Abstract
In addition to direct neutralization and other classical effector functions, IgG possesses a little recognized and thus under-utilized effector function at mucosal surfaces: Fc-mucin bonds enable IgG to trap viruses in mucus. Due to the paucity of envelope glycoproteins that limits the number of IgG that can bind HIV, it remains poorly understood whether IgG-mucin interactions can effectively immobilize HIV in human cervicovaginal mucus (CVM). Here, we obtained 54 fresh, undiluted CVM specimens from 17 different women, and employed high-resolution multiple particle tracking to quantify the mobility of fluorescent HIV virus-like-particles in CVM treated with various HIV-specific IgG. We observed consistent and effective trapping of HIV by broadly neutralizing antibodies (VRC01, PGT121, and 2F5) in a subset of women. While trapping efficacy was not affected by the menstrual cycle, it was positively correlated with appreciable L. Crispatus populations in the microbiome, and negatively correlated with appreciable L. Iners or G. Vaginalis populations. Our work demonstrates for the first time that IgG-mucin crosslinking is capable of reinforcing the mucosal barrier against HIV, and motivates further investigation of passive immunization against vaginal transmission of STIs.
Graphical Abstract
Statement of Significance
HIV transmission in women primarily occurs vaginally, yet the 3-way interactions between mucins and HIV virions mediated by HIV-binding antibodies in cervicovaginal mucus (CVM) is not well understood. While IgG-Fc possess weak affinity to mucins that trap virus/IgG complexes in mucus, the effectiveness against HIV remains unclear, due to the low number of virion-bound IgG. Here, we discovered that IgG can trap HIV consistently in CVM from select individuals regardless of their birth control status or menstrual cycle phase. IgG-mediated trapping of HIV was moderately associated with microbiome composition. These results suggest that IgG-mucin interactions could potentially reduce HIV transmission and highlight the importance of mucosal secretions in antibody-mediated prevention of HIV and other sexually transmitted infections.
1. Introduction
HIV-1 remains a significant global health burden. Nearly 2 million new infections occur each year, and AIDs-related illnesses continue to claim ~ 1 million lives annually [1]. As a cure remains elusive, there continues to be considerable interest in developing effective methods to prevent HIV transmission. A large proportion of new HIV infections occur in women via male-to-female vaginal transmission [2–4], which has led to a concerted effort to reduce vaginal transmission using microbicides delivered in a variety of formats [5]. Unfortunately, the vast majority of these methods have not been effective in clinical trials [6–9] with the most effective interventions conferring only very modest protection [10]. These realities underscore the need to continue to explore novel approaches to prevent vaginal HIV transmission.
The vaginal epithelium is coated with a layer of mucus gel; this is frequently referred to as cervicovaginal mucus (CVM) to reflect its origin [11,12]. In addition to minimizing physical trauma to the vaginal epithelium during coitus, CVM also serves as a diffusional barrier that limits pathogens from contacting the underlying epithelium following ejaculation [13,14]. Viruses in semen must traverse CVM to reach and infect target cells. Despite CVM’s natural role as the first line of defense against vaginal STI transmission, mucus is generally not considered a target for enhancing STI prevention. CVM contains an array of host defense proteins such as antimicrobial peptides and antibodies which contribute to the innate and adaptive immune response to sexually transmitted pathogens at the mucosal surface [13–15]. In theory, effective reinforcement of the CVM barrier should directly reduce the viral load that contacts target cells in the vaginal epithelium, lead to more complete inactivation before viruses reach target cells, and/or facilitate quicker and more complete elimination of viruses from the vagina via natural clearance mechanisms. Trapping viruses in mucus and blocking their access to target cells offers the potential for sterilizing immunity, as previously shown for vaginal Herpes [16].
Although there is far more IgG than secretory IgA in the vagina [17], IgG was long thought incapable of crosslinking pathogens to mucins, due to the seemingly negligible affinity between individual IgG and mucins. The diffusion of IgG is slowed only ~10% in cervical mucus compared to in buffer [18,19], indicating that most IgG remains unbound to mucins at any given moment in time. It was not until recently that we showed multiple IgGs accumulated on a single virion can generate sufficient avidity to crosslink the virus-IgG complexes to mucins and immobilize the virions in mucus [20,21]. Indeed, anti-Herpes IgG traps Herpes Simplex Virus (HSV-1) in CVM even at sub-neutralizing concentrations [16,18,19], and consistently does so regardless of the composition of vaginal microbiota or changes to CVM across the menstrual cycle [22]. Additionally, trapping HSV-1 in mucus even with a non-neutralizing IgG offers protection against vaginal HSV transmission in vivo [16]. Due to the limited number of envelope glycoproteins (Env) [23] that severely restrict the number of potential IgG-mucin crosslinks on HIV, it is unclear if monoclonal IgG antibodies can effectively immobilize HIV in CVM. To answer this question, we used high-resolution multiple-particle tracking to quantify the effect of exogenously administered broadly neutralizing antibodies (bnAb) against HIV-1 on the diffusion of HIV-1 virus-like particles (VLPs) in fresh, undiluted CVM specimens spanning the menstrual cycle and microbiota types.
2. Materials and Methods
2.1. Ethics Statement
Written informed consent was obtained from all participants after the nature and potential consequences of the study were explained. All studies were performed in accordance with protocol as approved by the Institutional Review Board of the University of North Carolina at Chapel Hill (IRB 10–1817).
2.2. CVM Collection and Characterization
CVM was collected and characterized as previously described [24]. Briefly, undiluted CVM secretions, averaging 0.3 g per sample, were obtained from 17 reproductive age women ranging in age from 18 to 36 using a self-sampling menstrual collection cup (Instead Softcup). Donors inserted the device into the vagina for 30 sec, were instructed to twist to collect mucus from the vaginal wall as it was removed, then placed it into a 50-mL centrifuge tube. Samples were centrifuged at 200 × g for 5 min to collect secretions. Donors stated that they had not used vaginal products or had unprotected intercourse within 3 days prior to donation, and also reported whether they had used a hormonal contraceptive within the 3 weeks prior to donation.
