscarriage, stillbirth, or induced abortion) or preterm birth (PTB); (3) Meta III, the association between interoutcome interval (IOI) and risk of adverse outcomes after a live birth (general population); and (4) Meta IV, the association between IOI and risk of adverse outcomes subsequent to pregnancy loss (miscarriage, stillbirth, or induced abortion) or PTB. Free icons were obtained from Flaticon.com.
2.4.1. Pooled OR
Meta‐analysis was applied for the association between birth spacing and specific adverse outcome with at least two eligible studies. Following the previous analytical procedure and taking into account the data availability in the original studies, birth spacing was categorized into six groups: 5 months or less, 6–11 months, 12–17 months, 18–23 months (18–35 months for Meta III), 24–59 months (36–59 months for Meta III), and 60 months or more. The reference category was set at 18 to 23 (35 for Meta III) months, as this interval has the lowest risk for most prevalent adverse outcomes (i.e., LBW, PTB, and SGA). To ensure the comparability between studies, risk estimates for studies using different reference categories were converted based on the Greenland and Longnecker method 26 using excel macro file. 27 If study categories did not match the above intervals, we assigned categories based on their midpoints and favored the reference interval in case the midpoints fell on the boundary.
2.4.2. Dose–response regression slopes
Under the assumption of the J‐shaped dose‐specific association, we evaluated the non‐linear dose–response regression slopes of each meta‐analytical synthesis (with eligible studies ≥5) using generalized least squares trend estimation based on the variance weighted least squares (VWLS) method. 28 This procedure requires the number of cases and population at risk (or controls), the midpoint of the exposure interval for at least three categories, and points that were half‐width of the adjacent interval from open ends for open‐ended intervals. 29 We examined a series of spline functions (3, 4, and 5 knots) and the significant model with highest goodness‐of‐fit chi‐squared score was selected.
The heterogeneity between studies was evaluated with Cochran's Q test (statistically significant for p value <0.10) and quantified with the I 2 metric. 30 Large heterogeneity was defined as an I 2 statistic of >50%. 31 In order to explore the potential modifying effects of study‐level variables on the association, subgroup, and univariable random‐effects, meta‐regression analyses were carried out for a specific association with eligible studies ≥10. Subgroup analyses were stratified by publication year (2005 and before or after 2005), study design (cross‐sectional, case–control, cohort study or trail), NOS score (≤8 or 9), adjustment of birth order/parity/ gravidity (Yes or No) and risk estimate (OR, risk ratio, or hazard ratio). Meta‐regression analyses were performed for the Sociodemographic Index (SDI), a composite indicator that effectively captures the level of development status. 32 SDI values for all estimated locations between 1950 and 2019 were obtained from the official website of the Global Burden of Disease Study 2019 (https://ghdx.healthdata.org/record/ihme‐data/gbd‐2019‐socio‐demographic‐index‐sdi‐1950‐2019). We assigned an SDI value to each study based on year of publication and study location. Publication bias was assessed using Egger's asymmetry tests, and was claimed at an Egger's p value of <0.10. 33 All statistical analyses were performed using Stata software version 14.0 (StataCorp). The level of statistical significance was set at p < 0.05, and p values were all two‐tailed.
3. RESULTS
3.1. Study selection and characteristics
A total of 9276 articles were initially identified through database search. After removing duplicates and assessing titles and abstracts, 397 full‐text articles were assessed for eligibility. Of these, articles were further excluded because of lack of risk estimates (n = 89), having two or fewer birth spacing strata (n = 80), birth spacing not measured (n = 36), irrelevant or nonspecific outcomes (n = 53), reviews or conference abstracts (n = 15), short birth spacing (n = 6), and overlapping population (n = 4). With additional manual search, 129 studies comprising 46 874 843 participants were ultimately included in the systematic review (Figure S1), including 90 cohort studies, 11 , 12 , 13 , 14 , 17 , 18 , 19 , 34 , 35 , 36 , 37 , 38 , 39 , 40 , 41 , 42 , 43 , 44 , 45 , 46 , 47 , 48 , 49 , 50 , 51 , 52 , 53 , 54 , 55 , 56 , 57 , 58 , 59 , 60 , 61 , 62 , 63 , 64 , 65 , 66 , 67 , 68 , 69 , 70 , 71 , 72 , 73 , 74 , 75 , 76 , 77 , 78 , 79 , 80 , 81 , 82 , 83 , 84 , 85 , 86 , 87 , 88 , 89 , 90 , 91 , 92 , 93 , 94 , 95 , 96 , 97 , 98 , 99 , 100 , 101 , 102 , 103 , 104 , 105 , 106 , 107 , 108 , 109 , 110 , 111 , 112 , 113 , 114 , 115 , 116 23 cross‐sectional studies, 10 , 117 , 118 , 119 , 120 , 121 , 122 , 123 , 124 , 125 , 126 , 127 , 128 , 129 , 130 , 131 , 132 , 133 , 134 , 135 , 136 , 137 , 138 15 case–control studies, 8 , 139 , 140 , 141 , 142 , 143 , 144 , 145 , 146 , 147 , 148 , 149 , 150 , 151 , 152 and 1 cluster‐randomized trial. 153 The baseline characteristics of the eligible studies are presented in Table S2. The quality assessments of eligible studies ranged from four to nine stars, and most studies (99/129) were awarded with eight stars or more (Table S3 and S4). Based on GRADE guidance, a moderate rating was given for the association between interpregnancy interval and pregnancy‐induced hypertension (PIH), whereas the certainty of evidence from other studies was deemed low.
Among studies eligible for systematic review, 23 studies were excluded from meta‐analytical synthesis because of unadjusted risk estimates, 39 , 72 , 77 , 113 , 125 , 128 , 129 , 140 , 147 , 148 lack of 95% CI, 50 , 51 , 63 , 68 , 117 , 118 , 127 and lack of adjustment for maternal age. 37 , 66 , 81 , 110 , 137 , 142 Under the premise of data availability of individual studies, 77 studies were included in dose–response analyses, and 89 studies were included in meta‐analyses (eight studies were further excluded from meta‐analyses because of broad reference interval: 12–47, 12–60, and 18–59 months). Results of studies that were not included in meta‐analytical synthesis are presented in Table S5.
3.2. Meta I: Interpregnancy interval and risk of adverse outcomes after a live birth
3.2.1. Adverse birth outcomes
Compared with an IPI of 18–23 months, extremely short intervals (<6 months) were associated with increased risk of PTB (pooled OR 1.55, 95% CI 1.47–1.63), SGA (pooled OR 1.17, 95% CI 1.12–1.23), LBW (pooled OR 1.42, 95% CI 1.31–1.55), fetal death (pooled OR 1.52, 95% CI 1.36–1.70), birth defects (pooled OR 1.12, 95% CI 1.04–1.22), and early neonatal death (pooled OR 1.32, 95% CI 1.04–1.67) (Table 1 and Figure S2). Likewise, there was an observed association between extremely long intervals (≥60 months) and an increased risk of PTB (pooled OR 1.28, 95% CI 1.20–1.35), SGA (pooled OR 1.30, 95% CI 1.22–1.40), LBW (pooled OR 1.37, 95% CI 1.27–1.48), and fetal death (pooled OR 1.14, 95% CI 1.04–1.24), birth defects (pooled OR 1.08, 95% CI 1.02–1.14), and early neonatal death (pooled OR 1.17, 95% CI 1.06–1.28) (Table 1 and Figure S2). We also observed an association between longer IPI (24–59 months) and an increased risk of cleft lip compared with the reference group (pooled OR 1.28, 95% CI 1.04–1.58).
TABLE 1.
Meta‐analysis I for the associations between interpregnancy interval and risk of adverse pregnancy and birth outcomes subsequent to live births.
