Abstract
Background:
The burden of diarrheal diseases remains high among children in low-income countries. Enteropathogens are challenging to control because they are transmitted via multiple pathways. Chickens are an important animal protein source, but live chickens and their products are often highly contaminated with enteropathogens.
Objectives:
We conducted this study to a) understand the contribution of multiple transmission pathways to the force of infection of Campylobacter spp. and nontyphoidal Salmonella spp., b) quantify the potential impact of reducing each pathway on human infection, and c) quantify hypothesized pathway reduction from the context of Maputo, Mozambique.
Methods:
We developed transmission models for Campylobacter and Salmonella that captured person-to-person, water-to-person, food-to-person, soil-to-person, animal-to-person, and all-other-sources-to-person in an urban, low-income setting in Mozambique. We calibrated these models using prevalence data from Maputo, Mozambique and estimates of attributable fraction of transmission pathways for the region. We simulated the prevalence of human infection after reducing transmission through each pathway.
Results:
Simulation results indicated that if foodborne transmission were reduced by 90%, the prevalence of Campylobacter and Salmonella infection would decline by [52.2%; 95% credible interval (CrI): 39.7, 63.8] and (46.9%; 95% CrI: 39, 55.4), respectively. Interruption of any other pathway did not have a substantial impact. Combined with survey and microbiology data, if contamination of broiler chicken meat at informal markets in Maputo could be reduced by 90%, the total infection of Campylobacter and Salmonella could be reduced by 21% (16–26%) and 12% (10–13%), respectively.
Discussion:
Our transmission models showed that the foodborne transmission has to be reduced to control enteropathogen infections in our study site, and likely in other similar contexts, but mitigation of this transmission pathway has not received sufficient attention. Our model can serve as a tool to identify effective mitigation opportunities to control zoonotic enteropathogens. https://doi.org/10.1289/EHP12314
Introduction
Diarrheal diseases are a substantial source of childhood morbidity and mortality.1 In contexts where enteropathogen transmission is high, repeated asymptomatic infections also contributed to undernutrition and growth faltering through gut inflammation and constant activation of immune response (environmental enteric disfunction), leading to long-term cognitive deficits, poorer academic performance and school retention, and lower economic productivity in adulthood.2,3 One-fifth of all child deaths worldwide is caused by undernutrition or stunting, although these disproportionately affect resource-limited settings.2,4
Improvements to water, sanitation, and hygiene (WASH) may help mitigate enteropathogen exposure but may not address all relevant pathways of infection, such as food and animal contact.5 The fraction of enteropathogen infections attributable to animal transmission is unknown but likely substantial; a previous study reported that over a quarter of childhood enteropathogen infections had a potential animal source.6 Currently, the amount of animal feces produced globally is four times that of human waste and growing.7 In many settings globally, food systems remain unregulated, leading to a considerable risk of infection among young children. Because of the close connections enteropathogens have with animals and food systems,6 a comprehensive One Health approach is needed to control them effectively.
Poultry production is a particularly important source of enteropathogens in low- and middle-income countries (LMICs).8 Poultry farming has been promoted as an important growth and development strategy and is expected to increase in LMICs.8 While poultry farming provides a source of nutrition and income,9 chickens also pose health risks because they are frequent carriers of those enteropathogens that cause the greatest fraction of the global burden of diarrheal disease, including Campylobacter, Salmonella, and Cryptosporidium.10 Poor sanitation practices and lack of biosecurity and resources throughout the chicken meat production system increase risks of exposure to these pathogens through direct contact with chickens, their feces, and/or their food products.11,12 As the consumption of poultry in LMICs increases,13 it will become increasingly important to examine foodborne transmission as part of a holistic One Health framework that incorporates all key transmission pathways.
Despite its importance, the One Health approach has often fallen between different sectors and regulatory agencies. For example, several recent trials and cohort studies evaluating the impact of WASH interventions revealed mixed results.14–17 One potential reason for these results was the focus on a selected set of transmission pathways (water and environment), while not incorporating other critical pathways, such as food and contact with live animals.14,18
Multiple transmission pathways, such as those through direct contact with infected people, contaminated water, soil, and food and contact with infected animals, affect each other through feedback loops. When infection prevalence increases, the number of pathogens shed into the environment (e.g., soil and water) from infected people also increases, resulting in an increased number of infections via contaminated environments. These nonlinear feedback loops make it difficult to predict the impact of potential interventions targeting different pathways on reducing total infection in the population without transmission dynamic models.
To better understand these nonlinear relationships between pathways and to better quantify the impact of potential control measures, we employed a mechanistic, systems approach using infectious disease transmission models to capture a comprehensive set of transmission pathways for zoonotic enteropathogens. Here, we focused on two key zoonotic enteropathogens of poultry origin, Campylobacter and nontyphoidal Salmonella. We built the models for Maputo, Mozambique, a country in southeastern Africa where poultry production has been increasing and the burden of enteric infections is high.8,19 Using these models, we evaluated the impact of reducing each transmission pathway on human infection and estimated how the reduction in one pathway would change the prevalence of total infection and pathway-specific infections. We then interpreted these simulation results based on survey and microbiology data collected locally in Maputo, Mozambique, to understand the impacts of control efforts reducing transmission along each pathway.
Methods
Research Questions
We aimed to a) understand the contribution of multiple transmission pathways to the force of infection of Campylobacter spp. and nontyphoidal Salmonella spp., b) quantify the potential impact of reducing each pathway on human infection prevalence, and c) quantify hypothesized pathway reduction from the context of Maputo, Mozambique.
Infectious Disease Transmission Models
We built susceptible-infectious-susceptible type models for Campylobacter spp. and nontyphoidal Salmonella spp. that capture a comprehensive set of transmission pathways, namely person-to-person (direct physical contact with infected people), water-to-person (drinking water and stored water), food-to-person (chicken meat, eggs, and contaminated raw product), animal-to-person (direct contact with live infected chickens), soil-to-person (contaminated soil around latrines), and all-other-sources-to-person (anything that was not captured by the aforementioned pathways) (Figure 1 and Equation 1–8).
| (1) |
| (2) |
| (3) |
| (4) |
| (5) |
| (6) |
| (7) |
| (8) |
These models track the fractions of children who are susceptible to infection (S) and children who are infected via direct contact with other infected people (), consumption of contaminated food () and water (), exposure to contaminated soil (), contacts with live animals (), and all other exposure sources () on day . Pathway-specific transmission rates are represented by , and the recovery rate from infection is denoted by (Table 1). Infections in these models may or may not be symptomatic. We did not model persistent acquired immunity because infection with these enteropathogens, while potentially causing immunity to disease,20 does not provide full protection against subsequent infections.21,22
Figure 1.