2.3. VLP Production and Purification
HIV pseudoviruses were produced by transfecting HEK293T cells with plasmids encoding Gag-mCherry and HIV glycoproteins (GP), generously provided by Dr. Suryaram Gummuluru (Department of Microbiology, Boston University School of Medicine). The cells were maintained in Dulbecco’s Modified Eagle’s Medium (DMEM; Sigma-Aldrich, St. Louis, MO) supplemented by 10% fetal bovine serum (FBS) and 2 mM L-glutamine (DMEM-10). Cell cultures were kept at 37°C in a humidified 5% CO2 atmosphere. 293T cells (2.0 × 106) were seeded in a 25 cm2 flask (Thermo Scientific, Rochester, NY) and transfected with the expression plasmids using XtremeGENE HP DNA Transfection Reagent (Roche Diagnostics, Indianapolis, IN). Gag-mCherry and HIV GP plasmids were mixed in a 1:1 ratio (1.5 μg of each), and added to 500 μL of DMEM with 9 μL of X-tremeGENE HP DNA Transfection Reagent. The mixture was incubated at room temperature for 30 min before being added to the culture of 293T cells. After 3 to 5 hr incubation at 37°C in 5% CO2, transfected cells were washed with DMEM and incubated for additional 24–48 hr with 2 mL of DMEM-10 at 37°C in 5% CO2. Supernatants from virus particle-producing cultures were then collected and clarified by centrifugation for 10 min at 300 × g, filtered through a low protein binding 0.45 μm syringe filter (Millipore, Bedford, MA) and partially purified through 25% w/v sucrose in Hepes-NaCl buffer by centrifugation at 221,630 × g at 4°C for 2.5 hr. The pellet was resuspended overnight at 4°C in 10% sucrose in Hepes-NaCl buffer, aliquoted, and stored at ‒ 80°C.
2.4. Multiple-particle tracking of HIV-1 in CVM
The neutralization of CVM by alkaline seminal fluid during intercourse was simulated by titrating CVM between pH 6.8‒7.2 by using small volumes of 3N NaOH (3% v/v). The pH was confirmed using a pH microelectrode (Microelectrodes, Bedford, NH) calibrated with pH 4, 7, and 10 buffers. HIV VLPs were mixed with anti-HIV mAbs (VRC01, PGT121, or 2F5) and CVM in a custom-made small-volume glass chamber, created by layering hole-punched electrical tape over a microscopy slide, so that the punched hole can accommodate 20μl in volume. The resulting final bnAb concentration was 5μg/mL and, and required roughly no more than ~10% total dilution of pH-neutralized CVM. Once mixed, samples were incubated for ~15 minutes at 37°C prior to microscopy. Particle motion was recorded using an EMCCD camera (Evolve 512; Photometrics, Tuscon, AZ) mounted on an inverted microscope (AxioObeserver D1; Zeiss, Thornwood, NY) equipped with an Alpha Plan-Apo 100x/1.46 NA objective, environmental (temperature and CO2) control chamber and an LED light source (Lumencor Light Engine DAPI/GFP/543/623/690). Videos (512 × 512, 16-bit image depth) were captured with MetaMorph imaging software (Molecular Devices, Sunnyvale, CA) at a temporal resolution of 66.7 ms and spatial resolution of 10 nm (nominal pixel resolution 0.156 μm/pixel). A minimum of 3 videos capturing a combined minimum of 100 particles on a frame-by-frame basis [25] were captured for each condition/specimen. Particle trajectories were obtained using a recently developed convolutional neural network [26], available at AI Tracking Solutions (http://www.aitracker.net). The coordinates of particle centroids were used to calculate the average mean-squared displacements (MSDs), calculated as <Δr2(τ)> = [x(t + τ) ‒ x(t)]2 + [y(t + τ) ‒ y(t)]2, where τ is the time lag [25]. Fast-moving particles were defined as those with effective diffusivity (Deff) ≥ 0.347 μm2/s, capable of crossing a 50μm mucus barrier within one hour.
2.5. First Passage Time Analysis
We performed a first passage time analysis as previously described [25], calculating the expected time for 50% of a particle population to pass through a 50 μm thick layer of mucus. Briefly, Given the diffusivity D of a particle, the probability that the particle has not passed through a layer of thickness L as of a given time t may be described by an explicit survival function. The fraction of remaining particles is treated as an average of particle survival functions weighted by the number of frames in which a given particle is observed.
2.6. 16s rRNA sequencing and analysis:
DNA extractions for microbiota analysis were performed as previously described [27,28] on aliquots of the same CVM samples used for microscopy. The method of Fadrosh et al. [29] was used to analyze the vaginal microbiota composition and structure, and relied on amplification and sequencing on an Illumina MiSeq instrument (300-bp paired-end reads) of the V3-to-V4 regions of the 16 S rRNA gene. Sequence analyses and taxonomic assignments were performed using a custom pipeline freely available on GitHub (https://github.com/cwzkevin/MiSeq16S). The resulting taxonomic assignments are shown in Table S1. To assess whether the vaginal microbiota affected the barrier properties and Ab-mediated trapping in CVM, we grouped the samples into Community State Types (CSTs) I–V according to the most dominant bacterial species within a sample. Samples were assigned to CSTs according to those identified by Ravel et.al. [28] L. Crispatus-dominated (CST I), L. Gasseri dominated (CST II), L. Iners-dominated (CST III), a diverse set of strict or facultative anaerobic bacteria such as G. Vaginalis (CST IV) and L. Jensenii-dominated (CST V). A sample grouped into a particular CST contained an average of ~80% of the class-defining species (Figure S2). However, microbial populations between the samples can be extremely diverse. In this sample set, the percentage of dominant bacterial species used to classify CST ranged from 34.26 to 99.9% of the total bacteria. Calculation of the Shannon diversity index on rarefied reads was done using the Qiime2 package [30].
2.7. Statistical Analysis
Statistical comparisons were performed using ANOVA with multiple comparisons between matched samples (Tukey’s multiple comparison’s test or Holm-Sidak corrections) or mixed effects analysis with multiple comparison between matched samples using GraphPad Prism version 8.0 (GraphPad Software, La Jolla, CA). Differences were deemed significant at an alpha of 0.05.
3. Results
3.1. Broadly neutralizing Ab (bnAb) trap HIV-1 in CVM
We first evaluated whether three different bnAb, can reduce the mobility of fluorescent HIV-1 VLPs in CVM pH-neutralized to mimic the buffering effects of alkaline semen. Each bnAb targets different parts of Env on HIV; VRC01 binds the CD4 binding portion of gp120 [31], PGT121 binds the V3 base of gp120 [32], and 2F5 binds gp41 which forms the transmembrane portion of the spike [33]. We observed heterogeneous traces reflecting a range of mobility for individual virions, not only between different CVM specimens but also within a given specimen and condition: both freely diffusive and immobilized virus can be readily found in the same videos. Despite this heterogeneity, the impact of bnAb on the diffusivity of HIV can be rigorously assessed when quantified over multiple videos and multiple unique specimens, and each of the bnAb appears to substantially restrict the free diffusion of HIV VLPs compared to HIV in untreated native CVM (Figure 1A; Supplemental Videos S1 and S2). Across the 54 individual CVM specimens collected from 17 unique donors, each of the bnAb (VRC01, PGT121, 2F5) reduced the ensemble-averaged effective diffusivity of HIV by an average of ~4-fold relative to native untreated CVM (Figure 1).