| Adverse outcomes | No. of studies | <6 months | 6–11 months | 12–17 months | 18–23 months | 24–59 months | ≥60 months | |||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Pooled OR (95% CI) | I 2 (%) | Pooled OR (95% CI) | I 2 (%) | Pooled OR (95% CI) | I 2 (%) | OR | Pooled OR (95% CI) | I 2 (%) | Pooled OR (95% CI) | I 2 (%) | ||
| Birth outcomes | ||||||||||||
| Preterm birth | 37 studies 10 , 12 , 14 , 19 , 36 , 41 , 42 , 46 , 47 , 54 , 62 , 69 , 76 , 78 , 80 , 82 , 86 , 88 , 89 , 92 , 94 , 95 , 97 , 100 , 101 , 102 , 107 , 114 , 115 , 119 , 122 , 123 , 132 , 139 , 143 , 150 , 151 | 1.55 (1.47–1.63) | 92.2 | 1.16 (1.10–1.23) | 99.7 | 1.05 (1.01–1.09) | 98.5 | 1.00 | 1.05 (1.02–1.07) | 87.8 | 1.28 (1.20–1.35) | 96.9 |
| Small for gestational age | 24 studies 10 , 14 , 19 , 34 , 35 , 46 , 47 , 59 , 62 , 69 , 75 , 78 , 85 , 86 , 88 , 89 , 92 , 95 , 97 , 102 , 115 , 119 , 122 , 123 | 1.17 (1.12–1.23) | 79.9 | 1.05 (1.03–1.08) | 82.7 | 1.01 (0.99–1.03) | 43.9 | 1.00 | 1.06 (1.03–1.09) | 79.9 | 1.30 (1.22–1.40) | 94.8 |
| Low birthweight | 21studies 10 , 14 , 34 , 41 , 42 , 46 , 49 , 69 , 78 , 82 , 86 , 88 , 89 , 92 , 102 , 107 , 119 , 122 , 123 , 132 , 135 | 1.42 (1.31–1.55) | 88.7 | 1.10 (1.06–1.13) | 48.8 | 1.01 (0.99–1.04) | 10.6 | 1.00 | 1.08 (1.05–1.12) | 70.9 | 1.37 (1.27–1.48) | 96.3 |
| Fetal death | 8 studies 48 , 53 , 55 , 82 , 98 , 122 , 123 , 149 | 1.52 (1.36–1.70) | 0.0 | 1.05 (0.93–1.19) | 61.7 | 1.02 (0.80–1.30) | 21.0 | 1.00 | 1.00 (0.97–1.03) | 27.6 | 1.14 (1.04–1.24) | 86.7 |
| Birth defects | 6 studies 8 , 10 , 62 , 73 , 79 , 87 | 1.12 (1.04–1.22) | 55.7 | 1.03 (0.97–1.10) | 56.0 | 1.01 (0.95–1.07) | 22.7 | 1.00 | 1.05 (1.02–1.09) | 0.0 | 1.08 (1.02–1.14) | 0.0 |
| Cleft lip with and without cleft palate | 4 studies 8 , 60 , 73 , 79 | 1.04 (0.68–1.60) | 0.0 | 1.20 (0.88–1.66) | 15.5 | 1.03 (0.74–1.45) | 0.0 | 1.00 | 1.28 (1.04–1.58) | 21.5 | — | — |
| Early neonatal death | 3 studies 48 , 62 , 122 | 1.32 (1.04–1.67) | 46.4 | 1.19 (1.03–1.36) | 32.0 | — | — | 1.00 | 1.01 (0.88–1.18) | 42.6 | 1.17 (1.06–1.28) | 0.0 |
| Large for gestational age | 3 studies 62 , 85 , 97 | 0.81 (0.78–0.84) | 18.1 | 0.91 (0.82–1.01) | 95.1 | — | — | 1.00 | 1.03 (0.93–1.13) | 97.1 | 0.94 (0.74–1.20) | 99.1 |
| Pregnancy outcomes | ||||||||||||
| Preeclampsia | 7 studies 13 , 82 , 89 , 107 , 109 , 112 , 120 | 0.96 (0.87–1.06) | 55.1 | 0.91 (0.81–1.03) | 85.1 | 0.94 (0.83–1.06) | 74.3 | 1.00 | 1.08 (0.99–1.18) | 89.5 | 1.35 (1.18, –1.54) | 95.2 |
| Gestational diabetes | 5 studies 89 , 99 , 100 , 107 , 120 | 1.07 (0.87–1.32) | 93.4 | 0.92 (0.91–0.94) | 0.0 | 0.98 (0.96–0.99) | 0.0 | 1.00 | 1.15 (1.08–1.22) | 16.8 | 1.37 (1.26–1.48) | 41.5 |
| Premature rupture of fetal membranes | 4 studies 96 , 107 , 120 , 123 | 1.56 (1.29–1.88) | 81.7 | 1.03 (0.96–1.11) | 0.0 | 1.02 (0.94–1.11) | 0.0 | 1.00 | 1.11 (0.96–1.28) | 80.0 | 1.53 (1.07–2.20) | 92.1 |
| Pregnancy‐induced hypertension | 3 studies 100 , 107 , 123 | 1.07 (0.77–1.49) | 66.9 | 0.93 (0.91–0.95) | 0.0 | 0.95 (0.93–0.96) | 0.0 | 1.00 | 1.04 (0.78–1.39) | 0.0 | 1.47 (1.01–2.14) | 28.4 |
| Anemia, maternal | 2 studies 109 , 120 | — | — | — | — | — | — | 1.00 | 1.09 (0.89–1.33) | 28.1 | 0.98 (0.76–1.25) | 14.3 |
| Postpartum hemorrhage | 2 studies 109 , 120 | — | — | — | — | — | — | 1.00 | 1.00 (0.85–1.17) | 40.2 | 0.89 (0.78–1.02) | 0.0 |
| Miscarriage | 2 studies 11 , 53 | — | — | 1.00 (0.80–1.24) | 0.0 | — | — | 1.00 | 1.23 (1.08–1.42) | 0.0 | — | — |
Abbreviations: CI, confidence interval; OR, odds ratio.
Dose–response analyses indicated significant J‐shaped relationships between IPI and risk of PTB, SGA, LBW, fetal death, and birth defects (p non‐linear for 3 knots ranged from <0.001 to 0.009, best goodness‐of‐fit chi‐squared scores ranged from 71.74 to 10 202.97) (Figure 2). The lowest risk estimates generally fell in the category of 30–40 months (Figure 2).
FIGURE 2.
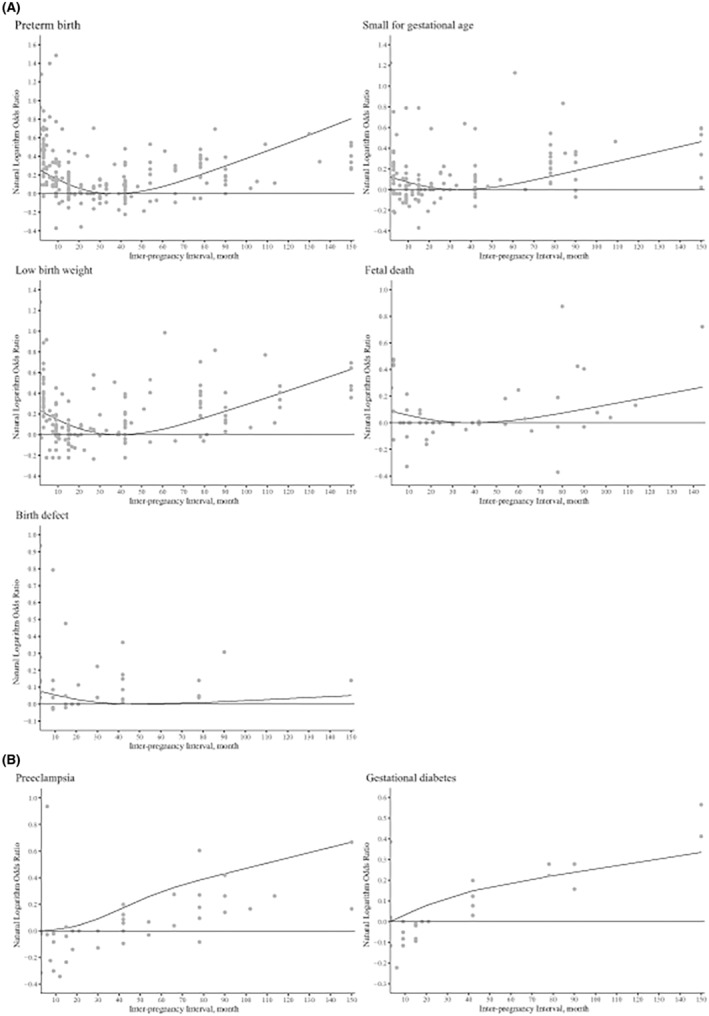
Dose–response relationships between interpregnancy interval and risk of adverse pregnancy and birth outcomes subsequent to live births.
3.2.2. Adverse pregnancy outcomes
Compared with an IPI of 18–23 months, shorter intervals were associated with an increased risk of premature rupture of fetal membranes (PROM) (pooled OR 1.56, 95% CI 1.29–1.88 for <6 months), but with a decreased risk of GDM (pooled OR 0.92, 95% CI 0.91–0.94 for 6–11 months) and PIH (pooled OR 0.93, 95% CI 0.91–0.95 for 6–11 months, 0.95, 95% CI 0.93–0.96 for 12–17 months). Except for maternal anemia and postpartum hemorrhage, longer IPI were associated with adverse pregnancy outcomes, including an increased risk of preeclampsia (pooled OR 1.35, 95% CI 1.18–1.54), GDM (pooled OR 1.37, 95% CI 1.26–1.48), PROM (pooled OR 1.53, 95% CI 1.07–2.20), PIH (pooled OR 1.47, 95% CI 1.01–2.14), and miscarriage (pooled OR 1.23, 95% CI 1.08–1.42) (Figure S2).
Dose–response analyses were performed for preeclampsia (p non‐linear < 0.001, 4 knots, best goodness‐of‐fit χ 2 score = 213.28) and GDM (p non‐linear < 0.001, 3 knots, best goodness‐of‐fit χ 2 score = 196.28). Consistent with the pooled estimates, the results showed that an increased IPI was positively associated with the risk of preeclampsia and GDM (Figure 2).
3.3. Meta II: Interpregnancy interval and risk of adverse outcomes after a PTB or pregnancy loss
In the analysis of subsequent to preterm birth, compared with an IPI of 18–23 months, intervals shorter than 12 months were significantly associated with increased risk of PTB (pooled OR 1.10, 95% CI 1.00–1.21 for 6–11 months; 1.45, 95% CI 1.25–1.68 for <6 months), and extremely long intervals (≥60 months) also indicated an increased risk (pooled OR 1.29, 95% CI 95% CI 1.03–1.63) (Table 2). In the analysis of subsequent to pregnancy loss, none of the pooled risk estimates reached statistical significance, except for LBW with intervals <6 months (pooled OR 1.64, 95% CI 1.10–2.44) (Table 2 and Figure S3).
TABLE 2.