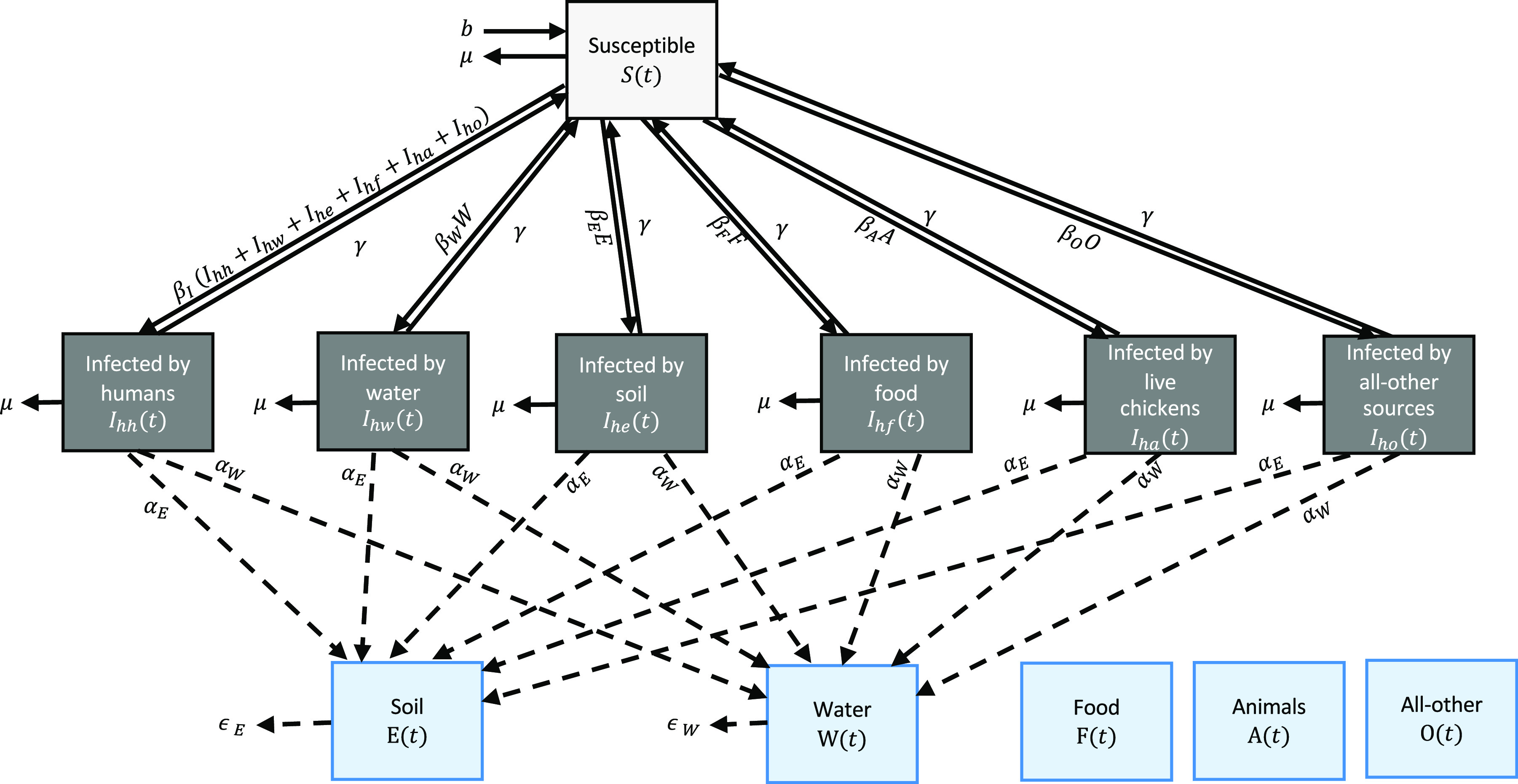
Structure of the infectious disease transmission model for Campylobacter spp. and nontyphoidal Salmonella spp. Model equations are given in Equation 1–8 and parameters are given in Table 1. These models track the fractions of children who are susceptible to infection (S) and children who are infected via direct contact with other infected people (), consumption of contaminated food () and water (), exposure to contaminated soil (), contacts with live animals (), and all other exposure sources () on day . The models also include water (W) and soil (E) compartments that track pathogen concentration in these two sources. The rates of pathogen shedding from infected people to water and soil are represented by and . The rates of natural pathogen clearance from water and soil are represented by and . Pathogen contamination in food (F) and all-other sources (O) and the prevalence of infection among live animals (A) were constant and not time-varying. The mortality rate and birth date are represented by μ and b, respectively.
Table 1.
Parameters for the Campylobacter and Salmonella transmission models.24
| Parameter | Definition and unit | Fixed or estimated |
|---|---|---|
| Transmission rate for the person-to-person pathway (1/day) | Estimated | |
| Transmission rate for the water-to-person pathway (1/day) | Estimated | |
| Transmission rate for the soil-to-person pathway (1/day) | Estimated | |
| Transmission rate for the food-to-person pathway (1/day) | Estimated | |
| Transmission rate for the live-animal-to-person pathway (1/day) | Estimated | |
| Transmission rate for the all-other-exposure-to-person pathway (1/day) | Estimated | |
| Rate of recovery from infection (1/day) | Fixed at 1 | |
| Mortality rate among children under 5 years of age in Mozambique (1/day) | Fixed at 70 per 1,000 children divided by 365.25 | |
| Birth rate (1/day) | Fixed at 70 per 1,000 children divided by 365.25 | |
| Rate of shedding into water and soil (1/day) | Fixed at 1 | |
| Rate of removal of pathogens from water and soil (1/day) | Fixed at 1 |
The models also include water (W) and soil (E) compartments that track pathogen concentration in these two sources. They are affected by the fraction of infected individuals on day through the rate of pathogen shedding from infected people to water and soil (α) and the rate of natural pathogen clearance from water and soil (ϵ) (Table 1). For the Campylobacter model, the soil pathway and soil compartment were not included because Campylobacter spp. are microaerophilic and cannot remain infectious in soil.23 Pathogen contamination in food (F) and all-other sources (O) and the prevalence of infection among live animals (A) were constant and not time-varying. The mortality rate (μ) was fixed to at the reported rate among children under 5 years of age in Mozambique in 2019 (Table 1).24 The birth rate (b) was also fixed and set to be the same as the mortality rate to generate a stable population over the simulation period.