Figure 1.
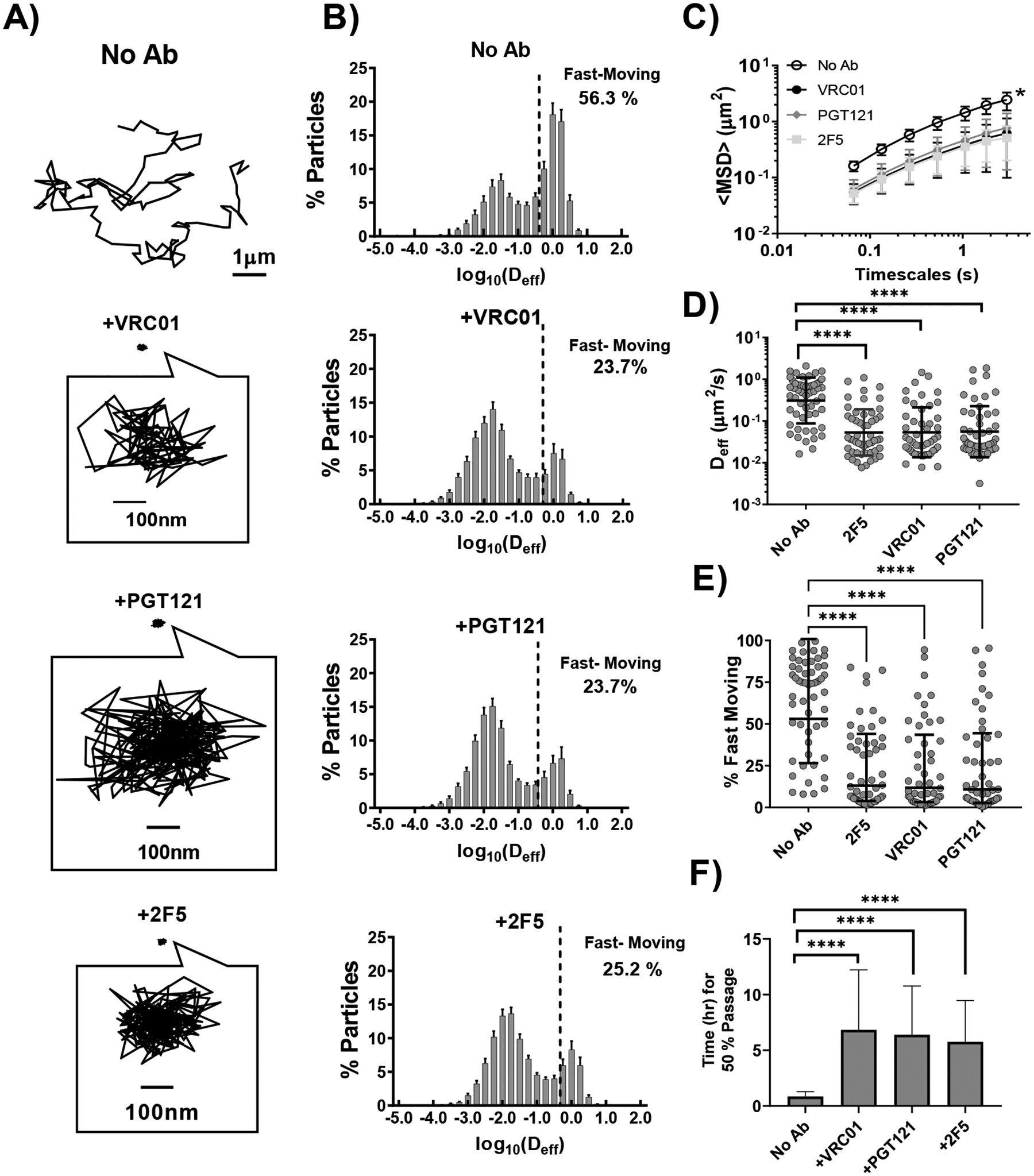
Diffusion of HIV VLPs in pH-neutralized human CVM that is untreated or treated with different HIV-binding bnAb (VRC01, PGT121,2F5). A) Representative traces with effective diffusivities within 1 standard error of the mean of the ensemble average at a time scale of 0.2667 seconds B) Distributions of logarithms of effective diffusivities <Deff> for HIV VLPs. Values right of the dashed line represent viruses that possess sufficient diffusivity to diffuse across a 50μm CVM layer within 1 hr. C) Ensemble-averaged geometric mean square displacements (<MSD>) as a function of timescale. * indicates statistically significant difference (p < 0.01) compared to HIV in untreated CVM by two-way repeated measures ANOVA with correction by Holm-Šidák test. D) Ensemble-averaged geometric Deff at a timescale of 0.2667 seconds for individual CVM specimens, with averages indicated by solid lines. Statistical significance is evaluated by repeated measures mixed effects analysis with correction by Holm-Šidák test on log-transformed data. E) Fraction of fast-moving particles (average <Deff> ≥ 0.347 μm2/s, capable of crossing a 50μm mucus barrier within one hour) with geometric mean and standard deviation indicated by solid lines. Evaluated by repeated measures mixed-effects analysis followed by with correction by Holm-Šidák test. F) Estimated time for 50% of pseudovirus particles to diffuse through a 50μm layer. Data represent the ensemble-averaged geometric mean of samples in each treatment. Error bars represent the 95% confidence interval. Evaluated by repeated measures mixed effect analysis on log-transformed data with correction by Holm-Šidák test. (* p<0.05, ** p<0.01, *** p <0.001, **** p <0.0001)
Logically, virions with the greatest mobility in CVM should pose the greatest risk of HIV transmission by more readily reaching and infecting target cells before they can be removed by natural mucus clearance. Additionally, commensal Lactobacilli rapidly re-acidfies the vaginal lumen after intercourse, at an estimated rate of 0.56–0.75 pH/hour [34]. As HIV begins to gradually lose infectivity below pH 7.4, and is nearly completely ablated by pH 6 [35], virions will quickly become inactivated by acidity as the native pH of the CVM layer is restored, with most viruses likely inactivated an hour after intercourse. We thus quantitatively defined a ‘fast-moving’ population, classified as those possessing sufficient mobility to penetrate a physiological thick CVM layer of ~50μm within an hour, i.e. a minimum effective diffusivity of ≥0.347μm2/s. All 3 bnAb substantially reduced this fraction of fast-moving HIV relative to no Ab control: whereas ~ 63% ± 28% (mean ± SD) of HIV in native, pH-neutralized CVM was classified as fast-moving, VRC01, PGT121 and 2F5 reduced this fraction by ~2.5-fold to ~24% ± 26%, ~24% ± 28% and ~24% ± 24%, respectively. Noted that only 45 of the 54 individual CVM samples yielded enough CVM to directly compare all three bnAb within the same sample; however, the averages for each bnAb condition are similar. To further understand how changes in mobility alter the flux of virions arriving at underlying epithelial cells, we performed a first passage time analysis that quantifies the fraction of HIV predicted to traverse a CVM layer of defined thickness over time. The predicted time for 50% of untreated HIV pseudoviruses to cross a 50μm thick mucus layer was ~0.8 [0.6, 1.3] hours (geometric mean [Lower CI, Upper CI]), compared to ~6.8 [3.8, 12.2], ~6.4 [3.8, 10.8], and ~5.8 [3.5, 9.5] hours for VRC01, PGT121, and 2F5 treatment respectively.
