Abstract
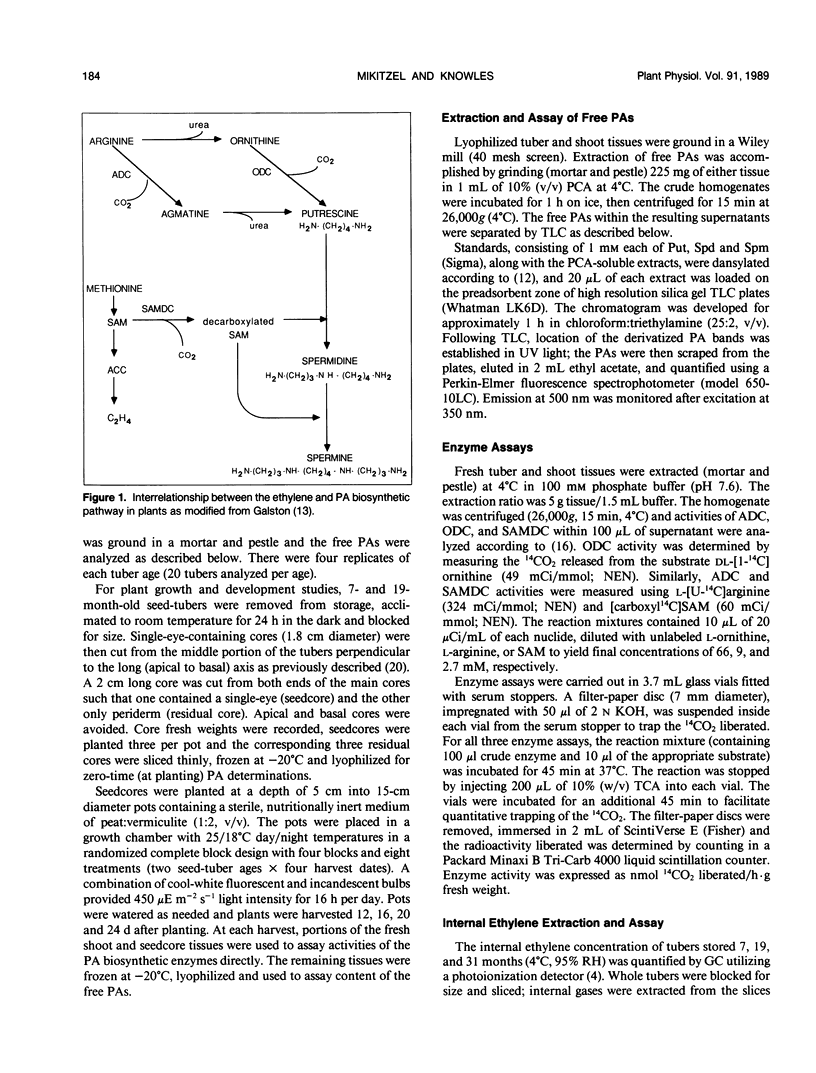
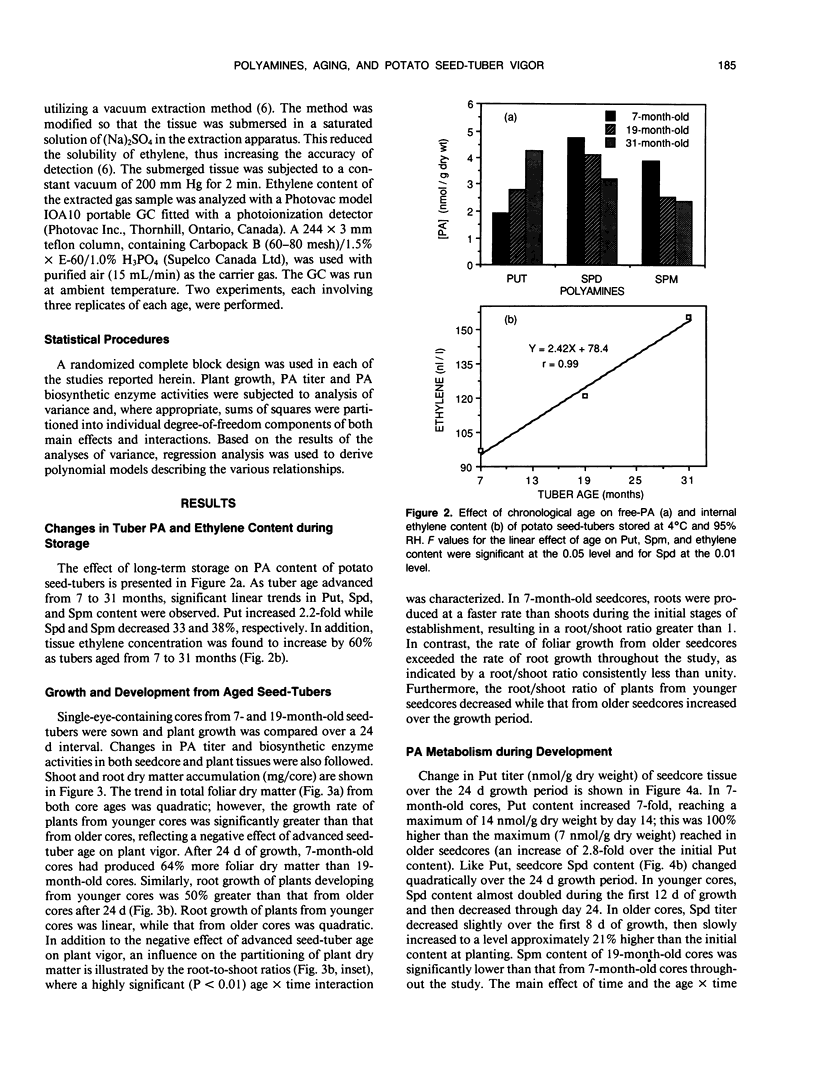
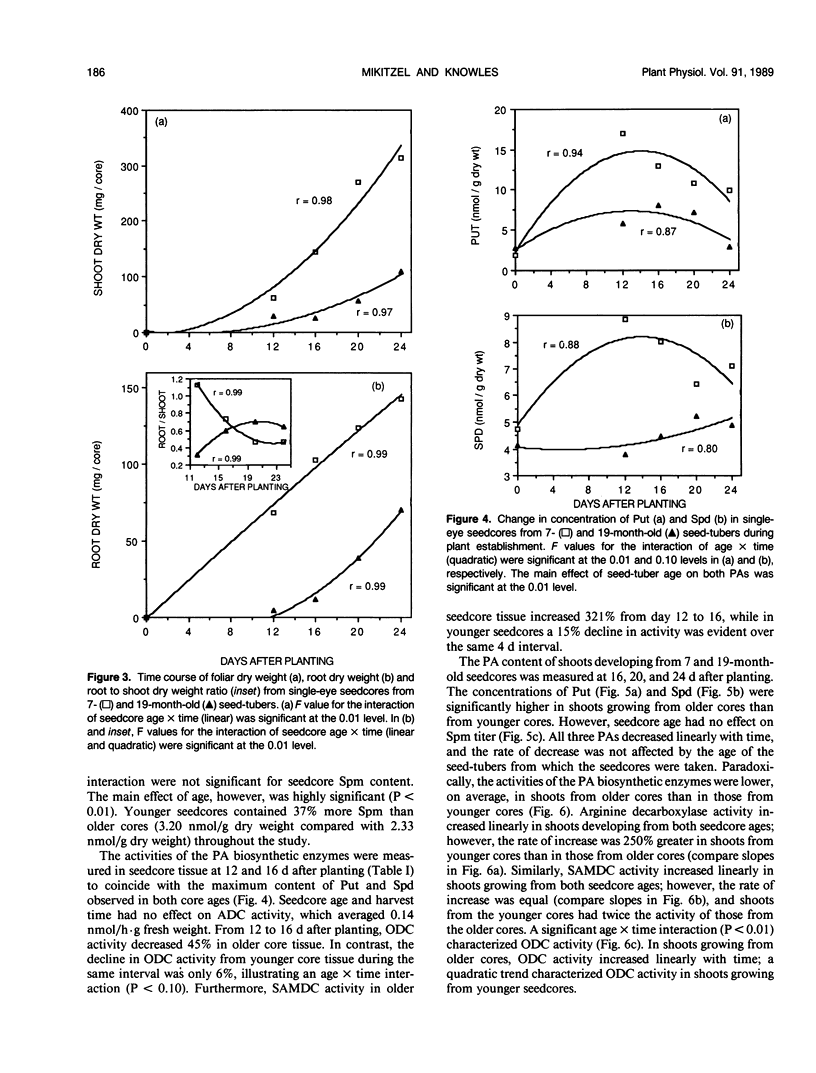
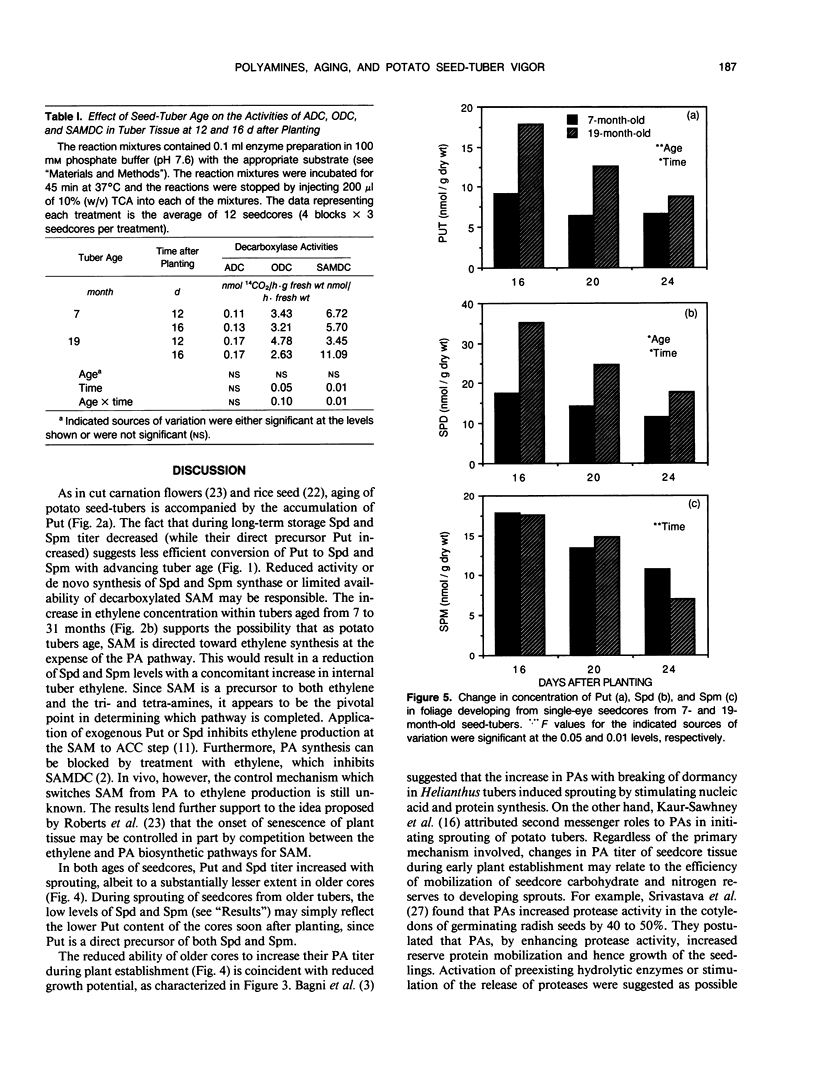
Growth potential of potato (Solanum tuberosum L.) plants is influenced by seed-tuber age. After 24 days of growth, single-eye seedcores from 7-month-old seed-tubers produced 64% more foliar dry matter than those from 19-month-old seed-tubers, reflecting a higher growth rate. This study was initiated to determine if differences in polyamine (PA) metabolism are associated with aging and age-reduced vigor of potato seed-tubers. As tubers aged in storage, putrescine (Put) increased 2.2-fold, while spermidine (Spd) and spermine (Spm) decreased 33% and 38%, respectively. Ethylene content of the tuber tissue also increased with advancing age, suggesting that during the aging process S-adenosylmethionine was directed toward ethylene biosynthesis at the expense of the PAs. Single-eye cores from 7- and 19-month-old tubers were sown and PA levels in core and shoot tissues were monitored during plant development. Put titer of younger cores increased 8.8-fold by 12 days. In contrast, the increase in Put over the initial titer in older cores was 2.9-fold. The reduced ability of older cores to synthesize Put during plant establishment is probably due to a 45% decline in ornithine decarboxylase activity between 12 and 16 days after planting. Lack of available Put substrate limited the biosynthesis of Spd and Spm, and thus their concentrations remained lower in older cores than in younger cores. Lower PA titer in older cores during plant establishment is thus coincident with reduced growth potential. Concentrations of Put and Spd were higher in shoots developing from older cores throughout the study, but there was no age-related difference in Spm content. In contrast, activities of arginine and S-adenosylmethionine decarboxylases were higher in shoots from younger cores during establishment. The results indicate that aging affects PA metabolism in both tuber and developing plant tissues, and this may relate to loss of growth potential with advancing seed-tuber age.