Identifiability, Reparameterization, and Parameter Estimation
To estimate transmission rates for the six pathways, we fit our models to previously reported data on the prevalence of infection among children in Maputo from February 2015 to February 2016 (8% for Campylobacter and 21% for nontyphoidal Salmonella),19 as well as estimates from the World Health Organization Estimates of the Global Burden of Foodborne Diseases (WHO FERG report) for the African region with high child mortality and very high adult mortality, which includes Mozambique (Table S1).25 Both the prevalence data and reported proportions of illness by pathways were cross-sectional estimates, so we connected the transmission models to these data through the prevalence of human infection at the simulated steady-state endemic equilibrium (i.e., when the simulated prevalence of infection reached a stable level).
Transmission models were connected to the reported prevalence of Campylobacter infection and Salmonella infection and the estimated proportion of illness caused by each pathway through the simulated prevalence of infection at endemic equilibrium. The force of infection at steady state scaled by the sum of the recovery rate and mortality rate [] is , where I* denotes the prevalence of human infection at equilibrium. We broke this scaled force of infection at steady state into attributable pieces as follows: , , , , , and , where asterisks denote equilibrium values. Due to the limited human infection estimates to fit the models, the following parameters were not separately identifiable from pathway-specific transmission rates (β), and thus, they were set to one and estimated as a part of betas: the recovery rate (γ), pathogen shedding rate from infected individuals to water and soil (α), the pathogen clearance rate from water and soil (ϵ) as well as pathogen contamination in food (F) and all other sources (O) and the prevalence of infection among live animals (A) (Table 1).
To estimate the contribution of each transmission pathway to the force of infection at the endemic equilibrium, as well as the uncertainty in the estimates, we used a sampling-importance resampling approach.26 This approach is useful when many model parameters will not be separately identifiable or will have large uncertainty around their estimates due to complicated model structure or limited data to fit the model, which is the case in our study. We first created a large number of potential values for the parameters that we estimate, namely the prevalence of infection at steady state and the contribution of each pathway to the force of infection at steady state. We sampled the contribution of each pathway to the force of infection by breaking a [0, 1] unit into five pieces for the Campylobacter model and six pieces for the Salmonella model. We also sampled the prevalence of human infection from a uniform distribution [Unif(0–0.2) for Campylobacter and Unif(0–0.4) for Salmonella]. We created a large number of such sets of values using a Sobol sequence (2.5 million sets for Campylobacter and 100 million sets for Salmonella).27 We ran the transmission model with each of these sets for (2 years) and estimated how likely it was for each set to be the “true” parameter values by comparing the simulated steady-state prevalence to the reported prevalence and contribution of each pathway to the force of infection at steady state. We calculated the negative log-likelihood based on the multinomial distribution by comparing the simulated prevalence at steady-state with the previously reported prevalence of Campylobacter (8%) and Salmonella (21%) among children in Maputo19 as well as the pathway-specific prevalence of infection, which is the product of the total infection and the WHO estimates of the proportion of illness caused by each pathway (Table S1).25 Finally, we resampled 10,000 sets of parameters using this likelihood as a probability of resampling. Each value of β was then calculated by dividing the product of the estimated contribution and () by the equilibrium values of I, W, C, F, and O. Medians and 2.5th and 97.5th percentiles of these resampled parameter sets (i.e., posterior distributions) were reported as point estimates and 95% credible intervals (CrIs) (which is an interval within which an unobserved parameter value falls with a particular probability in Bayesian statistics).
Simulation of Potential Mitigation Opportunities
To estimate the potential impact of controlling each pathway and to understand how the reduction in one pathway may affect infection through other pathways, we simulated the prevalence of infection from each pathway after reducing the transmission rate of each pathway . For each of 10,000 resampled parameter sets, we reduced the resampled value of for the ith pathway by multiplying them by 0.1, 0.2, 0.3, …, 0.9, and 0 (i.e., 90%, 80%, 70%, …, 10% reduction in the transmission rate). We ran the transmission model with each of these modified 10,000 parameter sets and obtained the total prevalence and pathway-specific prevalence at endemic equilibrium. We calculated the difference in the simulated steady-state prevalence before and after reducing the transmission rate for each of 10,000 sets.
Interpretation Based on Local Data
To meaningfully convey what it means to reduce each transmission pathway by 10%, 20%, and so on in the local context, we interpreted simulation results by incorporating empirical population-based survey and microbiology data that our team previously collected in Maputo, Mozambique.28
For the population survey, we randomly sampled 570 households in 14 neighborhoods in Maputo in March 2021 and asked questions about the purchase and consumption of broiler chicken meat (chickens raised specifically for meat production) in the previous week. Our in-person questionnaire asked whether each household purchased and/or ate chicken products (yes/no question) and also asked how many days each household ate poultry products in the previous week (frequency). The quantity of meat was not asked because respondents were less likely to know the accurate quantity. All households, regardless of the presence of children under 5 years of age, were included and interviewed. Survey response rate was high (no mention of refusals by fieldworkers), although we did not quantify this.
For the microbiology data, we sampled broiler carcass rinse samples () at various locations along the chicken value chain in Maputo, such as informal wet markets (selling wet and dry products, including eggs, live chickens, and fresh poultry meat butchered on site) and corner stores (small convenience shops that sell limited food products, including eggs and frozen chicken parts that are usually unwrapped and unlabeled). We tested them for Campylobacter jejuni/Campylobacter coli and nontyphoidal Salmonella spp. by quantitative real-time polymerase chain reaction (qPCR).