3.2. bnAb-mediated trapping of HIV is consistent within select individuals
While bnAb induced a definite reduction in mobility of HIV virions in CVM, there were substantial variations in the effectiveness of bnAb -mediated trapping, as reflected by the overall high (~30%) intra-sample standard deviation. Indeed, in select donors, none of the 3 bnAbs appreciably reduced either the ensemble-averaged effective diffusivities or the fraction of the fast-moving population. To begin to identify the factors that may contribute to such variations, we classified CVM specimens from the same donors into 2 categories: specimens from donors where bnAb failed to induce statistically significant reduction in mobility of HIV vs control, and those where bnAb induced a statistically significant effect. While there were minor variations depending on the bnAb, the groupings were overall generally consistent across all 3 bnAb studied. Overall, bnAbs were able to induce effective trapping of HIV in CVM from 10 of 17 donors enrolled in our study. Specifically, donors F13, F65, F66, F69, and F70 each donated 3–6 CVM specimens spanning 1–3 menstrual cycles, and the fast-moving viral population is markedly reduced by exogenously added bnAb in nearly all CVM samples donated by these donors, regardless of the specific bnAb added (Figure 2, Supplementary Figure S1). Altogether, HIV in these CVM specimens that afforded consistent trapping of HIV statistically (n=32 specimens) had an average effective diffusivity of 0.3 ± 0.4 μm/s with no bnAb compared to 0.05 ± 0.07 μm/s, 0.05 ± 0.08 μm/s, 0.04 ± 0.04 μm/s in the presence of VRC01, PGT121 and 2F5 respectively. The fast-moving HIV fraction was reduced from ~59 ± 31% with no bnAb to 13 ± 16%, 12 ± 16%, 12 ± 13% in CVM treated with VRC01, PGT121 and 2F5, respectively. In contrast, bnAb failed to provide consistent trapping of HIV in CVM from a subset of donors: F36, F54, and F64 each donated 2–3 samples, and most specimens from these donors show either limited or no appreciable reduction in HIV mobility in the presence of bnAb. These samples (n=14) had an average effective diffusivity of 0.5 ± 0.5 μm/s with no bnAb, vs. 0.3 ± 0.4 μm/s, 0.3 ± 0.5 μm/s, 0.2 ± 0.2 μm/s in the presence of VRC01, PGT121 and 2F5, respectively. Similarly, the fast-moving percentage was only modestly reduced from 67 ± 26% with no Ab to 46 ± 26%. 38 ± 28%, 43 ± 25% in these CVM treated with VRC01, PGT121 and 2F5, respectively.
Figure 2.
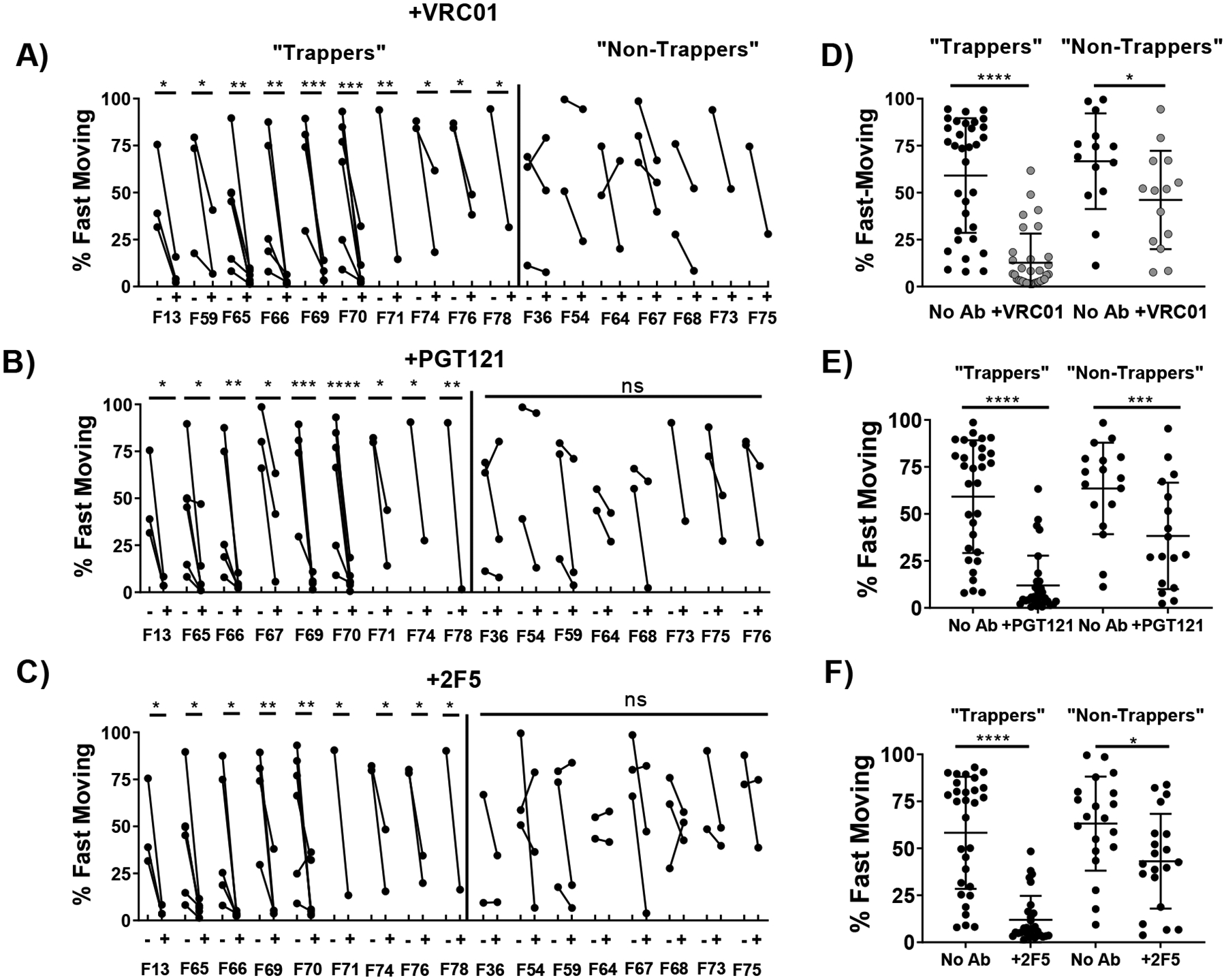
Diffusion of HIV VLPs in pH-neutralized CVM that is untreated or treated with VRC01, segregated by whether VRC01 induced a statistically significant reduction in the fast-moving populations of HIV VLPs in CVM from the same donor. (A/B/C) Fraction of fast-moving virus (average <Deff> ≥ 0.347 μm2/s, capable of crossing a 50μm mucus barrier within one hour in each donor with and without VRC01, PGT121, or 2F5 treatment respectively. Statistical difference