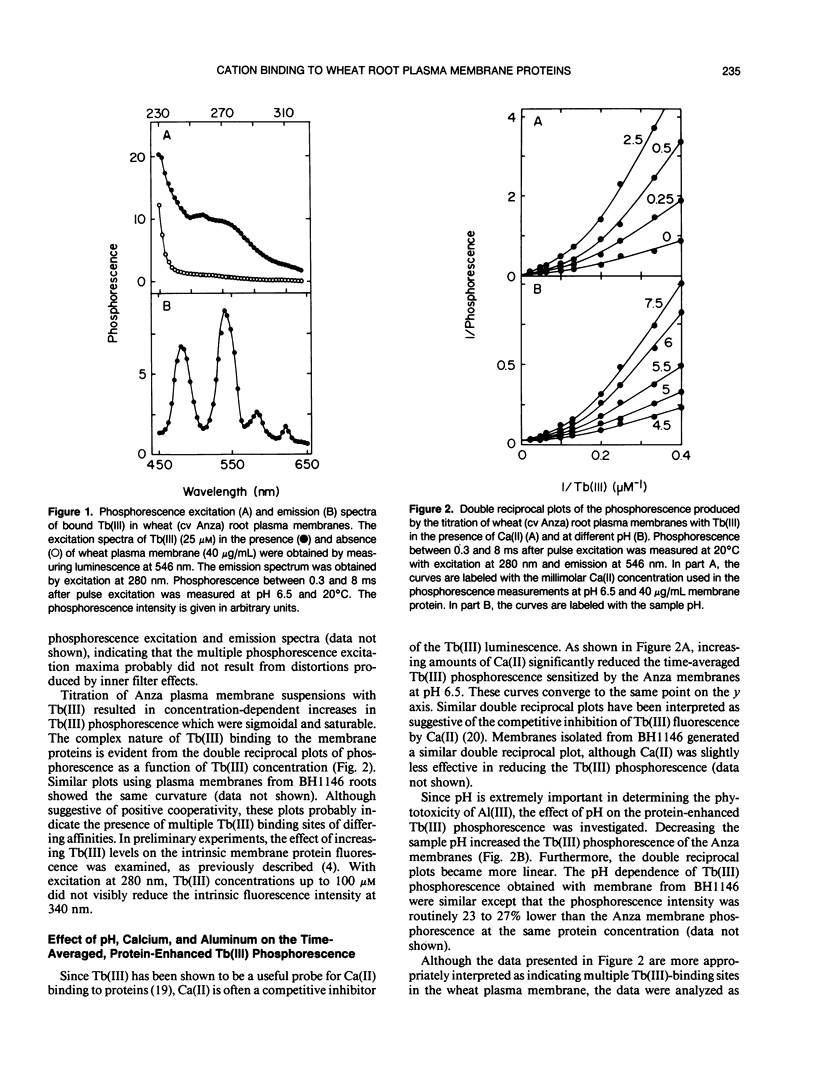
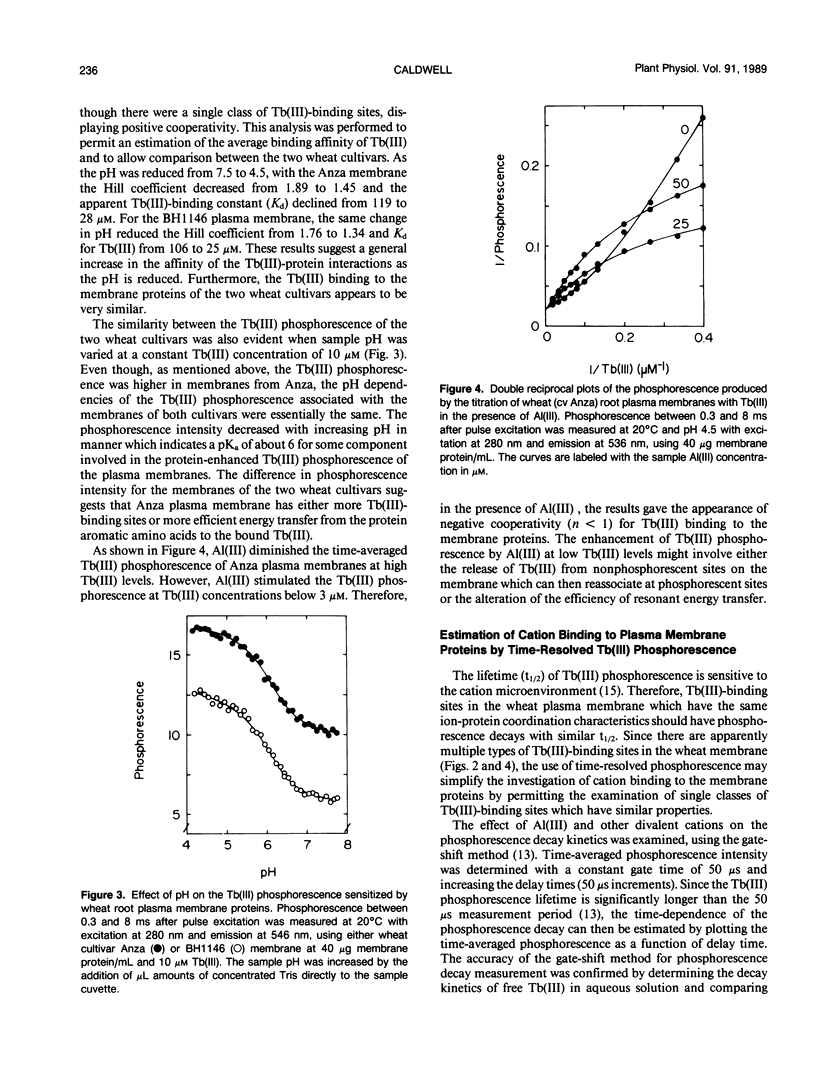
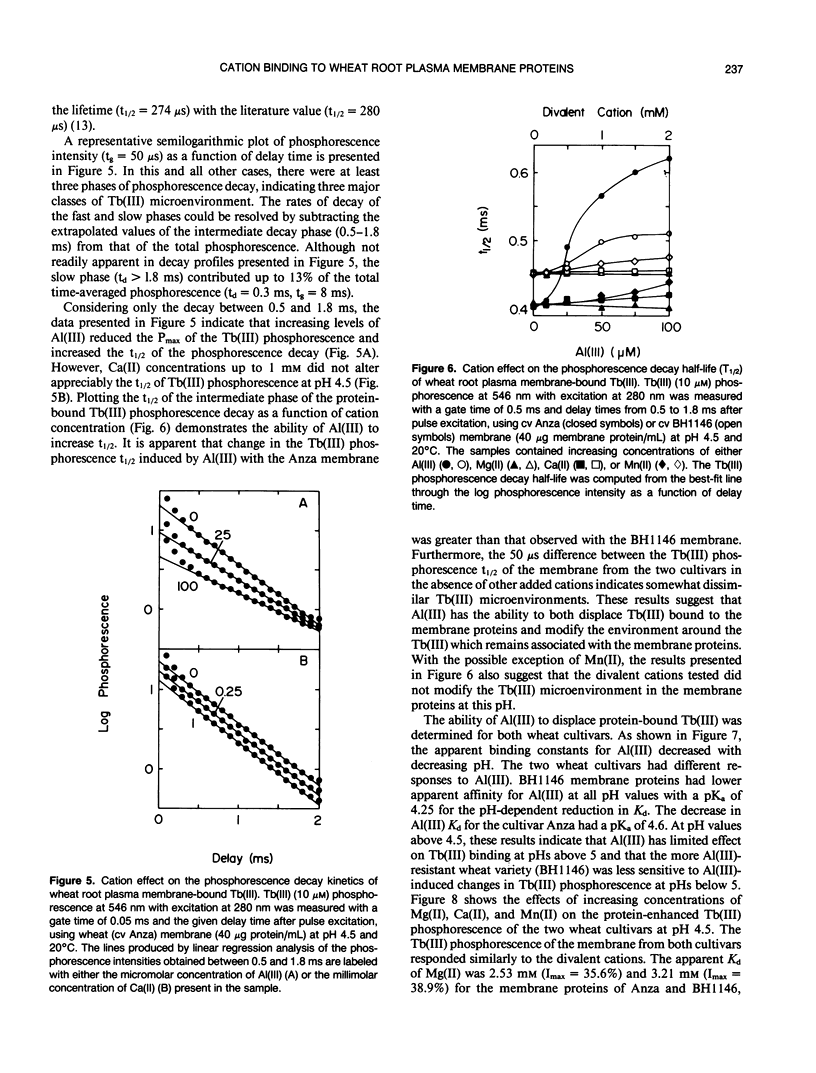
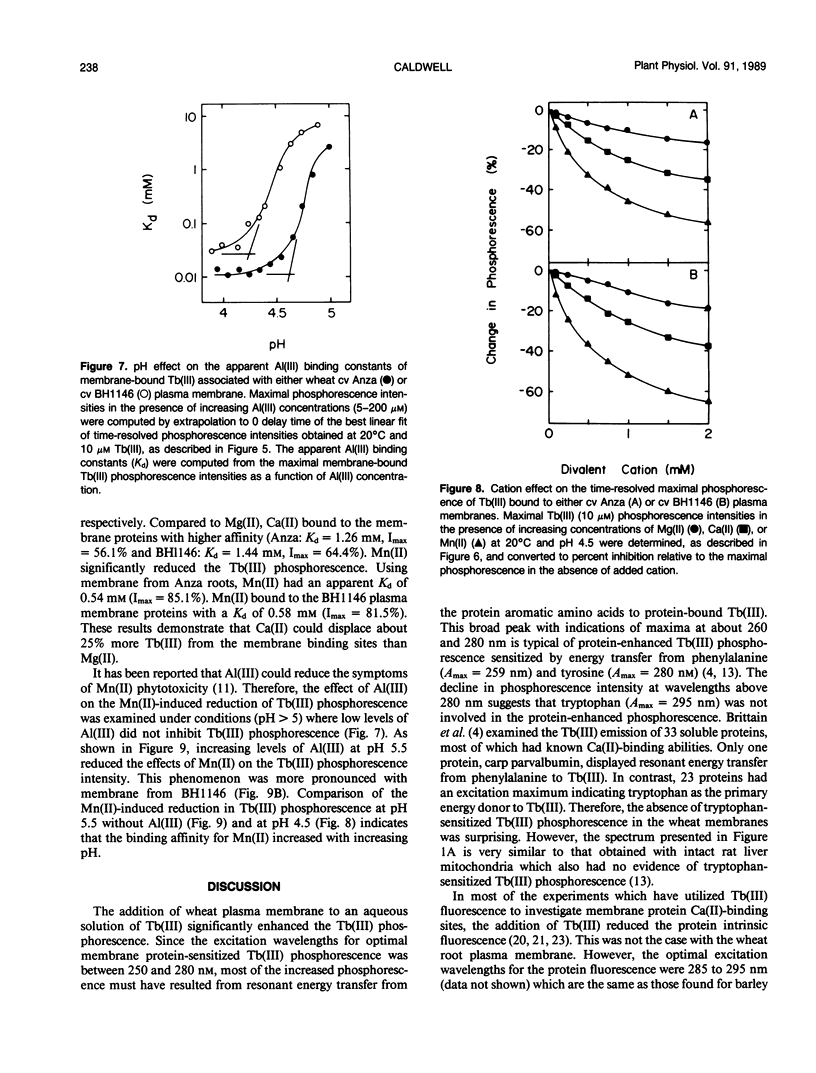
Abstract
A phosphorescent trivalent cation, terbium [Tb(III)], has been used to study the binding of different polyvalent cations to the proteins of wheat (Triticum aestivum L.) root plasma membranes. The phosphorescence emission intensity of Tb(III) was enhanced after Tb(III) binding to wheat root plasma membranes as a result of nonradiative resonance energy transfer from the membrane protein tyrosine and phenylalanine residues. Complex, saturable Tb(III) binding was observed, suggesting multiple binding sites. Bound Tb(III) could be displaced by divalent cations in the general order: Mn(II) > Ca(II) > Mg(II). Al(III) was very effective in reducing the protein-enhanced Tb(III) phosphorescence at pH values below 5. Al(III) also altered the Tb(III) phosphorescence lifetime, suggesting Al(III)-induced changes in membrane protein conformation. The more Al(III)-sensitive wheat cultivar (Anza) bound Al(III) with higher affinity than the more tolerant cultivar (BH 1146). At pH 5.5 where Al(III) did not displace bound Tb(III), low levels of Al(III) reduced the ability of Mn(II) to decrease Tb(III) phosphorescence. The significance of these results is discussed with respect to the mechanisms of Al(III) tolerance in wheat and the potential beneficial effects of Al(III) in reducing Mn(II) phytotoxicity.