Analysis for Shigella
We ran the analysis for Shigella that is mainly transmitted from person to person in order to compare results of the nonfoodborne enteropathogen to those of foodborne enteropathogens (Campylobacter and Salmonella). We fit a transmission model to previously reported data on the prevalence of Shigella infection among children in Maputo from 2015 to 2016 (44%)19 and the estimated proportion of illnesses caused by each transmission pathway (person-to-person: 50%, water: 26%, food: 15%, animal: 0%, soil: 0%, all-other: 9%).25
Software and Code
All analyses were conducted with R version 4 (R Center for Statistical Computing), and our code can be found in the supplementary material as well as in the following GitHub repository: https://github.com/KayokoShioda/ChEEPx2_TransmissionModel.
Ethics
The institutional review board at Emory University (IRB00108546) and the Research Council to the Veterinary Faculty at Eduardo Mondlane University determined that this research was exempt from human subjects review, and the Municipality of Maputo (reference number 754/SG/426/GP/2019) authorized this research.
Results
Estimated Contribution of Each Pathway to the Force of Infection
Our transmission models reflected the prevalence of total infection of Campylobacter and Salmonella in Maputo, Mozambique, and estimated the contribution of each pathway to the steady-state force of infection for both Campylobacter and Salmonella (Table 2). The food pathway was estimated to be attributable to (50.8%; 95% CrI: 38.2, 62.8) and (42.6%; 95% CrI: 35.2, 51.3) of the force of infection for Campylobacter and Salmonella, respectively. The sampling-importance resampling approach generated posterior distributions of the mechanistic model parameters (Figure S1). The resampled parameters underlying the fit to the data (the “factual” given in Table 2) served as the starting point for the counterfactual intervention simulations in the next section.
Table 2.
Prevalence of total infection and pathway-specific infection and contribution of each pathway to the steady-state force of infection and their 95% CrI for Campylobacter and Salmonella in Mozambique, estimated using the infectious disease transmission model described in the text.
| Campylobacter [% (95% CrI)] | Salmonella [% (95% CrI)] | |
|---|---|---|
| Estimated prevalence of infection at endemic equilibrium | ||
| Total prevalence of infection (all pathways combined) | 8.1 (6.3, 10.2) | 20.9 (18.2, 24.0) |
| Prevalence of infection via person-to-person | 0.4 (0.1, 1.0) | 3.8 (2.6, 5.3) |
| Prevalence of infection via water | 0.8 (0.3, 1.6) | 2.1 (1.3, 3.4) |
| Prevalence of infection via food | 4.1 (2.8, 5.7) | 8.9 (7.1, 11.2) |
| Prevalence of infection via live animals | 1.5 (0.8, 2.5) | 3.3 (2.2, 4.7) |
| Prevalence of infection via soil | NA | 0.3 (0.1, 0.9) |
| Prevalence of infection via all-other | 1.1 (0.5, 2.1) | 2.2 (1.2, 3.4) |
| Estimated contribution of pathway to the steady-state force of infection | ||
| Person-to-person | 5.0 (1.4, 12.5) | 18.0 (13.0, 24.6) |
| Water | 10.3 (4.0, 19.4) | 10.2 (6.3, 16.0) |
| Food | 50.8 (38.2, 62.8) | 42.6 (35.2, 51.3) |
| Live animals | 18.5 (9.9, 29.1) | 15.7 (10.6, 22.0) |
| Soil | NA | 1.5 (0.3, 4.1) |
| All-other sources | 14.2 (6.9, 24.5) | 10.3 (6.1, 15.8) |
Note: CrI, credible interval; NA, not applicable.
Simulated Impact of Potential Mitigation Opportunities
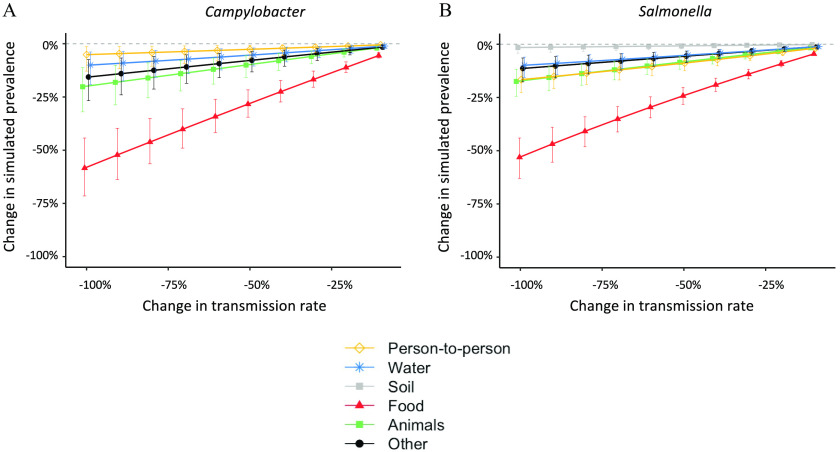
For both Campylobacter and Salmonella, our simulation analysis suggested that the modeled prevalence of infection would not decrease—given related bounds of statistical confidence—unless the foodborne pathway is reduced (Figure 2). Interruption of the remaining pathways resulted in at most a modest reduction of the total simulated prevalence of infection of both pathogens.
Figure 2.
Changes in the simulated steady-state prevalence of total infection after reducing the transmission rate for each pathway. Summary data of this figure can be found in Table 3 and 4 and Table S2 and S3.
When foodborne transmission was reduced by 90%, the simulated prevalence of total Campylobacter infection, combining infection from all pathways, decreased by (52.2%; 95% CrI: 39.7, 63.8) (Table 3). While person-to-person transmission and waterborne transmission were not directly targeted by the simulated intervention, the simulated prevalence of infection via these pathways also decreased by (50.1%; 95% CrI: 37.6, 62.0) due to the feedback loops in the model (Table 3). Because of reductions in the pathways that were not directly targeted by the simulated intervention, the overall reduction in the simulated total prevalence (52.2%) was larger than 90% of the estimated contribution of the foodborne pathway to the steady-state force of infection (Table 2; ). In contrast, both the simulated prevalence of infection via contact with infected live animals and all other sources increased by (4.5%; 95% CrI: 3.2, 6.3) after reducing the foodborne transmission by 90% (Table 3). This was because the force of infection through these pathways was constant and did not vary by the prevalence of human infection. As the availability of susceptible children increased in the simulation, a higher prevalence of infection was observed through pathways with the constant force of infections (Table 3). The same trend was observed for Salmonella (Table 4) and when reducing each pathway by 50% (Table S2 and Table S3).