is determined by two-way ANOVA followed by post-hoc uncorrected Fisher’s LSD test. 4. (D/E/F) Fraction of fast-moving virus in untreated or VRC01, PGT121, or 2F5-treated CVM samples respectively separated by trapping status. Statistical difference is determined by one-way repeated measures ANOVA corrected by post-hoc Sidak test. Trapping samples are those from donors with a significant decrease in fast-moving particles before and after treatment within each bnAb treatment condition. Statistical difference is determined by one-way repeated measures ANOVA corrected by post-hoc Sidak test. (* p<0.05, ** p<0.01, *** p <0.001, **** p <0.0001)
3.3. Effectiveness of bnAb-mediated trapping of HIV in CVM may be influenced by the vaginal microbiota
The vaginal microbiota is associated with susceptibility to various sexually transmitted infections [36–38], including HIV [39]. The vaginal microbiota can alter the rheological properties of vaginal secretions, and it has previously been shown that the native barrier properties of CVM against HIV is influenced by the dominant microbial species present [40,41]. Thus, we next investigated whether the vaginal microbiome may correlate with the observed variations in trapping potency of different bnAb-treated CVM specimens. We characterized the microbial communities in 50 of 54 CVM specimens using 16 s rRNA gene sequencing, and first classified the specimens into the five community state types (CSTs) based on the dominant bacterial species, following the standards established by Ravel et al [28]. In our cohort here, the vast majority of samples (~70%) were CST I (L. Crispatus dominant, n= 35), with a very limited number in CST II (L. Gasseri dominant, n=3), CST III (L. Iners dominant, n= 3), CST IV (G. Vaginalis and other dominant, n=4), or CST V (L. Gasseri dominant, n=2) bnAb-mediated trapping of HIV was statistically significant only for samples possessing CST I, likely due to limited sample size in the other categories (Table 1, Figure 3A).
Table 1:
Ensemble-averaged effective diffusivities and average percent fast-moving (FM) pseudoviruses in cervicovaginal mucus by community state type
| CST | No Ab | +VRC01 | +PGT121 | +2F5 | ||||
|---|---|---|---|---|---|---|---|---|
| Deff (μm/s) | % FM | Deff (μm/s) | % FM | Deff (μm/s) | % FM | Deff (μm/s) | % FM | |
| I (n= 35) | 0.5 ± 0.5 | 41 ± 29 | 0.1 ± 0.3 | 35 ± 24 | 0.1 ± 0.4 | 28 ± 22 | 0.06 ± 0.07 | 15 ± 16 |
| II (n= 3) | 1.0 ± 0.5 | 85 ± 10 | 0.3 ± 0.2 | 51 ± 15 | 0.3 ± 0.2 | 52 ± 22 | 0.1 ± 0.1 | 34 ± 14 |
| III (n= 3) | 0.8 ± 0.3 | 71 ± 20 | 0.08 ± 0.01 | 23 ± 7 | 0.1 ± 0.1 | 41 ± 12 | 0.3 ± 0.3 | 54 ± 19 |
| IV (n= 4) | 0.8 ± 0.9 | 65 ± 21 | 0.2 ± 0.3 | 35 ± 24 | 0.1 ± 0.04 | 35 ± 9 | 0.4 ± 0.4 | 49 ± 29 |
| V (n= 2) | 0.3 ± 0.3 | 39 ± 40 | 0.03 ± 0.02 | 7 ± 1 | 0.04 ± 0.02 | 7 ± 5 | 0.04 ± 0.004 | 13 ± 9 |
| All (n= 54) | 0.5 ± 0.5 | 63 ± 28 | 0.1 ± 0.2 | 24 ± 24 | 0.2 ± 0.4 | 24 ± 27 | 0.2 ± 0.3 | 24 ± 26 |
Figure 3.