Meta‐analysis II for the associations between interpregnancy interval and risk of adverse pregnancy and birth outcomes subsequent to preterm birth or pregnancy loss (miscarriage, stillbirth, or induced abortion).
| Adverse outcomes | No. of studies | <6 months | 6–11 months | 12–17 months | 18–23 months | 24–59 months | ≥60 months | |||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Pooled OR (95% CI) | I 2 (%) | Pooled OR (95% CI) | I 2 (%) | Pooled OR (95% CI) | I 2 (%) | OR | Pooled OR (95% CI) | I 2 (%) | Pooled OR (95% CI) | I 2 (%) | ||
| After preterm births | ||||||||||||
| Preterm birth | 5 studies 18 , 54 , 71 , 90 , 105 | 1.45 (1.25–1.68) | 71.3 | 1.10 (1.00–1.21) | 56.1 | 1.03 (0.97–1.09) | 3.8 | 1.00 | 1.03 (0.94–1.14) | 75.0 | 1.29 (1.03–1.63) | 70.9 |
| After pregnancy losses | ||||||||||||
| Birth outcomes | ||||||||||||
| Preterm birth | 7 studies 12 , 14 , 65 , 70 , 91 , 103 , 121 | 1.21 (0.7–1.86) | 98.2 | 0.97 (0.88–1.06) | 84.1 | 0.99 (0.95–1.03) | 0.0 | 1.00 | 0.99 (0.96–1.01) | 0.0 | 1.03 (0.96–1.10) | 63.9 |
| Low birthweight | 5 studies 14 , 65 , 70 , 91 , 121 | 1.64 (1.10–2.44) | 93.8 | 1.03 (0.93–1.14) | 57.5 | 1.00 (0.96–1.05) | 0.0 | 1.00 | 1.02 (0.97–1.06) | 0.0 | 1.08 (0.93–1.24) | 93.0 |
| Small for gestational age | 5 studies 14 , 70 , 91 , 103 , 121 | 1.03 (0.83–1.29) | 70.2 | 1.00 (0.87–1.15) | 89.7 | 1.00 (0.86–1.17) | 19.1 | 1.00 | 0.99 (0.94–1.04) | 34.1 | 1.07 (0.99–1.15) | 31.3 |
| Fetal death | 3 studies 65 , 103 , 121 | 1.07 (0.83–1.38) | 0.0 | 1.00 (0.87–1.16) | 0.0 | 1.01 (0.87–1.16) | 0.0 | 1.00 | 1.01 (0.88–1.16) | 0.0 | — | — |
| Pregnancy outcomes | ||||||||||||
| Miscarriage | 3 studies 11 , 65 , 93 | 0.76 (0.54–1.08) | 16.7 | — | — | 1.07 (0.81–1.41) | 2.2 | 1.00 | 0.98 (0.77–1.26) | 0.0 | — | — |
| Preeclampsia | 2 studies 65 , 121 | 1.04 (0.90–1.20) | 0.0 | 1.04 (0.87–1.23) | 33.2 | 1.10 (0.96–1.25) | 0.0 | 1.00 | 1.06 (0.94–1.20) | 0.0 | — | — |
Abbreviations: CI, confidence interval; OR, odds ratio.
Dose–response analyses were conducted for PTB (p non‐linear = 0.008, 5 knots, best goodness‐of‐fit χ 2 score = 722.07), LBW (p non‐linear < 0.001, 3 knots, best goodness‐of‐fit χ 2 score = 741.32), and SGA (p non‐linear = 0.013, 4 knots, best goodness‐of‐fit χ 2 score = 35.50). The results indicated that for women whose most recent pregnancy had ended in preterm birth, safe intervals were generally shorter than for the general population. Additionally, conception at 9 months following a pregnancy loss was not associated with an increased risk of PTB in the subsequent pregnancy (Figure 3).
FIGURE 3.
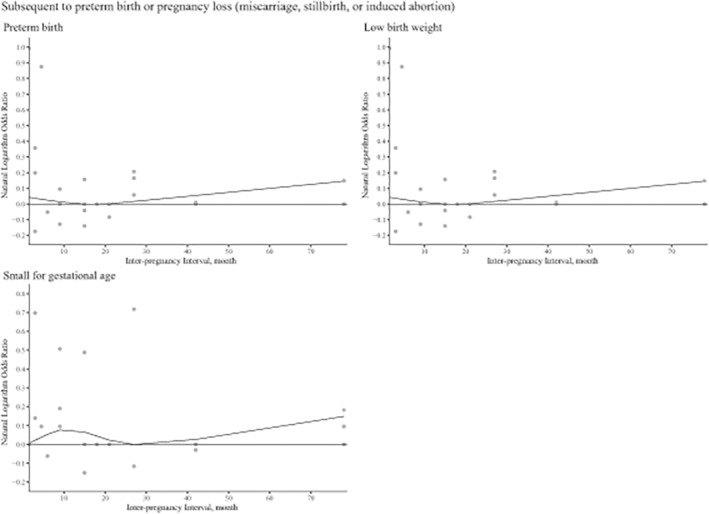
Dose–response relationships between interpregnancy interval and risk of adverse birth outcomes subsequent to pregnancy loss or preterm birth.
3.4. Meta III and IV: Interoutcome interval and risk of adverse pregnancy and birth outcomes
Compared with an IOI of 18–35 months, shorter intervals were associated with increased risk of LBW (pooled OR 1.20, 95% CI 1.01–1.41 for 12–17 months; 1.66, 95% CI 1.46–1.89 for 6–11 months), fetal death (pooled OR 2.35, 95% CI 1.60–3.46 for 12–17 months), and PTB (pooled OR 1.37, 95% CI 1.25–1.51 for 12–17 months; 3.10, 95% CI 2.32–4.14 for 6–11 months). Likewise, extremely long intervals (≥60 months) were associated with increased risk of LBW (pooled OR 1.20, 95% CI 1.15–1.26), PTB (pooled OR 1.11, 95% CI 1.02–1.20), and cesarean delivery (pooled OR 1.72, 95% CI 1.04–2.83; Figure S4 and S5). However, no significant association was found between IOI and risk of early neonatal death, GDM, postpartum hemorrhage, preeclampsia, SGA, and miscarriage. (Table 3 and Table S6).
TABLE 3.
Meta‐analysis III for the associations between interoutcome interval and risk of adverse pregnancy and birth outcomes subsequent to live births.
| Adverse outcomes | No. of studies | 6–11 months | 12–17 months | 18–35 months | 36–59 months | ≥60 months | ||||
|---|---|---|---|---|---|---|---|---|---|---|
| Pooled OR (95% CI) | I 2 (%) | Pooled OR (95% CI) | I 2 (%) | OR | Pooled OR (95% CI) | I 2 (%) | Pooled OR (95% CI) | I 2 (%) | ||
| Birth outcomes | ||||||||||
| Low birthweight | 6 studies 38 , 57 , 104 , 133 , 136 , 145 | 1.66 (1.46–1.89) | 0.8 | 1.20 (1.01–1.41) | 95 | 1.00 | 1.08 (0.97–1.20) | 89.8 | 1.20 (1.15–1.26) | 4.5 |
| Fetal death | 5 studies 61 , 104 , 124 , 130 , 138 | — | — | 2.35 (1.60–3.46) | 97.4 | 1.00 | 1.08 (0.79–1.46) | 94.6 | 1.19 (0.84–1.69) | 89.6 |
| Preterm birth | 4 studies 57 , 74 , 83 , 104 | 3.10 (2.32–4.14) | 96.4 | 1.37 (1.25–1.51) | 97.3 | 1.00 | 1.07 (0.95–1.19) | 97.8 | 1.11 (1.02–1.20) | 93.8 |
| Early neonatal death | 2 studies 124 , 133 | — | — | — | — | 1.00 | 0.75 (0.46–1.24) | 44.7 | 0.76 (0.56–1.02) | 0.0 |
| Small for gestational age | 2 studies 83 , 131 | — | — | 0.98 (0.62–1.55) | 78.7 | 1.00 | 1.07 (0.89–1.28) | 27.4 | — | — |
| Pregnancy outcomes | ||||||||||
| Cesarean delivery | 3 studies 67 , 83 , 134 | — | — | — | — | 1.00 | 1.03 (0.71–1.48) | 88.1 | 1.72 (1.04–2.83) | 87.8 |
| Gestational diabetes | 2 studies 83 , 131 | — | — | 1.00 (0.96–1.04) | 0.0 | 1.00 | 0.99 (0.95–1.04) | 1.0 | — | — |
| Preeclampsia | 2 studies 58 , 152 | — | — | 1.64 (0.34–8.02) | 0.0 | 1.00 | 1.24 (0.98–1.57) | 68.1 | — | — |
| Postpartum hemorrhage | 2 studies 104 , 144 | — | — | — | — | 1.00 | 1.01 (0.93–1.10) | 0.0 | 2.31 (0.55–9.75) | 89.9 |
Abbreviations: CI, confidence interval; OR, odds ratio.
Dose–response analyses were conducted for LBW (p non‐linear < 0.001, 3 knots, best goodness‐of‐fit χ 2 score = 117.5) and fetal death (p non‐linear < 0.001, 3 knots, best goodness‐of‐fit χ 2 score = 493.75). The analyses revealed a J‐shaped dose–response curve, indicating that the lowest risk was observed in the category of 30–40 months (Figure S6).
3.5. Subgroup analysis and meta‐regression
Subgroup analysis was carried out to explore the potential source of heterogeneity. In the preterm birth group of Meta I, the pooled OR were higher for cohort studies than cross‐sectional studies (OR 1.36 vs. 1.20; p interaction = 0.002), and higher for studies not adjusted for birth order/parity/ gravidity than studies adjusted for these variables (OR 1.47 vs. 1.25; p interaction < 0.001). Regarding the low‐birthweight group of Meta II, the pooled OR were higher for studies with a NOS score of 9 compared with studies with a score lower than 9 (OR 1.61 vs. 1.25; p interaction = 0.012). However, no significant interaction was observed for between published year, or risk estimate (p interaction range 0.066–1.000) (Table S7–S9). In addition, results of meta‐regression analysis indicated that SDI did not contribute to the inter‐study heterogeneity (p value range 0.052–0.265) (Table S10).
3.6. Publication bias
We conducted Egger's asymmetry tests for meta‐analytical syntheses with 10 or more eligible studies. Most meta‐analyses showed no significant publication bias (p Egger's range 0.107–0.826), except for pooled OR of LBW (p Egger's value was 0.006 for shortest IPI and 0.066 for longest IPI). Using the trim‐and‐fill method, the recalculated OR of LBW attenuated for ≥60 months (13 missing studies, imputed OR 1.17, 95% CI 1.09–1.27), and recalculated OR of LBW unchanged for <6 months (no trimming was performed, imputed OR 1.42, 95% CI 1.31–1.55).