Table 3.
Changes in the simulated steady-state prevalence of Campylobacter infection in Maputo, Mozambique after a simulated intervention that produces a 90% reduction in the selected transmission rate using the infectious disease transmission model described in the text.
| Intervention scenario | Changes in the simulated steady-state prevalence of Campylobacter infection after a simulated intervention | |||||
|---|---|---|---|---|---|---|
| Total infection | Infection via person-to-person | Infection via water | Infection via food | Infection via live animals | Infection via all-other sources | |
| Reducing the rate of person-to-person transmission by 90% | (, ) | (, ) | (, ) | 0.4% (0.1%, 1.1%) | 0.4% (0.1%, 1.1%) | 0.4% (0.1%, 1.1%) |
| Reducing the rate of waterborne transmission by 90% | (, ) | (, ) | (, ) | 0.8% (0.3%, 1.6%) | 0.8% (0.3%, 1.6%) | 0.8% (0.3%, 1.6%) |
| Reducing the rate of foodborne transmission by 90% | (, ) | (, ) | (, ) | (, ) | 4.5% (3.2%, 6.3%) | 4.5% (3.2%, 6.3%) |
| Reducing the rate of live animal transmission by 90% | (, ) | (, ) | (, ) | 1.6% (0.8%, 2.7%) | (, ) | 1.6% (0.8%, 2.7%) |
| Reducing the rate of all-other transmission by 90% | (, ) | (, ) | (, ) | 1.2% (0.6%, 2.2%) | 1.2% (0.6%, 2.2%) | (, ) |
Table 4.
Changes in the simulated steady-state prevalence of Salmonella infection in Maputo, Mozambique after a simulated intervention that produces a 90% reduction in the selected transmission rate using the infectious disease transmission model described in the text.
| Intervention scenario | Changes in the simulated steady-state prevalence of Salmonella infection after a simulated intervention | ||||||
|---|---|---|---|---|---|---|---|
| Total infection | Infection via person-to-person | Infection via water | Infection via food | Infection via live animals | Infection via soil | Infection via all-other sources | |
| Reducing the rate of person-to-person transmission by 90% | (, ) | (, ) | (, ) | 4.1% (2.8%, 5.8%) | 4.1% (2.8%, 5.8%) | (, ) | 4.1% (2.8%, 5.8%) |
| Reducing the rate of waterborne transmission by 90% | (, ) | (, ) | (, ) | 2.4% (1.5%, 3.9%) | 2.4% (1.5%, 3.9%) | (, ) | 2.4% (1.5%, 3.9%) |
| Reducing the rate of foodborne transmission by 90% | (, ) | (, ) | (, ) | (, ) | 12.4% (9.9%, 15.4%) | (, ) | 12.4% (9.9%, 15.4%) |
| Reducing the rate of live animal transmission by 90% | (, ) | (, ) | (, ) | 4.1% (2.7%, 6%) | (, ) | (, ) | 4.1% (2.7%, 6%) |
| Reducing the rate of soil transmission by 90% | (, ) | (, ) | (, ) | 0.4% (0.1%, 1%) | 0.4% (0.1%, 1%) | (, ) | 0.4% (0.1%, 1%) |
| Reducing the rate of all-other transmission by 90% | (, ) | (, ) | (, ) | 2.7% (1.5%, 4.3%) | 2.7% (1.5%, 4.3%) | (, ) | (, ) |
We ran the same analysis with an enteropathogen that is not frequently transmitted via food, Shigella. The major transmission pathway for Shigella is person-to-person contact,25 so the relative impact of controlling foodborne transmission on human infection was smaller (Figure S2).
Interpretation Based on Local Data from Maputo, Mozambique
In our population-based survey in Maputo, 123 of 261 households with children under 5 years of age reported that they purchased broiler meat in the past week (Table 5). Of these 123 households, 73 purchased broiler meat at corner stores, where 25% of broiler carcass rinse water was contaminated with Campylobacter jejuni/Campylobacter coli, suggesting that 18 households purchased contaminated meat at corner stores.28 Similarly, we calculated that 24 of the households purchased contaminated meat from informal markets (24 households purchasing meat that was 100% contaminated) and 12 from farmers (20 households, 61% contaminated). Assuming that the meat acquired from other sources (6 households) was not contaminated, a total of 54 households purchased contaminated meat.
Table 5.
Source of broiler meat and contamination with Campylobacter jejuni/Campylobacter coli and Salmonella in Maputo, Mozambique.28
| Location | Number (%) of households that purchased broiler meat at each location | Broiler meat contaminated with Campylobacter jejuni/coli (%) | Broiler meat contaminated with Salmonella (%) |
|---|---|---|---|
| Corner stores | 73 (59%) | 25% | 15% |
| Informal markets | 24 (20%) | 100% | 17% |
| Farmers | 20 (16%) | 61% | 0% |
| Others | 6 (5%) | NA | NA |
Note: Data on the source of broiler meat were collected through the in-person population-based survey. We randomly sampled 570 households in 14 neighborhoods in Maputo in March 2021 and asked questions about the purchase and consumption of broiler chicken meat (chickens raised specifically for meat production) in the previous week. Our questionnaire asked whether each household purchased and/or ate chicken products (yes/no question) and also asked how many days each household ate poultry products in the previous week (frequency). The quantity of meat was not asked because respondents were less likely to know the accurate quantity. All households, regardless of the presence of children under 5 years of age, were included and interviewed. Survey response rate was high (no mention of refusals by fieldworkers), although we did not quantify this.
If Campylobacter contamination at corner stores could be reduced by 90%, the number of households purchasing meat contaminated with Campylobacter from corner stores would decrease from 18 to 2, reducing the total number of households purchasing contaminated meat from 54 to 38 (30% reduction). If we assume that the majority of foodborne transmission is from broiler meat consumption, the model simulation showed that this 30% reduction in foodborne transmission would decrease the simulated total infection of Campylobacter by (17%; 95% CrI: 13, 20). If contamination at informal markets, instead, could be reduced by 90%, the total number of households purchasing meat contaminated with Campylobacter would decrease from 55 to 33 (40% reduction). The model estimated that the 40% reduction in foodborne transmission would decrease the simulated total infection of Campylobacter by (23%; 95% CrI: 17, 27).