Full text
PDF



Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Blanchard J. S. Buffers for enzymes. Methods Enzymol. 1984;104:404–414. doi: 10.1016/s0076-6879(84)04107-0. [DOI] [PubMed] [Google Scholar]
- Brittain H. G., Richardson F. S., Martin R. B. Terbium (III) emission as a probe of calcium(II) binding sites in proteins. J Am Chem Soc. 1976 Dec 8;98(25):8255–8260. doi: 10.1021/ja00441a060. [DOI] [PubMed] [Google Scholar]
- Caldwell C. R. Temperature-Induced Protein Conformational Changes in Barley Root Plasma Membrane-Enriched Microsomes: II. Intrinsic Protein Fluorescence. Plant Physiol. 1987 Jul;84(3):924–929. doi: 10.1104/pp.84.3.924. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Deleers M. Cationic atmosphere and cation competition binding at negatively charged membranes: pathological implications of aluminum. Res Commun Chem Pathol Pharmacol. 1985 Aug;49(2):277–294. [PubMed] [Google Scholar]
- Deschenes R. J., Hilt D. C., Marquis J. K., Mautner H. G. Terbium binding to axonal membrane vesicles from lobster (Homarus americanus) peripheral nerve. A probe of calcium binding sites. Biochim Biophys Acta. 1981 Feb 20;641(1):166–172. doi: 10.1016/0005-2736(81)90580-0. [DOI] [PubMed] [Google Scholar]
- Epstein M., Levitzki A., Reuben J. Binding of lanthanides and of divalent metal ions to porcine trypsin. Biochemistry. 1974 Apr 9;13(8):1777–1782. doi: 10.1021/bi00705a034. [DOI] [PubMed] [Google Scholar]
- Good N. E., Winget G. D., Winter W., Connolly T. N., Izawa S., Singh R. M. Hydrogen ion buffers for biological research. Biochemistry. 1966 Feb;5(2):467–477. doi: 10.1021/bi00866a011. [DOI] [PubMed] [Google Scholar]
- Hashimoto K., Rottenberg H. Surface potential in rat liver mitochondria: terbium ion as a phosphorescent probe for surface potential. Biochemistry. 1983 Dec 6;22(25):5738–5745. doi: 10.1021/bi00294a010. [DOI] [PubMed] [Google Scholar]
- Kinraide T. B., Parker D. R. Cation amelioration of aluminum toxicity in wheat. Plant Physiol. 1987 Mar;83(3):546–551. doi: 10.1104/pp.83.3.546. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lux B., Baudier J., Gerard D. Tyrosyl fluorescence spectra of proteins lacking tryptophan: effects of intramolecular interactions. Photochem Photobiol. 1985 Sep;42(3):245–251. doi: 10.1111/j.1751-1097.1985.tb08938.x. [DOI] [PubMed] [Google Scholar]
- Martin R. B., Richardson F. S. Lanthanides as probes for calcium in biological systems. Q Rev Biophys. 1979 May;12(2):181–209. doi: 10.1017/s0033583500002754. [DOI] [PubMed] [Google Scholar]
- Mikkelsen R. B., Wallach D. F. Binding of fluorescent lanthanides to rat liver mitochondrial membranes and calcium ion-binding proteins. Biochim Biophys Acta. 1976 May 21;433(3):674–683. doi: 10.1016/0005-2736(76)90290-x. [DOI] [PubMed] [Google Scholar]
- Mikkelsen R. B., Wallach D. F. High affinity calcium binding sites on erythrocyte membrane proteins. Use of lanthanides as fluorescent probes. Biochim Biophys Acta. 1974 Sep 6;363(2):211–218. doi: 10.1016/0005-2736(74)90060-1. [DOI] [PubMed] [Google Scholar]
- O'Sullivan W. J., Smithers G. W. Stability constants for biologically important metal-ligand complexes. Methods Enzymol. 1979;63:294–336. doi: 10.1016/0076-6879(79)63014-8. [DOI] [PubMed] [Google Scholar]
- Ohyashiki T., Chiba K., Mohri T. Terbium as a fluorescent probe for analysis of the nature of Ca2+-binding sites of rat intestinal mucosal membranes. J Biochem. 1979 Nov;86(5):1479–1485. doi: 10.1093/oxfordjournals.jbchem.a132666. [DOI] [PubMed] [Google Scholar]
- Sommerville L. E., Thomas D. D., Nelsestuen G. L. Tb3+ binding to bovine prothrombin and bovine prothrombin fragment 1. J Biol Chem. 1985 Sep 5;260(19):10444–10452. [PubMed] [Google Scholar]
- Wang C., Smith R. L. Lowry determination of protein in the presence of Triton X-100. Anal Biochem. 1975 Feb;63(2):414–417. doi: 10.1016/0003-2697(75)90363-2. [DOI] [PubMed] [Google Scholar]