4. DISCUSSION
In this comprehensive systematic review and meta‐analysis, we found that, compared with a reference interval of 18–23 months, extreme IPI (<6 and ≥60 months) in the general population had adverse effects on several pregnancy and birth outcomes, including PTB, SGA, LBW, fetal death, birth defects, early neonatal death, and PROM. Similar associations were also observed between IOI and risk of LBW and PTB (<6 and ≥60 months), as well as longer than 60 months and risk of preeclampsia, GDM, PIH, and cesarean delivery. In addition, extreme IPI after a pregnancy loss or PTB (<6 and ≥60 months) were associated with an increased risk of PTB and LBW, with the optimum interval for conceiving again after a PTB being 9 months. Additionally, according to meta‐regression analyses, the magnitude of increased risks of PTB, SGA, and LBW outcomes in Meta I was not correlated with the SDI, suggesting that the development status of the population from different countries may not be the reason for the inter‐study heterogeneity. For adverse birth outcomes, results of three primary outcomes (i.e., PTB, SGA, and LBW) were consistent with previous meta‐analyses. 4 , 154 , 155 Large cohort studies considered the birth spacing of 18 to 23 months as an appropriate reference interval, which was in line with our quantitative analysis. 78 , 88 , 89 , 102 However, dose–response analyses suggested that the minimum risk might occur after 23 months, possibly as a result of the wide intervals used in original studies (eg 18–35 months, 24–59 months, 36–59 months, and ≥60 months). For rare events such as fetal death and birth defects, the results of meta‐analyses and dose–response analyses showed adverse effects of extremely short and long birth spacing. Therefore, the optimal intervals are associated with potential benefits for both common and rare adverse birth outcomes. Additionally, we observed a decreased risk of LGA in birth spacing of <18 months, which was consistent with the results of each individual study. 62 , 85 , 97 This finding is reasonable as short birth spacing is associated with various disadvantages of fetal growth.
There is relatively limited evidence focusing on the association between birth spacing and maternal health. However, our results suggest that both short and long birth spacing are associated with an increased risk of PROM (one type of PTB), and that long intervals are associated with adverse effects on preeclampsia, GDM, PIH, and miscarriage. Consistent with the systematic review by Conde‐Agudelo et al., 16 our analysis found that long intervals were independently associated with increased risk of preeclampsia. Large population‐based studies have shown that women with birth spacing longer than 23 months are at significantly increased risk of preeclampsia. 13 , 82 , 112 , 120 Similar effects were observed with long intervals (≥23 months) in GDM. 89 , 99 , 107 In addition, previous studies have suggested that intervals of 6–11 and 12–17 months have been associated with a lower risk on GDM and PIH. 99 , 100 Therefore, when considering birth spacing, it may be necessary to take into account its impact on maternal and infant health.
The findings of the meta‐analysis by Kangatharan et al. 7 suggested that an IPI of <6 months following a miscarriage was not associated with adverse outcomes in the next pregnancy. Our study confirmed that IPI of <6 months might have no effect on SGA, fetal death, miscarriage, or preeclampsia. However, the meta‐analytical synthesis indicated that both short and long IPI were associated with increased risk of recurrent PTB, which is consistent with previous cohort studies. 90 , 107 According to the WHO recommendation 3 that women should wait at least 6 months after a pregnancy loss before conceiving another child, our results further suggest that an IPI of 9 months after a PTB might be optimal for conceiving again.
Conde‐Agudelo et al., in 2012, provided a comprehensive theoretical framework for possible causal mechanisms of birth spacing. 156 The most widely accepted interpretation of adverse outcomes due to short birth spacing includes postpartum nutritional depletion (especially folate deficiency), 157 vertical transmission of infection, 158 , 159 and cervical insufficiency (the major cause of spontaneous PTB). 160 Folate depletion is one of the most convincing causal mechanisms. The concentrations of folate levels in maternal serum and erythrocytes begin to decline in the fifth month of pregnancy and persist at low levels after delivery. 157 Low folate levels have been reported in women from 4 weeks to 12 months postpartum. 161 , 162 , 163 , 164 Data from a cohort study of 48 855 pairs of pregnancies with the second pregnancy found lower preconception folic acid use in women with both short and long inter‐pregnancy intervals. 165 Folate depletion increases the risk of poor fetal growth associated with short birth spacing, therefore, postnatal folate supplementation may be beneficial. 59 , 166 For the adverse outcomes associated with long birth spacing, the physiological regression hypothesis and reproductive wastage hypothesis are possible drivers. 167 Physiological regression suggests that the benefits attainable during pregnancy would gradually diminish after delivery, thereby affecting fetal growth 119 and maternal cardiovascular adaptation. 168 Population‐based studies found the protective effect of previous pregnancy against preeclampsia was transient. 120 , 169 In addition, long birth spacing might reflect the deficiency in health and fertility of women who have been pregnant. 103
The finding of a shorter optimum interval after pregnancy loss can be explained by two aspects. On the one hand, pregnancy loss may have less of an effect on the body's reserves of folate than live birth. 7 Most abortions often occur in the first trimester of pregnancy, when breastfeeding has not started, so women conceiving again soon after may not deplete vital nutrients. 53 On the other hand, women conceiving again within a short interval after pregnancy loss may not necessarily be poorer in education and family planning resources compared with women with a short interpregnancy interval after a live childbirth. 65 Additionally, women in this situation may have higher fecundity and be less likely to be obese, representing a higher level of reproductive health. 93
The WHO recommends that women prioritize achieving ideal birth spacing before conceiving again. What is certain is that both short and long intervals are not conducive to the health of pregnant women and newborns. The previous recommendation of waiting at least 2 years after a livebirth before conceiving again may have been too long, although other factors such as breastfeeding were taken into account. 170 Given the great significance for guiding the postpartum pregnancy, more research is needed to evaluate the association between birth spacing after pregnancy loss and maternal and infant health, while markers of physiological depletion also warrant further exploration.
The main limitations of this systematic review are related to the primary studies that were included in the review. The measurements of birth spacing used in the primary studies may not reflect the true value because of the memory bias or the omission of pregnancies that ended in loss between two live births. Additionally, some studies failed to report the starting point for birth spacing, which could result in a study population that includes women who have experienced pregnancy loss. Moreover, adjustments for confounding variables varied among the included studies. One recent study suggested that women of all ages may be at increased risk of adverse pregnancy outcomes because of short birth spacing, 95 but we attempted to ensure that at least maternal age and other socioeconomic factors were adjusted in the maximally adjusted models. According to the NOS assessments, most cohort studies were rated as high quality, suggesting that the NOS might have limited power to identify potential risk of bias in these studies. Despite its widespread use, this topic warrants careful consideration when assessing the overall validity and reliability of the study. Finally, despite the fact that subgroup and meta‐regression analyses were conducted, sources of heterogeneity were not well identified and findings should be interpreted with caution.
5. CONCLUSION
Our results confirm a non‐linear dose–response relationship between birth spacing and multiple adverse pregnancy and birth outcomes. Extreme IPI and IOI had adverse effects on PTB, SGA, LBW, fetal death, early neonatal death, birth defects, PROM, preeclampsia, GDM, PIH, and cesarean delivery. An IPI of 18–23 months could be optimal for the general population, and an IPI of 9 months after a preterm birth could be optimal for conceiving another child. Conceiving again and choosing an optimal birth spacing is a multifactorial decision, our findings can be used to assist policy‐makers and healthcare providers in developing guidelines for postpartum family planning.
AUTHOR CONTRIBUTIONS
ZFF and CWT designed the study. NWZ a GXP performed the literature search and screening, and extracted the data. NWZ, GXP, SX, and ZSW conducted the data analyses. NWZ, GXP, ZL, LJZ, FYH, CSY, and MJR created the figures and tables, and drafted the manuscript. All authors participated in the interpretation of results, and critically revised the manuscript. All authors approved the final version of the manuscript for publication. NWZ and GXP contributed equally to the manuscript as joint first authors. ZFF and CWT jointly take responsibility for the integrity of the data and the accuracy of the data analysis.
FUNDING INFORMATION
This research was supported by grants from National Natural Science Foundation of China (81602853, ZFF). The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
CONFLICT OF INTEREST STATEMENT
None.
Supporting information
Appendix S1–S6.
Table S1–S10.
Figure S1–S6.
Ni W, Gao X, Su X, et al. Birth spacing and risk of adverse pregnancy and birth outcomes: A systematic review and dose–response meta‐analysis. Acta Obstet Gynecol Scand. 2023;102:1618‐1633. doi: 10.1111/aogs.14648
Wanze Ni and Xuping Gao contributed equally to this work and are co‐first authors.
Fangfang Zeng and Wenting Cao contibuted equally to this work.
DATA AVAILABILITY STATEMENT
All relevant data are within the manuscript and its Supporting Information files.