Similarly, we estimated that a 90% reduction in Salmonella contamination at corner stores would reduce households purchasing meat contaminated with Salmonella by 65% (from 15 to 5 households). Using the model simulation, this would decrease the simulated total infection of Salmonella by (32%; 95% CrI: 27, 38). A 90% reduction in Salmonella contamination at informal markets would reduce the number of households purchasing meat contaminated with Salmonella by 25% (from 15 to 11 households), which would reduce the simulated total infection of Salmonella by (12%; 95% CrI: 10, 13).
Discussion
We developed a novel approach leveraging multipathway infectious disease transmission models and sampling-importance resampling to explain infection prevalence of and pathway-attributable fractions for Campylobacter and Salmonella in Maputo, Mozambique and to estimate the impact of interventions. Building off a multipathway modeling approach to analyzing randomized controlled trial data,26 we showed that this approach can be used on observational data and may be widely applied across contexts to estimate the reduction in relevant pathways of transmission. Our simulation modeling suggested that if foodborne transmission was reduced by 90%, the prevalence of Campylobacter and Salmonella infection in Maputo would be reduced by approximately half. These results are likely generalizable to other LMIC contexts where regulation of food systems is limited27,29; they highlighted that without controlling foodborne transmission, it would be highly implausible to substantively reduce infection with these enteropathogens. Even a 90% reduction—a very ambitious reduction target for real-world settings—in contamination of chicken meat in informal markets would only result in a reduction of 23% and 12% in the prevalence of Campylobacter and Salmonella infection, respectively, in Maputo, highlighting the challenges of creating effective real-world change in enteropathogen infections. Our findings suggested that zoonotic enteropathogen infection via the foodborne transmission pathway was a major contributor to the burden of diarrheal diseases in LMICs and supported the hypothesis that recent null and mixed WASH trial results could in part be explained by a lack of attention to food, particularly contamination that occurs outside of the home, and animal transmission pathways.18,26
The importance of foodborne transmission of zoonotic enteropathogens is well established. The Food and Agriculture Organization of the United Nations predicted that consumption of poultry meat in Mozambique would increase from 34,200 metric tons in 2000 to 127,800 metric tons in 2030,13 and similar increases were expected in many other parts of the world. As such, the control of foodborne transmission of enteropathogens of poultry origin would become even more critical in the coming years. In rural Ethiopia, consumption of animal-source foods increased the odds of Campylobacter detection among children.30 In the low-income area of Dhaka, Bangladesh, high contamination of Escherichia coli was reported in leftover food.31 Our group also found high contamination of Campylobacter jejuni/Campylobacter coli and some contamination for nontyphoidal Salmonella in chicken feces and chicken carcass rinse samples throughout the production to consumption chain in Maputo, Mozambique.28 In high-income countries, various regulatory and management practices are in place to control foodborne transmission, but the burden of foodborne illness remains high, suggesting that more attention and research is needed not only in low-income settings but across the world.32
A One Health approach that incorporates and distinguishes the contribution of all key transmission pathways is critical for controlling zoonotic enteropathogens.14,18 While a report by the WHO FERG provided the best currently available estimates on the proportion of illnesses caused by each pathway,25 they were based on expert opinions and had large uncertainties. As relationships between transmission pathways are not linear, they cannot be simply calculated using traditional epidemiological methods.33 The transmission model approach is useful in this situation.33 We built transmission models with multiple pathways that reflected the transmission dynamics in Mozambique.
Our transmission models provided interesting insights on nonlinear feedback loops between multiple transmission pathways. There were two types of transmission pathways in our model as follows: those influenced by the proportion of infected individuals in the population (person-to-person and waterborne pathways) and those that were not (food, animals, and all-other pathways). We used this approach because data are not available on how the prevalence of infection among humans might affect contamination in food, infection among animals, and all other sources, which makes it impossible to estimate parameters for feedback loops. Including different characteristics of transmission provided a unique opportunity to understand how pathways with and without feedback loops respond to the reduction in a selected pathway. When one pathway was reduced in the simulation, infection via pathways with feedback loops also decreased due to the decreased probability of encountering infected individuals in the community or decreased pathogen shedding into the environment. In contrast, infection via pathways without feedback loops (i.e., those with constant force of infection) slightly increased after reducing one of the pathways, which may be counterintuitive. Our findings emphasized the complexity of nonlinear dynamic transmission via multiple pathways for zoonotic enteropathogens and highlighted the capacity of infectious disease transmission models to generate important hypotheses about the complex outcomes of potential interventions in a cost-effective way that can be further evaluated in future empirical studies.
Our approach had some limitations. Our model found it was important to control foodborne pathways to reduce transmission, but this finding did not apply to all enteropathogens, such as Shigella whose major transmission pathway is person-to-person contact (Figure S2). Due to the lack of time-series data on human infection as well as pathogen contamination in each source, we fixed the recovery rate, pathogen shedding rate into water and soil, and pathogen clearance rate from water and soil in the models. Despite this limitation, the sampling-importance resampling approach enabled us to develop distributions of key mechanistic parameters that were consistent with the data, and the model provided essential insights that could not be gained from purely empirical work. However, it does not undermine the importance of longitudinal data on pathogen contamination in environmental sources to structure and parameterize transmission models, which is a critical gap in the field. We included feedback loops for the water-to-person and soil-to-person pathways, reflecting our assumption that pathogen concentrations in water and soil are impacted by local human infections. We did not include the feedback from animal infections to pathogen contamination in water and soil, which may have nonnegligible impacts on transmission dynamics. More empirical data on the influence of animal infections on the contamination level in water and soil could help us address this point. This study can be readily applied to other enteropathogens, settings, and animal species with similar data. More detailed behavior data and associated pathogen contamination data at informal live markets (e.g., data on butchering processes, such as contamination due to inappropriate evisceration, unclean surfaces, and inefficient rinsing of chicken meat) as well as at households (e.g., cooking, cleaning, handwashing, storage) may allow us to develop a quantitative microbial risk assessment model that could evaluate the impact of more detailed, specific interventions targeting the food pathway. A strength of our study is that we interpreted these simulation results and were able to understand what it means to reduce each pathway in the real world based on locally collected data in Maputo.