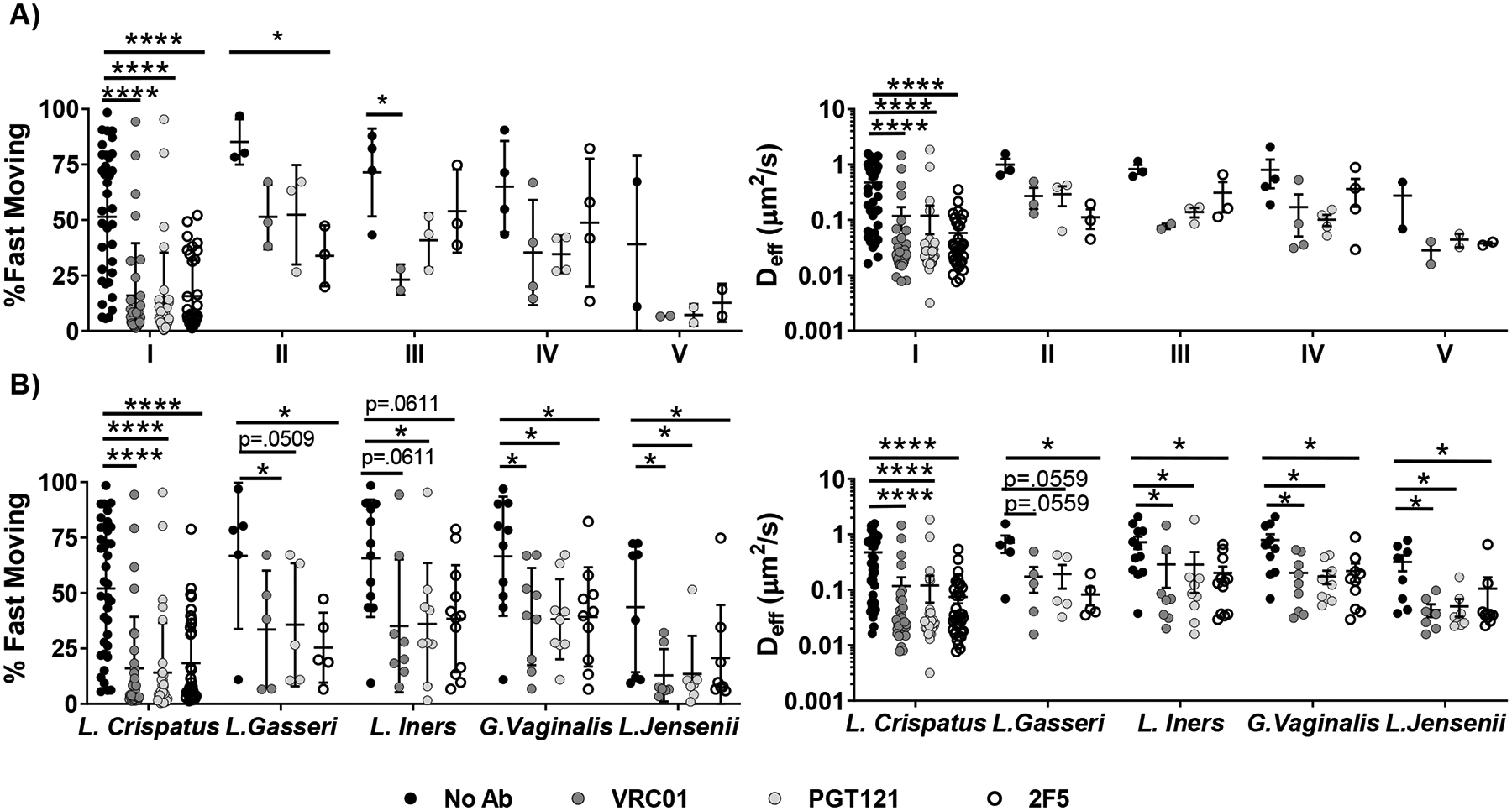
A) bnAb-mediated trapping of HIV VLPs, Ensemble-averaged <Deff> in ph-neutralized CVM treated with different bnAb and percent fast-moving viruses (Deff) ≥ 0.347 μm2/s. Samples are grouped by microbial Community State Type (CST). Samples were sequenced with 16 s rRNA whole - genome sequencing analysis and classified into CSTs depending on the predominant microbial species, according to the following groups: Class I (L.crispatus, n = 35), Class II (L.gasseri, n = 3), Class III (L.iners, n = 3), Class IV (G.vaginalis, n = 4), or Class V (L.Jensenii, n = 2). B) Ensemble-averaged <Deff> and percent fast-moving viruses (<Deff> ≥ 0.347 μm2/s) in CVM samples whose microbiome contained at least 5% of each bacteria, treated with different bnAb. Statistical significance is measured by repeated measures mixed effects analysis with post-hoc Sidak test. (*p < 0.05, **p < 0.01, ***p < 0.001, ****p < 0.0001)
While it is convenient to consider only the dominant bacterial species present, the actual microbial makeup within any given CST category can vary substantially (Supplementary Figure S2). For example, samples classified as CST I had L. Crispatus populations that ranged from 42.6–99.9% of the overall microbes detected in the sample. It is possible that the activity of certain minority species (e.g. G. vaginalis) may influence the observed potencies of bnAb-mediated trapping. Thus, for the five most common bacterial species in the vaginal microbiota, we further classified the specimens according to whether they contained any appreciable population (>5%) of a given bacteria (Figure 3B, Figure 4, Table 2).
Figure 4.
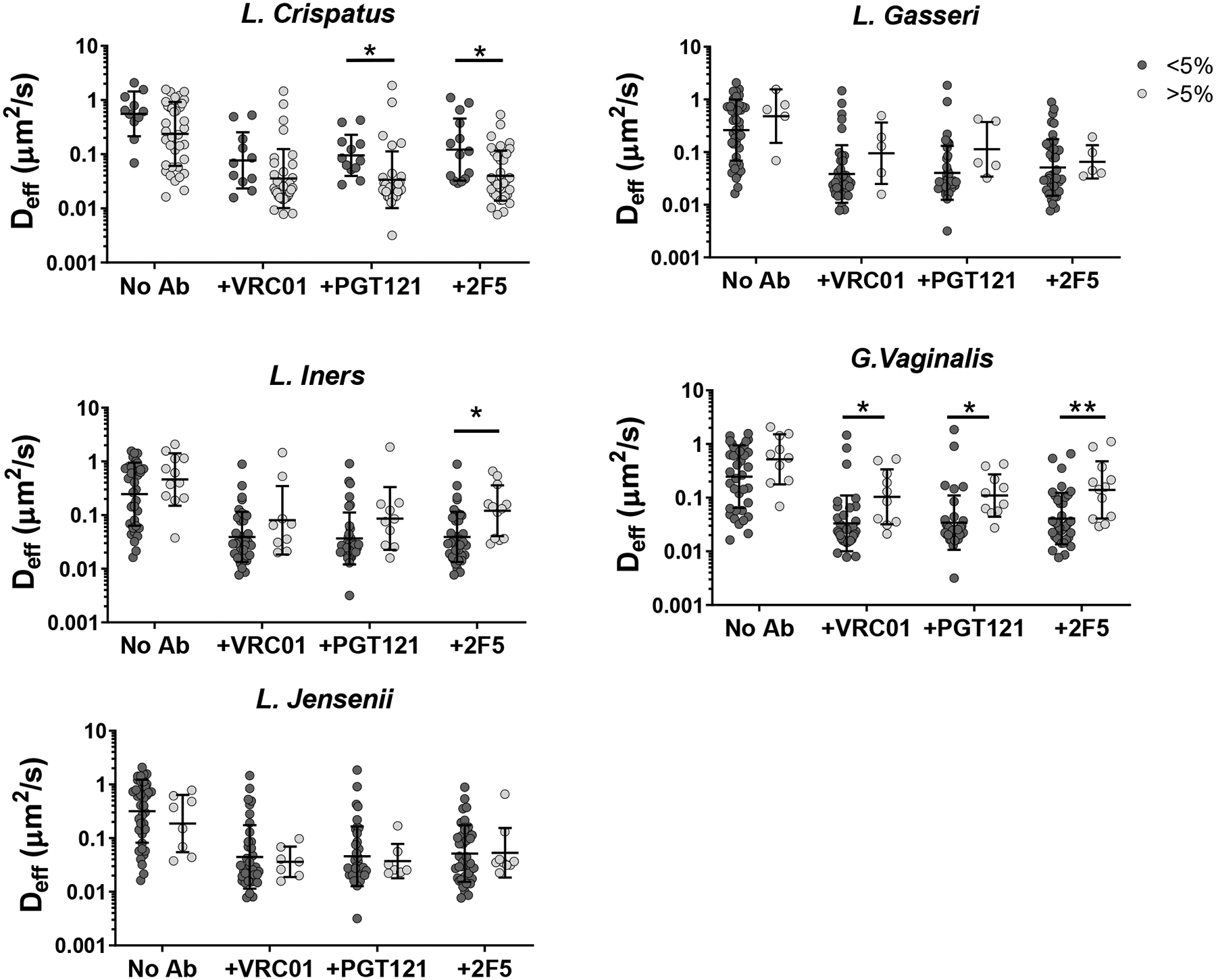
Ab-mediated Trapping of HIV VLPs based on presence of large bacterial populations. Samples were sorted by whether each bacteria composed greater than 5% of the sample’s bacterial population. Analysis was performed by two-way ANOVA on log-transformed data with post-hoc Holm-Sidak’s test. (*p < 0.05, **p < 0.01) Presence of L. Crispatus exhibited a small protective effect, while presence of G.Vaginalis and other non-lactobacillus species exhibited a small negative effect.