REFERENCES
- 1. Dorney E, Mazza D, Black KI. Interconception care. Aust J Gen Pract. 2020;49:317‐322. [DOI] [PubMed] [Google Scholar]
- 2. Louis JM, Bryant A, Ramos D, Stuebe A, Blackwell SC. Interpregnancy care. Am J Obstet Gynecol. 2019;220(1):B2‐b18. [DOI] [PubMed] [Google Scholar]
- 3. Organization WH . Report of a WHO Technical Consultation on Birth Spacing: Geneva, Switzerland 13–15 June 2005. World Health Organization; 2007. [Google Scholar]
- 4. Conde‐Agudelo A, Rosas‐Bermúdez A, Kafury‐Goeta AC. Birth spacing and risk of adverse perinatal outcomes: a meta‐analysis. JAMA. 2006;295(15):1809‐1823. [DOI] [PubMed] [Google Scholar]
- 5. Wendt A, Gibbs CM, Peters S, Hogue CJ. Impact of increasing inter‐pregnancy interval on maternal and infant health. Paediatr Perinat Epidemiol. 2012;26:239‐258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Cormick G, Betrán AP, Ciapponi A, Hall DR, Hofmeyr GJ. Inter‐pregnancy interval and risk of recurrent pre‐eclampsia: systematic review and meta‐analysis. Reprod Health. 2016;13(1):83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Kangatharan C, Labram S, Bhattacharya S. Interpregnancy interval following miscarriage and adverse pregnancy outcomes: systematic review and meta‐analysis. Hum Reprod Update. 2017;23(2):221‐231. [DOI] [PubMed] [Google Scholar]
- 8. Kwon S, Lazo‐Escalante M, Villaran MV, Li CI. Relationship between interpregnancy interval and birth defects in Washington state. J Perinatol. 2012;32(1):45‐50. [DOI] [PubMed] [Google Scholar]
- 9. Gebremedhin AT, Tessema GA, Regan AK, Pereira GF. Association between interpregnancy interval and pregnancy complications by history of complications: a population‐based cohort study. BMJ Open. 2021;11(12):e046962. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Shi G, Zhang B, Kang Y, Dang S, Yan H. Association of short and long interpregnancy intervals with adverse birth outcomes: evidence from a cross‐sectional study in Northwest China. Int J Gen Med. 2021;14:2871‐2881. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Buss L, Tolstrup J, Munk C, et al. Spontaneous abortion: a prospective cohort study of younger women from the general population in Denmark. Validation, occurrence and risk determinants. Acta Obstet Gynecol Scand. 2006;85:467‐475. [DOI] [PubMed] [Google Scholar]
- 12. Shachar BZ, Mayo JA, Lyell DJ, et al. Interpregnancy interval after live birth or pregnancy termination and estimated risk of preterm birth: a retrospective cohort study. BJOG. 2016;123:2009‐2017. [DOI] [PubMed] [Google Scholar]
- 13. Basso O, Christensen K, Olsen J. Higher risk of pre‐eclampsia after change of partner. An effect of longer interpregnancy intervals? Epidemiology. 2001;12(6):624‐629. [DOI] [PubMed] [Google Scholar]
- 14. Palmsten K, Homer MV, Zhang Y, et al. In vitro fertilization, interpregnancy interval, and risk of adverse perinatal outcomes. Fertil Steril. 2018;109:840‐848.e1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Interval between pregnancies. Lancet. 1978;2(8095):879‐880. [PubMed] [Google Scholar]
- 16. Conde‐Agudelo A, Rosas‐Bermudez A, Kafury‐Goeta AC. Effects of birth spacing on maternal health: a systematic review. Am J Obstet Gynecol. 2007;196:297‐308. [DOI] [PubMed] [Google Scholar]
- 17. De Silva DA, Thoma ME. The association between interpregnancy interval and severe maternal morbidities using revised national birth certificate data: a probabilistic bias analysis. Paediatr Perinat Epidemiol. 2020;34(4):469‐480. [DOI] [PubMed] [Google Scholar]
- 18. Marinovich ML, Regan AK, Gissler M, et al. Associations between interpregnancy interval and preterm birth by previous preterm birth status in four high‐income countries: a cohort study. BJOG. 2021;128:1134‐1143. [DOI] [PubMed] [Google Scholar]
- 19. Tessema G, Håberg S, Gissler M, et al. Interpregnancy intervals and adverse birth outcomes in high‐income countries: an international study. Paediatr Perinat Epidemiol. 2021;35(SUPPL 1):28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Moher D, Liberati A, Tetzlaff J, Altman DG. Preferred reporting items for systematic reviews and meta‐analyses: the PRISMA statement. BMJ. 2009;339:b2535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. McGowan J, Sampson M, Salzwedel DM, Cogo E, Foerster V, Lefebvre C. PRESS peer review of electronic search strategies: 2015 guideline statement. J Clin Epidemiol. 2016;75:40‐46. [DOI] [PubMed] [Google Scholar]
- 22. Wells GA, Wells G, Shea B, et al. The Newcastle‐Ottawa Scale (NOS) for Assessing the Quality of Nonrandomised Studies in Meta‐Analyses2014.
- 23. Hultcrantz M, Rind D, Akl EA, et al. The GRADE working group clarifies the construct of certainty of evidence. J Clin Epidemiol. 2017;87:4‐13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. DerSimonian R, Laird N. Meta‐analysis in clinical trials. Control Clin Trials. 1986;7(3):177‐188. [DOI] [PubMed] [Google Scholar]
- 25. Riley RD, Higgins JP, Deeks JJ. Interpretation of random effects meta‐analyses. BMJ. 2011;342:d549. [DOI] [PubMed] [Google Scholar]
- 26. Greenland S, Longnecker MP. Methods for trend estimation from summarized dose‐response data, with applications to meta‐analysis. Am J Epidemiol. 1992;135:1301‐1309. [DOI] [PubMed] [Google Scholar]
- 27. Hamling J, Lee P, Weitkunat R, Ambühl M. Facilitating meta‐analyses by deriving relative effect and precision estimates for alternative comparisons from a set of estimates presented by exposure level or disease category. Stat Med. 2008;27:954‐970. [DOI] [PubMed] [Google Scholar]
- 28. Orsini N, Bellocco R, Greenland S. Generalized least squares for trend estimation of summarized dose–response data. Stata J. 2006;6:40‐57. [Google Scholar]
- 29. Aune D, Saugstad OD, Henriksen T, Tonstad S. Maternal body mass index and the risk of fetal death, stillbirth, and infant death: a systematic review and meta‐analysis. JAMA. 2014;311(15):1536‐1546. [DOI] [PubMed] [Google Scholar]
- 30. Higgins JP, Thompson SG. Quantifying heterogeneity in a meta‐analysis. Stat Med. 2002;21(11):1539‐1558. [DOI] [PubMed] [Google Scholar]
- 31. Zeng X, Zhang Y, Kwong JS, et al. The methodological quality assessment tools for preclinical and clinical studies, systematic review and meta‐analysis, and clinical practice guideline: a systematic review. J Evid Based Med. 2015;8:2‐10. [DOI] [PubMed] [Google Scholar]
- 32. Global age‐sex‐specific fertility . Mortality, healthy life expectancy (HALE), and population estimates in 204 countries and territories, 1950‐2019: a comprehensive demographic analysis for the global burden of disease study 2019. Lancet. 2020;396(10258):1160‐1203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Egger M, Smith GD, Schneider M, Minder C. Bias in meta‐analysis detected by a simple, graphical test. BMJ. 1997;315(7109):629‐634. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Klebanoff MA. Short interpregnancy interval and the risk of low birthweight. Am J Public Health. 1988;78(6):667‐670. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Lieberman E, Lang JM, Ryan KJ, Monson RR, Schoenbaum SC. The association of inter‐pregnancy interval with small for gestational age births. Obstet Gynecol. 1989;74:1‐5. [PubMed] [Google Scholar]
- 36. Lang JM, Lieberman E, Ryan KJ, Monson RR. Interpregnancy interval and risk of preterm labor. Am J Epidemiol. 1990;132:304‐309. [DOI] [PubMed] [Google Scholar]
- 37. Barros FC, Huttly SR, Victora CG, Kirkwood BR, Vaughan JP. Comparison of the causes and consequences of prematurity and intrauterine growth retardation: a longitudinal study in southern Brazil. Pediatrics. 1992;90(2 Pt 1):238‐244. [PubMed] [Google Scholar]
- 38. Gribble JN. Birth intervals, gestational age, and low birth weight: are the relationships confounded? Popul Stud. 1993;47(1):133‐146. [Google Scholar]
- 39. Leong WP, Viegas OA, Ratnam SS. Premature childbirth: social and behavioural risks in Singapore. J Biosoc Sci. 1993;25(4):465‐472. [DOI] [PubMed] [Google Scholar]
- 40. Fikree FF, Berendes HW. Risk factors for term intrauterine growth retardation: a community‐based study in Karachi. Bull World Health Organ. 1994;72:581‐587. [PMC free article] [PubMed] [Google Scholar]
- 41. Adams MM, Delaney KM, Stupp PW, McCarthy BJ, Rawlings JS. The relationship of interpregnancy interval to infant birthweight and length of gestation among low‐risk women, Georgia. Paediatr Perinat Epidemiol. 1997;11:48‐62. [DOI] [PubMed] [Google Scholar]
- 42. Basso O, Olsen J, Knudsen LB, Christensen K. Low birth weight and preterm birth after short interpregnancy intervals. Am J Obstet Gynecol. 1998;178:259‐263. [DOI] [PubMed] [Google Scholar]
- 43. Adams MM, Elam‐Evans LD, Wilson HG, Gilbertz DA. Rates of and factors associated with recurrence of preterm delivery. JAMA. 2000;283(12):1591‐1596. [DOI] [PubMed] [Google Scholar]
- 44. Fuentes‐Afflick E, Hessol NA. Interpregnancy interval and the risk of premature infants. Obstet Gynecol. 2000;95:383‐390. [DOI] [PubMed] [Google Scholar]
- 45. Trogstad LIS, Eskild A, Magnus P, Samuelsen SO, Nesheim BI. Changing paternity and time since last pregnancy; the impact on pre‐eclampsia risk. A study of 547 238 women with and without previous pre‐eclampsia. Int J Epidemiol. 2001;30:1317‐1322. [DOI] [PubMed] [Google Scholar]
- 46. Zhu BP, Haines KM, Le T, McGrath‐Miller K, Boulton ML. Effect of the interval between pregnancies on perinatal outcomes among white and black women. Am J Obstet Gynecol. 2001;185(6):1403‐1410. [DOI] [PubMed] [Google Scholar]