Conclusions
Enteropathogens are transmitted via multiple pathways; zoonotic enteropathogens are even more complex given the ubiquitous role of animals in livelihoods and nutrition in low-income settings and the limited regulation of food systems.6 Better quantification of these complex and interrelated transmission pathways will support policy-makers, implementers, and researchers to design effective One Health interventions.26 Our results suggested that reductions in Campylobacter and Salmonella infection would not be achieved unless foodborne transmission was substantially reduced. The importance of the One Health approach, including control of foodborne transmission, has been underappreciated, and our study highlighted the need for more attention to this pathway. Testing different interventions targeting each pathway in a randomized controlled trial is expensive, so it is helpful to generate hypotheses using this type of simulation analysis in advance to understand the scope of reductions a trial might reasonably expect to produce.
Supplementary Material
Acknowledgments
K.S., K.L., and M.F. conceptualized the study. K.S., A.B., K.L., and M.F. developed study methods. F.L., H.N.M., K.L., and M.F. collected survey data and microbiology data in Maputo, Mozambique. F.L. and K.S. analyzed survey data. F.L., H.N.M., and K.L. ran molecular tests. K.S. ran the modeling analysis, which A.B. supervised. K.S. drafted the paper and created figures and tables, which A.B., K.L., and M.F. supervised. All authors reviewed, revised, and approved the paper. All authors had full access to all of the data and fully agreed with the decision to submit the paper for publication. The corresponding author (M.F.) had final responsibility for the decision to submit for publication after obtaining approval from all co-authors.
We would like to thank our in-country collaborators from the Biotechnology Centre at Eduardo Mondlane University, especially our enumerators, José Fafetine, Joaquim Saíde, Teresa A. Cuinhane, Hagnésio C. Chiponde, and Amélia Mondlane Milisse. We are grateful to the Maputo City Municipality for allowing us to conduct this study and to the study participants for their contributions. We would also like to thank Molly Miller-Petrie for constructive criticism of the manuscript.
This research was supported by the Bill & Melinda Gates Foundation. A.B. received funding from the Bill and Melinda Gates Foundation (INV-005081) and the National Science Foundation (DMS1853032). The funder of the study had no role in study design, data collection, data analysis, data interpretation, or writing of the report.
Data can be shared for research purposes upon reasonable request. Researchers can refer to the authors for information on accessing the data.
References
- 1.Troeger C, Blacker BF, Khalil IA, Rao PC, Cao S, Zimsen SR, et al. 2018. Estimates of the global, regional, and national morbidity, mortality, and aetiologies of diarrhoea in 195 countries: a systematic analysis for the global burden of disease study 2016. Lancet Infect Dis 18(11):1211–1228, 10.1016/S1473-3099(18)30362-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Humphrey JH. 2009. Child undernutrition, tropical enteropathy, toilets, and handwashing. Lancet 374(9694):1032–1035, PMID: , 10.1016/S0140-6736(09)60950-8. [DOI] [PubMed] [Google Scholar]
- 3.Owino V, Ahmed T, Freemark M, Kelly P, Loy A, Manary M, et al. 2016. Environmental enteric dysfunction and growth failure/stunting in global child health. Pediatrics 138(6):e20160641, PMID: , 10.1542/peds.2016-0641. [DOI] [PubMed] [Google Scholar]
- 4.Victora CG, Adair L, Fall C, Hallal PC, Martorell R, Richter L, et al. 2008. Maternal and child undernutrition: consequences for adult health and human capital. Lancet 371(9609):340–357, PMID: , 10.1016/S0140-6736(07)61692-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Prüss-Ustün A, Wolf J, Bartram J, Clasen T, Cumming O, Freeman MC, et al. 2019. Burden of disease from inadequate water, sanitation and hygiene for selected adverse health outcomes: an updated analysis with a focus on low- and middle-income countries. Int J Hyg Environ Health 222(5):765–777, PMID: , 10.1016/j.ijheh.2019.05.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Delahoy MJ, Wodnik B, McAliley L, Penakalapati G, Swarthout J, Freeman MC, et al. 2018. Pathogens transmitted in animal feces in low- and middle-income countries. Int J Hyg Environ Health 221(4):661–676, PMID: , 10.1016/j.ijheh.2018.03.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Berendes D, Capone D, Knee J, Holcomb D, Sultana S, Pickering AJ, et al. 2020. Associations between enteric pathogen carriage and height-for-age, weight-for-age and weight-for-height in children under 5 years old in urban Dhaka, Bangladesh. Epidemiol Infect 148:e39, PMID: , 10.1017/S0950268820000369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.FAO, Animal Production and Helath Division (AGA). 2018. Shaping the Future of Livestock: Sustainably, Responsibly, Efficiently. http://www.fao.org/publications/card/en/c/I8384EN/ [accessed 22 April 2021].