Table 2:
Ensemble-averaged effective diffusivities and average percent fast-moving (FM) pseudoviruses in cervicovaginal mucus when divided based on presence of a substantial population (>5%) of each bacteria
| Bacteria | No ab | +VRC01 | +PGT121 | +2F5 | |||||
|---|---|---|---|---|---|---|---|---|---|
| Deff (μm/s) | % FM | Deff (μm/s) | % FM | Deff (μm/s) | % FM | Deff (μm/s) | % FM | ||
| L.Crispatus | > 5% | 0.5 ± 0.5 | 52 ± 29 | 0.1 ± 0.3 | 16 ± 23 | 0.1 ± 0.1 | 15 ± 23 | 0.07 ± 0.08 | 16 ± 17 |
| < 5% | 0.8 ± 0.6 | 69 ± 25 | 0.2 ± 0.2 | 31 ± 23 | 0.1 ± 0.1 | 34 ± 22 | 0.3 ± 0.4 | 40 ± 25 | |
| L.Gasseri | > 5% | 0.7 ± 0.5 | 67 ± 33 | 0.2 ± 0.2 | 34 ± 27 | 0.2 ± 0.2 | 36 ± 28 | 0.08 ± 0.07 | 25 ± 16 |
| < 5% | 0.5 ± 0.5 | 55 ± 28 | 0.1 ± 0.3 | 18 ± 23 | 0.1 ± 0.3 | 17 ± 22 | 0.1 ± 0.2 | 23 ± 23 | |
| L.Iners | > 5% | 0.6 ± 0.5 | 66 ± 26 | 0.3 ± 0.6 | 35 ± 29 | 0.4 ± 0.8 | 36 ± 28 | 0.2 ± 0.2 | 38 ± 24 |
| < 5% | 0.5 ± 0.5 | 56 ± 27 | 0.1 ± 0.2 | 16 ± 21 | 0.1 ± 0.2 | 16 ± 21 | 0.1 ± 0.1 | 18 ± 20 | |
| L.Jensenii | > 5% | 0.3 ± 0.3 | 44 ± 29 | 0.04 ± 0.03 | 13 ± 12 | 0.1 ± 0.1 | 14 ± 17 | 0.1 ± 0.2 | 21 ± 24 |
| < 5% | 0.6 ± 0.5 | 57 ± 28 | 0.1 ± 0.3 | 21 ± 26 | 0.1 ± 0.3 | 21 ± 25 | 0.1 ± 0.2 | 24 ± 23 | |
| G.Vaginalis (and other) | > 5% | 0.8 ± 0.7 | 67 ± 27 | 0.2 ± 0.2 | 39 ± 22 | 0.2 ± 0.1 | 39 ± 22 | 0.2 ± 0.3 | 39 ± 22 |
| < 5% | 0.5 ± 0.4 | 54 ± 29 | 0.1 ± 0.3 | 15 ± 22 | 0.1 ± 0.3 | 15 ± 22 | 0.1 ± 0.1 | 19 ± 21 | |
Based on this analysis, mobility of HIV in CVM treated with bnAb was reduced compared to untreated CVM regardless of what bacteria was considered (Table 2, Figure 3B). However, we found bnAb-mediated trapping of HIV appeared modestly more effective in CVM samples with at least 5% L.Crispatus (n=37) than in CVM samples with less than 5% L.Crispatus (n=12) (Figure 4). In contrast, those with at least 5% non-lactobacillus species (*G.Vaginalis and other; n=10), appeared to be less effective at trapping HIV than those with no substantial non-lactobacilli population (n=38). CVM samples containing at least 5% L.Iners also appeared to have less effective Ab-mediated trapping, although most conditions did not reach statistical significance. It remains uncertain if the microbial influence on the observed bnAb-mediated trapping potency in CVM can be solely attributed to differences in IgG-mucin crosslinking as a result of microbial influence on mucin composition/content, as similar trends in the average effective diffusivity for HIV are also observed in the absence of bnAb. We used Spearman’s correlation to assess the relationship between diffusivity of HIV VLPs in untreated and bnAb-treated CVM from the same sample. There was a moderate correlation between HIV-effective diffusivity with and without bnAb in the case of VRC01 and PGT121 (Supplementary Figure S3). It is possible that these populations can compromise the native barrier properties of CVM, resulting in faster-moving virions that may be more difficult to trap.
Polymicrobial conditions such as bacterial vaginosis (BV) are correlated with increased susceptibility to STIs compared to more homogeneous Lactobacillus-dominated microbiota [42–45]. Thus, we also assessed the impact of overall microbial diversity on trapping potency by correlating the measured HIV mobility to the Shannon diversity index calculated for each CVM specimen from rarefied sequence reads and assessing dependence of effect size on diversity via Spearman’s correlation. We found no correlation between sample diversity and the mobility of untreated HIV VLPs in pH-neutralized CVM, and a weak correlation between the Shannon diversity index and both diffusivity and fast-moving population across all conditions containing antibody (Supplementary Figure S4).