- 47. Smith GCS, Pell JP, Dobbie R. Interpregnancy interval and risk of preterm birth and neonatal death: retrospective cohort study. BMJ. 2003;327(7410):313‐316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Stephansson O, Dickman PW, Cnattingius S. The influence of interpregnancy interval on the subsequent risk of stillbirth and early neonatal death. Obstet Gynecol. 2003;102:101‐108. [DOI] [PubMed] [Google Scholar]
- 49. Zhu BP, Le T. Effect of interpregnancy interval on infant low birth weight: a retrospective cohort study using the Michigan maternally linked birth database. Mat Child Health J. 2003;7(3):169‐178. [DOI] [PubMed] [Google Scholar]
- 50. DaVanzo J, Razzaque A, Rahman M, et al. The Effects of Birth Spacing on Infant and Child Mortality, Pregnancy Outcomes, and Maternal Morbidity and Mortality in Matlab. Technical Consultation and Review of the Scientific Evidence for Birth Spacing; 2004. [Google Scholar]
- 51. Razzaque A, Da Vanzo J, Rahman M, et al. Pregnancy spacing and maternal morbidity in Matlab, Bangladesh. Int J Gynecol Obstet. 2005;89(SUPPL. 1):S41‐S49. [DOI] [PubMed] [Google Scholar]
- 52. Zhu BP, Grigorescu V, Le T, et al. Labor dystocia and its association with interpregnancy interval. Am J Obstet Gynecol. 2006;195(1):121‐128. [DOI] [PubMed] [Google Scholar]
- 53. DaVanzo J, Hale L, Razzaque A, Rahman M. Effects of interpregnancy interval and outcome of the preceding pregnancy on pregnancy outcomes in Matlab, Bangladesh. BJOG. 2007;114(9):1079‐1087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. DeFranco EA, Stamilio DM, Boslaugh SE, Gross GA, Muglia LJ. A short interpregnancy interval is a risk factor for preterm birth and its recurrence. Am J Obstet Gynecol. 2007;197(264):e1‐e6. [DOI] [PubMed] [Google Scholar]
- 55. Smith GC, Shah I, White IR, Pell JP, Dobbie R. Previous preeclampsia, preterm delivery, and delivery of a small for gestational age infant and the risk of unexplained stillbirth in the second pregnancy: a retrospective cohort study, Scotland, 1992‐2001. Am J Epidemiol. 2007;165:194‐202. [DOI] [PubMed] [Google Scholar]
- 56. Stamilio DM, Defranco E, Paré E, et al. Short interpregnancy interval: risk of uterine rupture and complications of vaginal birth after cesarean delivery. Obstet Gynecol. 2007;110(5):1075‐1082. [DOI] [PubMed] [Google Scholar]
- 57. Mohsin M, Jalaludin B. Influence of previous pregnancy outcomes and continued smoking on subsequent pregnancy outcomes: an exploratory study in Australia. BJOG. 2008;115:1428‐1435. [DOI] [PubMed] [Google Scholar]
- 58. Mostello D, Kallogjeri D, Tungsiripat R, Leet T. Recurrence of preeclampsia: effects of gestational age at delivery of the first pregnancy, body mass index, paternity, and interval between births. Am J Obstet Gynecol. 2008;199(1):55.e1‐55.e7. [DOI] [PubMed] [Google Scholar]
- 59. Van Eijsden M, Smits LJM, Van Der Wal MF, Bonsel GJ. Association between short interpregnancy intervals and term birth weight: the role of folate depletion. Am J Clin Nutr. 2008;88(1):147‐153. [DOI] [PubMed] [Google Scholar]
- 60. Villamor E, Sparen P, Cnattingius S. Risk of oral clefts in relation to prepregnancy weight change and interpregnancy interval. Am J Epidemiol. 2008;167(11):1305‐1311. [DOI] [PubMed] [Google Scholar]
- 61. Williams EK, Hossain MB, Sharma RK, Kumar V, Pandey CM, Baqui AH. Birth interval and risk of stillbirth or neonatal death: findings from rural North India. J Trop Pediatr. 2008;54(5):321‐327. [DOI] [PubMed] [Google Scholar]
- 62. Grisaru‐Granovsky S, Gordon ES, Haklai Z, Samueloff A, Schimmel MM. Effect of interpregnancy interval on adverse perinatal outcomes—a national study. Contraception. 2009;80(6):512‐518. [DOI] [PubMed] [Google Scholar]
- 63. Partington SN, Steber DL, Blair KA, Cisler RA. Second births to teenage mothers: risk factors for low birth weight and preterm birth. Perspect Sex Reprod Health. 2009;41:101‐109. [DOI] [PubMed] [Google Scholar]
- 64. Bujold E, Gauthier RJ. Risk of uterine rupture associated with an interdelivery interval between 18 and 24 months. Obstet Gynecol. 2010;115:1003‐1006. [DOI] [PubMed] [Google Scholar]
- 65. Love ER, Bhattacharya S, Smith NC, Bhattacharya S. Effect of interpregnancy interval on outcomes of pregnancy after miscarriage: retrospective analysis of hospital episode statistics in Scotland. BMJ. 2010;341:c3967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66. de Weger FJ, Hukkelhoven C, Serroyen J, Velde ERT, Smits LJM. Advanced maternal age, short interpregnancy interval, and perinatal outcome. Am J Obstet Gynecol. 2011;204(421):e1‐e9. [DOI] [PubMed] [Google Scholar]
- 67. Miller ES, Grobman WA. Interbirth interval with frequency of cesarean delivery. Obstet Gynecol. 2011;118(1):39‐42. [DOI] [PubMed] [Google Scholar]
- 68. DaVanzo J, Hale L, Rahman M. How long after a miscarriage should women wait before becoming pregnant again? Multivariate analysis of cohort data from Matlab, Bangladesh. BMJ Open. 2012;2:e001591. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69. Salihu HM, August EM, Mbah AK, et al. The impact of birth spacing on subsequent feto‐infant outcomes among community enrollees of a federal healthy start project. J Com Health. 2012;37:137‐142. [DOI] [PubMed] [Google Scholar]
- 70. Huo XX, Gao ES, Cheng YM, et al. Effect of interpregnancy interval after a mifepristone‐induced abortion on neonatal outcomes in subsequent pregnancy. Contraception. 2013;87:38‐44. [DOI] [PubMed] [Google Scholar]
- 71. Simonsen SE, Lyon JL, Stanford JB, Porucznik CA, Esplin MS, Varner MW. Risk factors for recurrent preterm birth in multiparous Utah women: a historical cohort study. BJOG. 2013; 120(7):863–872. [DOI] [PubMed] [Google Scholar]
- 72. Blumenfeld YJ, Baer RJ, Druzin ML, et al. Association between maternal characteristics, abnormal serum aneuploidy analytes, and placental abruption. Am J Obstet Gynecol. 2014;211(144):e1‐e9. [DOI] [PubMed] [Google Scholar]
- 73. Chen I, Jhangri GS, Chandra S. Relationship between interpregnancy interval and congenital anomalies. Am J Obstet Gynecol. 2014;210(6):564.e1‐564.e8. [DOI] [PubMed] [Google Scholar]
- 74. DeFranco EA, Ehrlich S, Muglia LJ. Influence of interpregnancy interval on birth timing. BJOG. 2014;121(13):1633‐1640. [DOI] [PubMed] [Google Scholar]
- 75. Hinkle SN, Albert PS, Mendola P, et al. Differences in risk factors for incident and recurrent small‐for‐ gestational‐age birthweight: a hospital‐based cohort study. BJOG. 2014;121:1080‐1089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76. Naimi AI, Moodie EEM, Auger N, Kaufman JS. Stochastic mediation contrasts in epidemiologic research: interpregnancy interval and the educational disparity in preterm delivery. Am J Epidemiol. 2014;180:436‐445. [DOI] [PubMed] [Google Scholar]
- 77. Baer RJ, Chambers CD, Jones KL, et al. Maternal factors associated with the occurrence of Gastroschisis. Am J Medl Genett A. 2015;167(7):1534‐1541. [DOI] [PubMed] [Google Scholar]
- 78. Chen I, Jhangri GS, Lacasse M, Kumar M, Chandra S. Relationship between interpregnancy interval and adverse perinatal and neonatal outcomes in northern Alberta. J Obstet Gynaecol Can. 2015;37(7):598‐605. [DOI] [PubMed] [Google Scholar]
- 79. Mburia‐Mwalili A, Yang W. Interpregnancy interval and birth defects. Birth Defects Res A Clin Mol Teratol. 2015;103:904‐912. [DOI] [PubMed] [Google Scholar]
- 80. Nerlander LM, Callaghan WM, Smith RA, Barfield WD. Short Interpregnancy interval associated with preterm birth in US adolescents. Mat Child Health J. 2015;19(4):850‐858. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81. Cofer FG, Fridman M, Lawton E, Korst LM, Nicholas L, Gregory KD. Interpregnancy interval and childbirth outcomes in California, 2007‐2009. Mat Child Health J. 2016;20:S43‐S51. [DOI] [PubMed] [Google Scholar]
- 82. Mignini LE, Carroli G, Betran AP, et al. Interpregnancy interval and perinatal outcomes across Latin America from 1990 to 2009: a large multi‐country study. BJOG. 2016;123(5):730‐737. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83. Yee LM, Truong YN, Caughey AB, Cheng YW. The association between interdelivery interval and adverse perinatal outcomes in a diverse US population. J Perinatol. 2016;36(8):593‐597. [DOI] [PubMed] [Google Scholar]
- 84. Appareddy S, Pryor J, Bailey B. Inter‐pregnancy interval and adverse outcomes: evidence for an additional risk in health disparate populations. J Mat Fetal Neonat Med. 2017;30(21):2640‐2644. [DOI] [PubMed] [Google Scholar]
- 85. Atreya MR, Muglia LJ, Greenberg JM, DeFranco EA. Racial differences in the influence of interpregnancy interval on fetal growth. Mat Child Health J. 2017;21(3):562‐570. [DOI] [PubMed] [Google Scholar]
- 86. Class QA, Rickert ME, Oberg AS, et al. Within‐family analysis of interpregnancy interval and adverse birth outcomes. Obstet Gynecol. 2017;130:1304‐1311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87. Coo H, Brownell MD, Ruth C, Flavin M, Au W, Day AG. Interpregnancy interval and congenital anomalies: a record‐linkage study using the Manitoba population research data repository. J Obstet Gynaecol Can. 2017;39(11):996‐1007. [DOI] [PubMed] [Google Scholar]
- 88. Coo H, Brownell MD, Ruth C, Flavin M, Au W, Day AG. Interpregnancy interval and adverse perinatal outcomes: a record‐linkage study using the Manitoba population research data repository. J Obstet Gynaecol Can. 2017;39(6):420‐433. [DOI] [PubMed] [Google Scholar]