Full text
PDF

Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Baxter C., Coscia C. J. In vitro synthesis of spermidine in the higher plant, Vinca rosea. Biochem Biophys Res Commun. 1973 Sep 5;54(1):147–154. doi: 10.1016/0006-291x(73)90901-7. [DOI] [PubMed] [Google Scholar]
- Beyer E. M., Morgan P. W. A method for determining the concentration of ethylene in the gas phase of vegetative plant tissues. Plant Physiol. 1970 Aug;46(2):352–354. doi: 10.1104/pp.46.2.352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cohen E., Arad S. M., Heimer Y. M., Mizrahi Y. Participation of ornithine decarboxylase in early stages of tomato fruit development. Plant Physiol. 1982 Aug;70(2):540–543. doi: 10.1104/pp.70.2.540. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cohen E., Heimer Y. M., Mizrahi Y. Ornithine decarboxylase and arginine decarboxylase activities in meristematic tissues of tomato and potato plants. Plant Physiol. 1982 Aug;70(2):544–546. doi: 10.1104/pp.70.2.544. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dumortier F. M., Flores H. E., Shekhawat N. S., Galston A. W. Gradients of polyamines and their biosynthetic enzymes in coleoptiles and roots of corn. Plant Physiol. 1983 Aug;72(4):915–918. doi: 10.1104/pp.72.4.915. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Even-Chen Z., Mattoo A. K., Goren R. Inhibition of ethylene biosynthesis by aminoethoxyvinylglycine and by polyamines shunts label from 3,4-[C]methionine into spermidine in aged orange peel discs. Plant Physiol. 1982 Feb;69(2):385–388. doi: 10.1104/pp.69.2.385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Flores H. E., Galston A. W. Analysis of polyamines in higher plants by high performance liquid chromatography. Plant Physiol. 1982 Mar;69(3):701–706. doi: 10.1104/pp.69.3.701. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaur Sawhney R., Shekhawat N. S., Galston A. W. Polyamine levels as related to growth, differentiation and senescence in protoplast-derived cultures of Vigna aconitifolia and Avena sativa. Plant Growth Regul. 1985;3:329–337. doi: 10.1007/BF00117590. [DOI] [PubMed] [Google Scholar]
- Kaur-Sawhney R., Shih L. M., Galston A. W. Relation of polyamine biosynthesis to the initiation of sprouting in potato tubers. Plant Physiol. 1982 Feb;69(2):411–415. doi: 10.1104/pp.69.2.411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Slocum R. D., Kaur-Sawhney R., Galston A. W. The physiology and biochemistry of polyamines in plants. Arch Biochem Biophys. 1984 Dec;235(2):283–303. doi: 10.1016/0003-9861(84)90201-7. [DOI] [PubMed] [Google Scholar]
- Suzuki Y., Hirasawa E. S-adenosylmethionine decarboxylase of corn seedlings. Plant Physiol. 1980 Dec;66(6):1091–1094. doi: 10.1104/pp.66.6.1091. [DOI] [PMC free article] [PubMed] [Google Scholar]