- 9.Wong JT, de Bruyn J, Bagnol B, Grieve H, Li M, Pym R, et al. 2017. Small-scale poultry and food security in resource-poor settings: a review. Glob Food Secur 15:43–52, 10.1016/j.gfs.2017.04.003. [DOI] [Google Scholar]
- 10.Havelaar AH, Kirk MD, Torgerson PR, Gibb HJ, Hald T, Lake RJ, et al. 2015. World Health Organization global estimates and regional comparisons of the burden of foodborne disease in 2010. PLoS Med 12(12):e1001923, PMID: , 10.1371/journal.pmed.1001923. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Carron M, Alarcon P, Karani M, Muinde P, Akoko J, Onono J, et al. 2017. The broiler meat system in Nairobi, Kenya: using a value chain framework to understand animal and product flows, governance and sanitary risks. Prev Vet Med 147:90–99, PMID: , 10.1016/j.prevetmed.2017.08.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Carron M, Chang Y-M, Momanyi K, Akoko J, Kiiru J, Bettridge J, et al. 2018. Campylobacter, a zoonotic pathogen of global importance: prevalence and risk factors in the fast-evolving chicken meat system of Nairobi, Kenya. PLoS Negl Trop Dis 12(8):e0006658, PMID: , 10.1371/journal.pntd.0006658. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Robinson TP, Pozzi F. 2011. Mapping Supply and Demand for Animal-Source Foods to 2030. Rome, Italy: Food and Agriculture Organization of the United Nations. [Google Scholar]
- 14.Pickering AJ, Null C, Winch PJ, Mangwadu G, Arnold BF, Prendergast AJ, et al. 2019. The WASH benefits and SHINE trials: interpretation of WASH intervention effects on linear growth and diarrhoea. Lancet Glob Health 7(8):e1139–e1146, PMID: , 10.1016/S2214-109X(19)30268-2. [DOI] [PubMed] [Google Scholar]
- 15.Reese H, Routray P, Torondel B, Sinharoy SS, Mishra S, Freeman MC, et al. 2019. Assessing longer-term effectiveness of a combined household-level piped water and sanitation intervention on child diarrhoea, acute respiratory infection, soil-transmitted helminth infection and nutritional status: a matched cohort study in rural Odisha, India. Int J Epidemiol 48(6):1757–1767, PMID: , 10.1093/ije/dyz157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Luby SP, Rahman M, Arnold BF, Unicomb L, Ashraf S, Winch PJ, et al. 2018. Effects of water quality, sanitation, handwashing, and nutritional interventions on diarrhoea and child growth in rural Bangladesh: a cluster randomised controlled trial. Lancet Glob Health 6(3):e302–e315, PMID: , 10.1016/S2214-109X(17)30490-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Null C, Stewart CP, Pickering AJ, Dentz HN, Arnold BF, Arnold CD, et al. 2018. Effects of water quality, sanitation, handwashing, and nutritional interventions on diarrhoea and child growth in rural Kenya: a cluster-randomised controlled trial. Lancet Glob Health 6(3):e316–e329, PMID: , 10.1016/S2214-109X(18)30005-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Cumming O, Arnold BF, Ban R, Clasen T, Esteves Mills J, Freeman MC, et al. 2019. The implications of three major new trials for the effect of water, sanitation and hygiene on childhood diarrhea and stunting: a consensus statement. BMC Med 17(1):173, PMID: , 10.1186/s12916-019-1410-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Knee J, Sumner T, Adriano Z, Berendes D, de Bruijn E, Schmidt W-P, et al. 2018. Risk factors for childhood enteric infection in urban Maputo, Mozambique: a cross-sectional study. PLoS Negl Trop Dis 12(11):e0006956, PMID: , 10.1371/journal.pntd.0006956. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Havelaar AH, van Pelt W, Ang CW, Wagenaar JA, van Putten JPM, Gross U, et al. 2009. Immunity to Campylobacter: its role in risk assessment and epidemiology. Crit Rev Microbiol 35(1):1–22, PMID: , 10.1080/10408410802636017. [DOI] [PubMed] [Google Scholar]
- 21.Rogawski McQuade ET, Liu J, Kang G, Kosek MN, Lima AAM, Bessong PO, et al. 2020. Protection from natural immunity against enteric infections and etiology-specific diarrhea in a longitudinal birth cohort. J Infect Dis 222(11):1858–1868, PMID: , 10.1093/infdis/jiaa031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Amour C, Gratz J, Mduma E, Svensen E, Rogawski ET, McGrath M, et al. 2016. Epidemiology and impact of Campylobacter infection in children in 8 low-resource settings: results from the MAL-ED study. Clin Infect Dis 63(9):1171–1179, PMID: , 10.1093/cid/ciw542. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Percival SL, Williams DW. 2014. Chapter 4. Campylobacter. In: Microbiology of Waterborne Diseases. Percival SL, Yates MV, Williams DW, Chalmers R, Gray NF, eds. 2nd ed. Amsterdam, the Netherlands: Academic Press, 65–78. [Google Scholar]
- 24.Wang H, Abbas KM, Abbasifard M, Abbasi-Kangevari M, Abbastabar H, Abd-Allah F, et al. 2020. Global age-sex-specific fertility, mortality, healthy life expectancy (HALE), and population estimates in 204 countries and territories, 1950–2019: a comprehensive demographic analysis for the global burden of disease study 2019. Lancet 396(10258):1160–1203, 10.1016/S0140-6736(20)30977-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.WHO (World Health Organization). 2015. WHO Estimates of the Global Burden of Foodborne Diseases. https://www.who.int/publications/i/item/9789241565165 [accessed 27 October 2023].
- 26.Brouwer AF, Eisenberg MC, Bakker KM, Boerger SN, Zahid MH, Freeman MC, et al. 2022. Leveraging infectious disease models to interpret randomized controlled trials: controlling enteric pathogen transmission through water, sanitation, and hygiene interventions. PLoS Comput Biol 18(12):e1010748, 10.1371/journal.pcbi.1010748. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Sobol’ IM. 1967. On the distribution of points in a cube and the approximate evaluation of integrals. USSR Computational Mathematics and Mathematical Physics 7(4):86–112, 10.1016/0041-5553(67)90144-9. [DOI] [Google Scholar]
- 28.Lamar F. 2021. ChickFlows in Maputo, Mozambique: High-Risk Behaviors, Management Practices, and Pathways for Childhood Exposure to Enteropathogens from Chickens. Atlanta, Georgia: Emory University. [Google Scholar]
- 29.Grace D. 2015. Food safety in low and middle income countries. Int J Environ Res Public Health 12(9):10490–10507, PMID: , 10.3390/ijerph120910490. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Chen D, McKune SL, Singh N, Yousuf Hassen J, Gebreyes W, Manary MJ, et al. 2020. Campylobacter colonization, environmental enteric dysfunction, stunting, and associated risk factors among young children in rural Ethiopia: a Cross-Sectional study from the Campylobacter genomics and environmental enteric dysfunction (CAGED) project. Front Public Health 8:615793, PMID: , 10.3389/fpubh.2020.615793. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Jensen PKM, Hossain ZZ, Ferdous J, Sultana R, Almeida S, Koch EB, et al. 2022. Escherichia coli ingested via food may overshadow the positive effects of clean drinking water: an example from Dhaka. Am J Trop Med Hyg 106(4):1163–1169, PMID: , 10.4269/ajtmh.20-1530. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Pires SM, Fischer-Walker CL, Lanata CF, Devleesschauwer B, Hall AJ, Kirk MD, et al. 2015. Aetiology-specific estimates of the global and regional incidence and mortality of diarrhoeal diseases commonly transmitted through food. PLoS One 10(12):e0142927, PMID: , 10.1371/journal.pone.0142927. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Eisenberg MC, Robertson SL, Tien JH. 2013. Identifiability and estimation of multiple transmission pathways in cholera and waterborne disease. J Theor Biol 324:84–102, PMID: , 10.1016/j.jtbi.2012.12.021. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.