- 89. Hanley GE, Hutcheon JA, Kinniburgh BA, Lee L. Interpregnancy interval and adverse pregnancy outcomes an analysis of successive pregnancies. Obstet Gynecol. 2017;129(3):408‐415. [DOI] [PubMed] [Google Scholar]
- 90. Koullali B, Kamphuis EI, Hof MHP, et al. The effect of interpregnancy interval on the recurrence rate of spontaneous preterm birth: a retrospective cohort study. Am J Perinatol. 2017;34:174‐182. [DOI] [PubMed] [Google Scholar]
- 91. Mannisto J, Bloigu A, Mentula M, Gissler M, Heikinheimo O, Niinimaki M. Interpregnancy interval after termination of pregnancy and the risks of adverse outcomes in subsequent birth. Obstet Gynecol. 2017;129(2):347‐354. [DOI] [PubMed] [Google Scholar]
- 92. Qin C, Mi C, Xia A, et al. A first look at the effects of long inter‐pregnancy interval and advanced maternal age on perinatal outcomes: a retrospective cohort study. Birth (Berkeley, Calif). 2017;44(3):230‐237. [DOI] [PubMed] [Google Scholar]
- 93. Sundermann AC, Hartmann KE, Jones SH, Torstenson ES, Velez Edwards DR. Interpregnancy interval after pregnancy loss and risk of repeat miscarriage. Obstet Gynecol. 2017;130(6):1312‐1318. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94. Delara RMM, Madden E, Bryant AS. Short interpregnancy intervals and associated risk of preterm birth in Asians and Pacific islanders. J Mat Fetal Neonat Med. 2018;31(14):1894‐1899. [DOI] [PubMed] [Google Scholar]
- 95. Schummers L, Hutcheon JA, Hernandez‐Diaz S, et al. Association of short interpregnancy interval with pregnancy outcomes according to maternal age. JAMA Internal Med. 2018;178:1661‐1670. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96. Shree R, Caughey AB, Chandrasekaran S. Short interpregnancy interval increases the risk of preterm premature rupture of membranes and early delivery. J Mat Fetal Neonat Med. 2018;31(22):3014‐3020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97. Zhang LF, Shen SY, He JR, et al. Effect of Interpregnancy interval on adverse perinatal outcomes in southern China: a retrospective cohort study, 2000‐2015. Paediatr Perinat Epidemiol. 2018;32:131‐140. [DOI] [PubMed] [Google Scholar]
- 98. Carmichael SL, Blumenfeld YJ, Mayo JA, et al. Stillbirth and live birth at Periviable gestational age: a comparison of prevalence and risk factors. Am J Perinatol. 2019;36(5):537‐544. [DOI] [PubMed] [Google Scholar]
- 99. Gebremedhin AT, Regan AK, Ball S, et al. Effect of interpregnancy interval on gestational diabetes: a retrospective matched cohort study. Ann Epidemiol. 2019;39:33‐38. [DOI] [PubMed] [Google Scholar]
- 100. Haight SC, Hogue CJ, Raskind‐Hood CL, Ahrens KA. Short interpregnancy intervals and adverse pregnancy outcomes by maternal age in the United States. Ann Epidemiol. 2019;31:38‐44. [DOI] [PubMed] [Google Scholar]
- 101. Lonhart JA, Mayo JA, Padula AM, Wise PH, Stevenson DK, Shaw GM. Short interpregnancy interval as a risk factor for preterm birth in non‐Hispanic Black and White women in California. J Perinatol. 2019;39(9):1175‐1181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102. Regan AK, Ball SJ, Warren JL, et al. A population‐based matched‐sibling analysis estimating the associations between first interpregnancy interval and birth outcomes. Am J Epidemiol. 2019;188:9‐16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103. Regan AK, Gissler M, Magnus MC, et al. Association between interpregnancy interval and adverse birth outcomes in women with a previous stillbirth: an international cohort study. Lancet. 2019;393(10180):1527‐1535. [DOI] [PubMed] [Google Scholar]
- 104. Bauserman M, Nowak K, Nolen TL, et al. The relationship between birth intervals and adverse maternal and neonatal outcomes in six low and lower‐middle income countries. Reprod Health. 2020;17:157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105. Kalengo NH, Sanga LA, Philemon RN, Obure J, Mahande MJ. Recurrence rate of preterm birth and associated factors among women who delivered at Kilimanjaro Christian medical Centre in Northern Tanzania: a registry based cohort study. PloS One. 2020;15(9):e0239037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106. Kawakita T, Franco S, Ghofranian A, Thomas A, Landy HJ. Association between long interpregnancy intervals and cesarean delivery due to arrest disorders. Am J Obstet Gynecol MFM. 2020;2:100103. [DOI] [PubMed] [Google Scholar]
- 107. Lin J, Liu H, Wu DD, et al. Long interpregnancy interval and adverse perinatal outcomes: a retrospective cohort study. Sci China Life Sci. 2020;63(6):898‐904. [DOI] [PubMed] [Google Scholar]
- 108. Rohde RL, Luong L, Adjei Boakye E, Chang JJ. Effect of interpregnancy interval after a first pregnancy complicated by placental abruption, on adverse maternal and fetal outcomes in a second pregnancy. J Matern Fetal Neonatal Med. 2020;33(22):3809‐3815. [DOI] [PubMed] [Google Scholar]
- 109. Sanga LA, Mtuy T, Philemon RN, Mahande MJ. Inter‐pregnancy interval and associated adverse maternal outcomes among women who delivered at Kilimanjaro Christian medical Centre in Tanzania, 2000‐2015. PloS One. 2020;15:e0228330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110. Zhang L, Li H, Li J, et al. Prediction of iatrogenic preterm birth in patients with scarred uterus: a retrospective cohort study in Northeast China. BMC Pregnancy Childbirth. 2020;20:490. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111. Cunningham S, Algeo CE, DeFranco EA. Influence of interpregnancy interval on uterine rupture. J Mat Fetal Neonatal Med. 2021;34:2848‐2853. [DOI] [PubMed] [Google Scholar]
- 112. Gebremedhin AT, Regan AK, Ball S, et al. Interpregnancy interval and hypertensive disorders of pregnancy: a population‐based cohort study. Paediatr Perinat Epidemiol. 2021;35:404‐414. [DOI] [PubMed] [Google Scholar]
- 113. Mantel Ä, Ajne G, Lindblad Wollmann C, Stephansson O. Previous preterm cesarean delivery and risk of uterine rupture in subsequent trial of labor‐a national cohort study. Am J Obstet Gynecol. 2021;224:380.e1‐380.e13. [DOI] [PubMed] [Google Scholar]
- 114. Tanigawa K, Ikehara S, Cui M, et al. Association between interpregnancy interval and risk of preterm birth and its modification by folate intake: the Japan environment and children's study. J Epidemiol. 2023;33:113‐119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115. Congdon JL, Baer RJ, Arcara J, et al. Interpregnancy interval and birth outcomes: a propensity matching study in the California population. Matern Child Health J. 2022;26(5):1115‐1125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116. Rao J, Fan D, Ma H, et al. Is there an optimal inter‐delivery interval in women who underwent trial of labor after cesarean delivery (TOLAC)? Reprod Health. 2022;19:14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117. Miller JE. Determinants of intrauterine growth retardation: evidence against maternal depletion. J Biosocl Sci. 1989;21(2):235‐243. [DOI] [PubMed] [Google Scholar]
- 118. Kallan JE. Reexamination of interpregnancy intervals and subsequent birth outcomes: evidence from U.S. linked birth/infant death records. Soc Biol. 1997;44(3–4):205‐212. [PubMed] [Google Scholar]
- 119. Zhu BP, Rolfs RT, Nangle BE, Horan JM. Effect of the interval between pregnancies on perinatal outcomes. N Engl J Med. 1999;340(8):589‐594. [DOI] [PubMed] [Google Scholar]
- 120. Conde‐Agudelo A, Belizan JM. Maternal morbidity and mortality associated with interpregnancy interval: cross sectional study. BMJ. 2000;321(7271):1255‐1259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121. Conde‐Agudelo A, Belizan JM, Breman R, Brockman SC, Rosas‐Bermudez A. Effect of the interpregnancy interval after an abortion on maternal and perinatal health in Latin America. Int J Gynecol Obstet. 2005;89:S34‐S40. [DOI] [PubMed] [Google Scholar]
- 122. Conde‐Agudelo A, Belizan JM, Norton MH, Rosas‐Bermudez A. Effect of the interpregnancy interval on perinatal outcomes in Latin America. Obstet Gynecol. 2005;106(2):359‐366. [DOI] [PubMed] [Google Scholar]
- 123. Cecatti JG, Correa‐Silva EP, Milanez H, Morais SS, Souza JP. The associations between inter‐pregnancy interval and maternal and neonatal outcomes in Brazil. Matern Child Health J. 2008;12(2):275‐281. [DOI] [PubMed] [Google Scholar]
- 124. de Jonge HCC, Azad K, Seward N, et al. Determinants and consequences of short birth interval in rural Bangladesh: a cross‐sectional study. BMC Pregnancy Childbirth. 2014;14:427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125. Firdous N, Manzoor R, Qureshi A, Pandit B. Impact of interpregnancy interval on perinatal outcome. JK Practitioner. 2014;19(3–4):75‐79. [Google Scholar]
- 126. Kader M, Perera NKP. Socio‐economic and nutritional determinants of low birth weight in India. N Am J Med Sci. 2014;6:302‐308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127. Habimana‐Kabano I, Broekhuis A, Hooimeijer P. Inter‐pregnancy intervals and maternal morbidity: new evidence from Rwanda. Afr J Reprod Health. 2015;19(3):77‐86. [PubMed] [Google Scholar]
- 128. Bhagwan D, Kumar A, Rao CR, Kamath A. Prevalence of anaemia among postnatal mothers in coastal Karnataka. J Clin Diagn Res. 2016;10:LC17‐LC20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129. Foto TG, Chapman RS, Lashari AG. Risk factors for low birth weight: bivariate analysis of findings from the Zimbabwe 2014 multiple indicator cluster survey. J Health Res. 2016;30(Suppl. 1):S1‐S8. [Google Scholar]
