Abstract
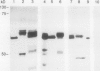
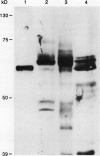
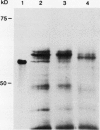
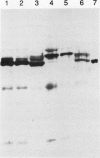
The chiA gene of Serratia marcescens codes for a secreted protein, bacterial chitinase (ChiA). We have investigated the modifications and the cellular location of ChiA when it is expressed in transgenic tobacco plants. Immunoblots on total leaf protein probed with antibody to ChiA showed that when the bacterial chitinase is expressed in plants, it migrates as a series of discrete bands with either the same or a slower mobility than the secreted bacterial protein. Analysis of the vacuum infiltrate of leaves expressing ChiA showed that the modified forms of the protein are enriched in the intercellular fluid. Media recovered from suspension cultures of cell lines expressing the chiA gene were also enriched for the modified forms of ChiA. Washed protoplasts, however, contained only the nonmodified form. The molecular weight of these polypeptides is reduced by treatment with glycopeptidase F but not with endoglycosidase H. Treatment of the suspension cultures with tunicamycin also leads to reduction in the molecular weight of the chitinase bands. We suggest that some of the ChiA protein is N-glycosylated and secreted when expressed in plants, and that the modifications are complex glycans. These results show that a bacterial signal sequence can function in plant cells, and that protein secretion from plant cells probably operates by a default pathway.
Full text
PDF




Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bird P., Gething M. J., Sambrook J. Translocation in yeast and mammalian cells: not all signal sequences are functionally equivalent. J Cell Biol. 1987 Dec;105(6 Pt 2):2905–2914. doi: 10.1083/jcb.105.6.2905. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bradford M. M. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem. 1976 May 7;72:248–254. doi: 10.1016/0003-2697(76)90527-3. [DOI] [PubMed] [Google Scholar]
- Burgess T. L., Kelly R. B. Constitutive and regulated secretion of proteins. Annu Rev Cell Biol. 1987;3:243–293. doi: 10.1146/annurev.cb.03.110187.001331. [DOI] [PubMed] [Google Scholar]
- Chen J., Varner J. E. An extracellular matrix protein in plants: characterization of a genomic clone for carrot extensin. EMBO J. 1985 Sep;4(9):2145–2151. doi: 10.1002/j.1460-2075.1985.tb03908.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garoff H. Using recombinant DNA techniques to study protein targeting in the eucaryotic cell. Annu Rev Cell Biol. 1985;1:403–445. doi: 10.1146/annurev.cb.01.110185.002155. [DOI] [PubMed] [Google Scholar]
- Gatenby A. A., Boccara M., Baulcombe D. C., Rothstein S. J. Expression of a wheat alpha-amylase gene in Escherichia coli: recognition of the translational initiation site and the signal peptide. Gene. 1986;45(1):11–18. doi: 10.1016/0378-1119(86)90126-5. [DOI] [PubMed] [Google Scholar]
- Harpster M. H., Townsend J. A., Jones J. D., Bedbrook J., Dunsmuir P. Relative strengths of the 35S cauliflower mosaic virus, 1', 2', and nopaline synthase promoters in transformed tobacco sugarbeet and oilseed rape callus tissue. Mol Gen Genet. 1988 Apr;212(1):182–190. doi: 10.1007/BF00322463. [DOI] [PubMed] [Google Scholar]
- Jefferson R. A., Kavanagh T. A., Bevan M. W. GUS fusions: beta-glucuronidase as a sensitive and versatile gene fusion marker in higher plants. EMBO J. 1987 Dec 20;6(13):3901–3907. doi: 10.1002/j.1460-2075.1987.tb02730.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jones J. D., Grady K. L., Suslow T. V., Bedbrook J. R. Isolation and characterization of genes encoding two chitinase enzymes from Serratia marcescens. EMBO J. 1986 Mar;5(3):467–473. doi: 10.1002/j.1460-2075.1986.tb04235.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaiser C. A., Preuss D., Grisafi P., Botstein D. Many random sequences functionally replace the secretion signal sequence of yeast invertase. Science. 1987 Jan 16;235(4786):312–317. doi: 10.1126/science.3541205. [DOI] [PubMed] [Google Scholar]
- Lee K. Y., Townsend J., Tepperman J., Black M., Chui C. F., Mazur B., Dunsmuir P., Bedbrook J. The molecular basis of sulfonylurea herbicide resistance in tobacco. EMBO J. 1988 May;7(5):1241–1248. doi: 10.1002/j.1460-2075.1988.tb02937.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lord J. M. Precursors of ricin and Ricinus communis agglutinin. Glycosylation and processing during synthesis and intracellular transport. Eur J Biochem. 1985 Jan 15;146(2):411–416. doi: 10.1111/j.1432-1033.1985.tb08667.x. [DOI] [PubMed] [Google Scholar]
- Palade G. Intracellular aspects of the process of protein synthesis. Science. 1975 Aug 1;189(4200):347–358. doi: 10.1126/science.1096303. [DOI] [PubMed] [Google Scholar]
- Pfeffer S. R., Rothman J. E. Biosynthetic protein transport and sorting by the endoplasmic reticulum and Golgi. Annu Rev Biochem. 1987;56:829–852. doi: 10.1146/annurev.bi.56.070187.004145. [DOI] [PubMed] [Google Scholar]
- Pfitzner U. M., Goodman H. M. Isolation and characterization of cDNA clones encoding pathogenesis-related proteins from tobacco mosaic virus infected tobacco plants. Nucleic Acids Res. 1987 Jun 11;15(11):4449–4465. doi: 10.1093/nar/15.11.4449. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rathmell W. G., Sequeira L. Soluble peroxidase in fluid from the intercellular spaces of tobacco leaves. Plant Physiol. 1974 Feb;53(2):317–318. doi: 10.1104/pp.53.2.317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rothman J. E. Protein sorting by selective retention in the endoplasmic reticulum and Golgi stack. Cell. 1987 Aug 14;50(4):521–522. doi: 10.1016/0092-8674(87)90024-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tarentino A. L., Plummer T. H., Jr Oligosaccharide accessibility to peptide:N-glycosidase as promoted by protein-unfolding reagents. J Biol Chem. 1982 Sep 25;257(18):10776–10780. [PubMed] [Google Scholar]
- Trimble R. B., Maley F. The use of endo-beta-N-acetylglucosaminidase H in characterizing the structure and function of glycoproteins. Biochem Biophys Res Commun. 1977 Oct 10;78(3):935–944. doi: 10.1016/0006-291x(77)90512-5. [DOI] [PubMed] [Google Scholar]
- Voelker T. A., Florkiewicz R. Z., Chrispeels M. J. Secretion of phytohemagglutinin by monkey COS cells. Eur J Cell Biol. 1986 Dec;42(2):218–223. [PubMed] [Google Scholar]
- Wiedmann M., Huth A., Rapoport T. A. Xenopus oocytes can secrete bacterial beta-lactamase. Nature. 1984 Jun 14;309(5969):637–639. doi: 10.1038/309637a0. [DOI] [PubMed] [Google Scholar]