- 130. Lakew D, Tesfaye D, Mekonnen H. Determinants of stillbirth among women deliveries at Amhara region, Ethiopia. BMC Pregnancy Childbirth. 2017;17:375. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131. Huber LRB, Smith K, Sha W, Vick T. Interbirth interval and pregnancy complications and outcomes: findings from the pregnancy risk assessment monitoring system. J Midwifery Womens Health. 2018;63:436‐445. [DOI] [PubMed] [Google Scholar]
- 132. Ihongbe TO, Wallenborn JT, Rozario S, Masho SW. Short interpregnancy interval and adverse birth outcomes in women of advanced age: a population‐based study. Ann Epidemiol. 2018;28(9):605‐611. [DOI] [PubMed] [Google Scholar]
- 133. Nisha MK, Alam A, Islam MT, Huda T, Raynes‐Greenow C. Risk of adverse pregnancy outcomes associated with short and long birth intervals in Bangladesh: evidence from six Bangladesh demographic and health surveys, 1996‐2014. BMJ Open. 2019;9:e024392. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134. Barbosa R, Alves M, Nathasje I, Chagas D, Simoes VF, Silva L. Factors associated with inadequate birth intervals in the BRISA birth cohort, Brazil. Rev Bras Ginecol Obstet. 2020;42(2):67‐73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135. Kannaujiya AK, Kumar K, Upadhyay AK, McDougal L, Raj A, Singh A. Short interpregnancy interval and low birth weight births in India: evidence from National Family Health Survey 2015‐16. SSM Popul Health. 2020;12:100700. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136. Yaya S, Uthman OA, Ekholuenetale M, Bishwajit G, Adjiwanou V. Effects of birth spacing on adverse childhood health outcomes: evidence from 34 countries in sub‐Saharan Africa. J Matern Fetal Neonatal Med. 2020;33:3501‐3508. [DOI] [PubMed] [Google Scholar]
- 137. Myo T, Hong SA, Thepthien BO, Hongkrailert N. Prevalence and factors associated with postpartum depression in primary healthcare centres in Yangon, Myanmar. Malays J Med Sci. 2021;28:71‐86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138. Tesema GA, Tessema ZT, Tamirat KS, Teshale AB. Prevalence of stillbirth and its associated factors in East Africa: generalized linear mixed modeling. BMC Pregnancy Childbirth. 2021;21:414. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139. Ferraz EM, Gray RH, Fleming PL, Maia TM. Interpregnancy interval and low birth weight: findings from a case‐control study. Am J Epidemiol. 1988;128(5):1111‐1116. [DOI] [PubMed] [Google Scholar]
- 140. Chumnijarakij T, Nuchprayoon T, Chitinand S, et al. Maternal risk factors for low birth weight newborn in Thailand. J Med Assoc Thai. 1992;75(8):445‐452. [PubMed] [Google Scholar]
- 141. Smits LJ, Nelen WL, Wouters MG, Straatman H, Jongbloet PH, Zielhuis GA. Conditions at conception in women with recurrent miscarriage. Soc Biol. 1998;45:143‐149. [DOI] [PubMed] [Google Scholar]
- 142. Al‐Jasmi F, Al‐Mansoor F, Alsheiba A, Carter AO, Carter TP, Hossain MM. Effect of interpregnancy interval on risk of spontaneous preterm birth in Emirati* women, United Arab Emirates. Bull World Health Organ. 2002;80(11):871‐875. [PMC free article] [PubMed] [Google Scholar]
- 143. Arafa MA, Alkhouly A, Youssef ME. Influence of inter‐pregnancy interval on preterm delivery. Paediatr Perinat Epidemiol. 2004;18(4):248‐252. [DOI] [PubMed] [Google Scholar]
- 144. Wandabwa J, Doyle P, Todd J, Ononge S, Kiondo P. Risk factors for severe post partum haemorrhage in Mulago hospital, Kampala, Uganda. East Afr Med J. 2008;85(2):64‐71. [DOI] [PubMed] [Google Scholar]
- 145. Coutinho PR, Cecatti JG, Surita FG, Souza JP, Morais SS. Factors associated with low birth weight in a historical series of deliveries in Campinas, Brazil. Rev Assoc Med Bras (1992). 2009;55(6):692‐699. [DOI] [PubMed] [Google Scholar]
- 146. Getz KD, Anderka MT, Werler MM, Case AP. Short interpregnancy interval and gastroschisis risk in the national birth defects prevention study. Birth Defects Res A Clin Mol Teratol. 2012;94(9):714‐720. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147. Zhang YP, Liu XH, Gao SH, et al. Risk factors for preterm birth in five maternal and child health hospitals in Beijing. PloS One. 2012;7(12):e52780. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148. Ahankari A, Bapat S, Myles P, Fogarty A, Tata L. Factors associated with preterm delivery and low birth weight: a study from rural Maharashtra, India. F1000Res. 2017;6:72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149. Gupta PM, Freedman AA, Kramer MR, et al. Interpregnancy interval and risk of stillbirth: a population‐based case control study. Ann Epidemiol. 2019;35:35‐41. [DOI] [PubMed] [Google Scholar]
- 150. Asgharnia M, Varasteh T, Pourmarzi D. Inter‐pregnancy interval and the incidence of preterm birth. J Fam Reprod Health. 2020;14(1):52‐56. [PMC free article] [PubMed] [Google Scholar]
- 151. Regasa MT, Hinkosa L, Besho M, et al. Predictors of preterm birth in Western Ethiopia: a case control study. PloS One. 2021;16(4):e0247927. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 152. Demissie M, Molla G, Tayachew A, Getachew F. Risk factors of preeclampsia among pregnant women admitted at labor ward of public hospitals, low income country of Ethiopia; case control study. Pregnancy Hypertens. 2022;27:36‐41. [DOI] [PubMed] [Google Scholar]
- 153. Nonyane BAS, Norton M, Begum N, et al. Pregnancy intervals after stillbirth, neonatal death and spontaneous abortion and the risk of an adverse outcome in the next pregnancy in rural Bangladesh. BMC Pregnancy Childbirth. 2019;19(1):62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 154. Kozuki N, Lee AC, Silveira MF, et al. The associations of birth intervals with small‐for‐gestational‐age, preterm, and neonatal and infant mortality: a meta‐analysis. BMC Public Health. 2013;13(Suppl 3):S3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 155. Ahrens KA, Nelson H, Stidd RL, Moskosky S, Hutcheon JA. Short interpregnancy intervals and adverse perinatal outcomes in high‐resource settings: an updated systematic review. Paediatr Perinat Epidemiol. 2019;33(1):O25‐O47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 156. Conde‐Agudelo A, Rosas‐Bermudez A, Castaño F, Norton MH. Effects of birth spacing on maternal, perinatal, infant, and child health: a systematic review of causal mechanisms. Stud Fam Plann. 2012;43(2):93‐114. [DOI] [PubMed] [Google Scholar]
- 157. Smits LJ, Essed GG. Short interpregnancy intervals and unfavourable pregnancy outcome: role of folate depletion. Lancet. 2001;358(9298):2074‐2077. [DOI] [PubMed] [Google Scholar]
- 158. Cheng PJ, Chueh HY, Liu CM, Hsu JJ, Hsieh TT, Soong YK. Risk factors for recurrence of group B streptococcus colonization in a subsequent pregnancy. Obstet Gynecol. 2008;111(3):704‐709. [DOI] [PubMed] [Google Scholar]
- 159. Leruez‐Ville M, Guilleminot T, Stirnemann J, et al. Quantifying the burden of congenital cytomegalovirus infection with long‐term sequelae in subsequent pregnancies of women seronegative at their first pregnancy. Clin Infect Dis. 2020;71:1598‐1603. [DOI] [PubMed] [Google Scholar]
- 160. Romero R, Espinoza J, Kusanovic JP, et al. The preterm parturition syndrome. BJOG. 2006;113:17‐42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 161. O'Rourke KM, Redlinger TE, Waller DK. Declining levels of erythrocyte folate during the postpartum period among Hispanic women living on the Texas‐Mexico border. J Womens Health Gend Based Med. 2000;9:397‐403. [DOI] [PubMed] [Google Scholar]
- 162. Cikot RJ, Steegers‐Theunissen RP, Thomas CM, de Boo TM, Merkus HM, Steegers EA. Longitudinal vitamin and homocysteine levels in normal pregnancy. Br J Nutr. 2001;85:49‐58. [DOI] [PubMed] [Google Scholar]
- 163. Doyle W, Srivastava A, Crawford MA, Bhatti R, Brooke Z, Costeloe KL. Inter‐pregnancy folate and iron status of women in an inner‐city population. Br J Nutr. 2001;86(1):81‐87. [DOI] [PubMed] [Google Scholar]
- 164. Zhou YB, Si KY, Li HT, Li XC, Meng Y, Liu JM. Trends and influencing factors of plasma folate levels in Chinese women at mid‐pregnancy, late pregnancy and lactation periods. Br J Nutr. 2021;126:885‐891. [DOI] [PubMed] [Google Scholar]
- 165. Nilsen RM, Mastroiacovo P, Gunnes N, et al. Folic acid supplementation and interpregnancy interval. Paediatr Perinat Epidemiol. 2014;28:270‐274. [DOI] [PubMed] [Google Scholar]
- 166. Petersen JM, Yazdy MM, Getz KD, Anderka MT, Werler MM. Short interpregnancy intervals and risks for birth defects: support for the nutritional depletion hypothesis. Am J Clin Nutr. 2021;113:1688‐1699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 167. Zhu BP. Effect of interpregnancy interval on birth outcomes: findings from three recent US studies. Int J Gynaecol Obstet. 2005;89(Suppl 1):S25‐S33. [DOI] [PubMed] [Google Scholar]
- 168. Mikolajczyk RT, Zhang J, Ford J, Grewal J. Effects of interpregnancy interval on blood pressure in consecutive pregnancies. Am J Epidemiol. 2008;168(4):422‐426. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 169. Skjaerven R, Wilcox AJ, Lie RT. The interval between pregnancies and the risk of preeclampsia. N Engl J Med. 2002;346(1):33‐38. [DOI] [PubMed] [Google Scholar]
- 170. Organization WH . Global Strategy for Infant and Young Child Feeding. World Health Organization; 2003. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Appendix S1–S6.
Table S1–S10.
Figure S1–S6.
Data Availability Statement
All relevant data are within the manuscript and its Supporting Information files.