3.4. bnAb-mediated trapping of HIV can be consistent across the menstrual cycle and independent of birth control status
The viscoelastic properties of genital secretions are thought to vary substantially across the menstrual cycle [46–48]. We thus assessed whether bnAb-mediated trapping of HIV may vary depending on the menstrual cycle. We categorized all CVM specimens into either follicular or luteal phase, based on normalization to a 28-day cycle from the end of the last reported menstrual phase. We included women using hormonal birth control in this analysis, as many hormonal contraceptives do not completely eliminate hormonal fluctuations, and many women using hormonal birth control still display degrees of ovarian activity and follicular development [48–50]. Of 17 donors, 8 were using birth control; of these, 7 were using a combination birth control pill and 1 a progestin-only IUD (Supplementary Table 1). In good agreement with our previous studies on IgG-mediated trapping of Herpes, we did not observe an appreciable difference in the trapping potency of bnAb as a function of the menstrual cycle phase or birth control status regardless of whether all samples were considered or if microbial state was limited to those within CSTI (Figure 5, Supplementary Figure S5 and S6). Finally, we investigated whether the muco-trapping potencies were consistent across the 28-day menstrual cycle. We plotted the fast-moving population percentage and effective diffusivity as a function of the cycle day and tested for linearity using Pearson’s correlation. We found no significant trend between virus mobility and cycle day, with or without the addition of bnAb (Supplementary Figure S7). The birth control types represented in this study are primarily combination estrogen-progestin pills. However, prior studies have found an increase in diffusivity of HIV virions in rhesus-macaques administered the high-dose, progestin-only birth control DMPA [51]. Thus, it remains unclear whether high-dose progestin-only birth controls may impact bnAb-mediated trapping.
Figure 5.
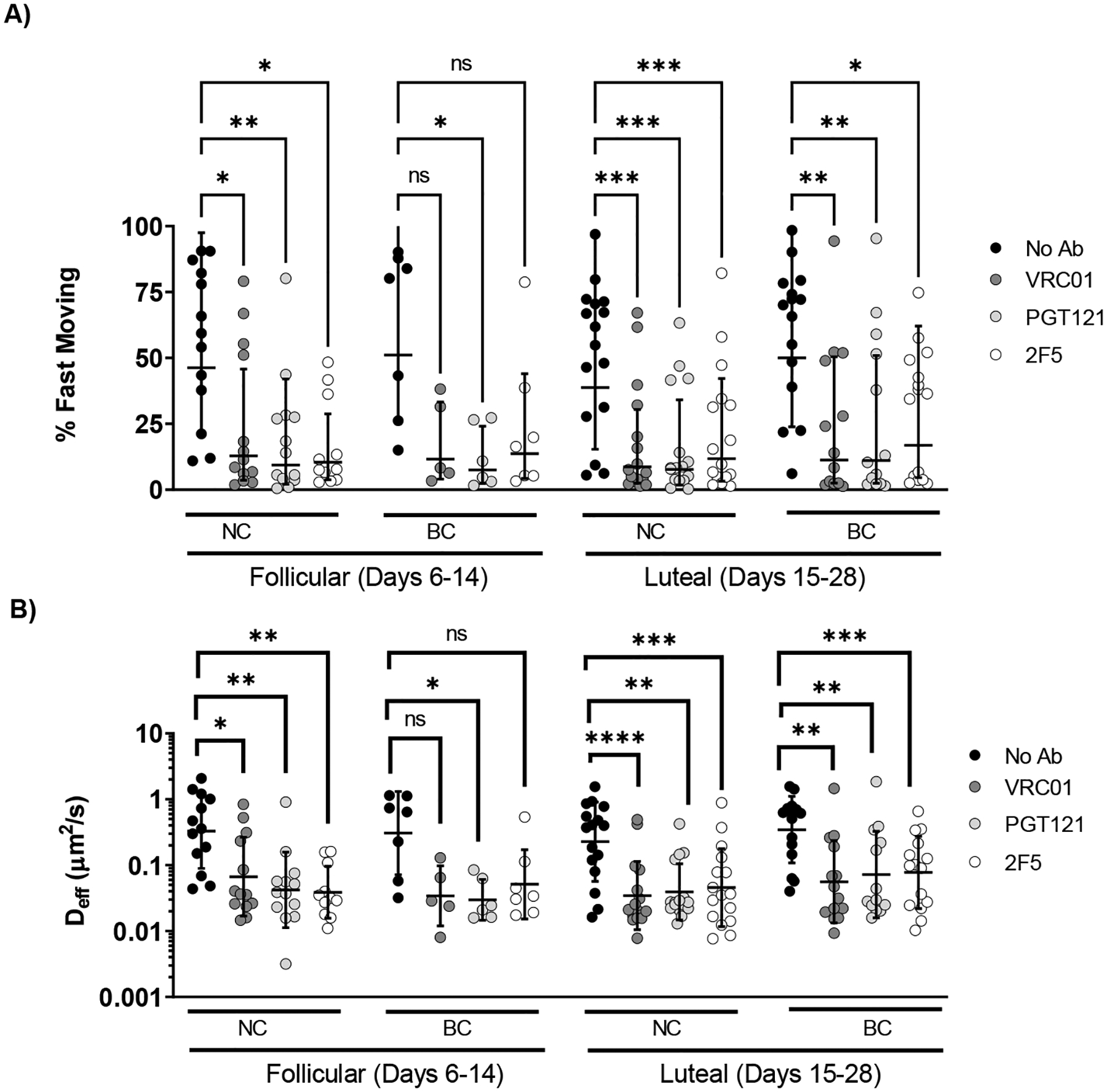
Effect of the menstrual cycle phase and birth control status on bnAb-mediated trapping of HIV VLPs in pH-neutralized CVM. Cycles were normalized to 28 days based on the donor reported number of days post-menses. CVM samples were grouped by the cycle phase and separated by birth control status. Samples classified as follicular phase were those collected 6–14 days post-menses, while those classified as luteal phase were from 15–28 days post-menses. A) Average <Deff> of virions in follicular versus luteal phase in normally cycling (NC) versus versus donors on birth control (BC) B) Percentage of fast-moving virions (average <Deff> ≥ 0.347 μm2/s) by phase in follicular versus luteal phase in normally cycling (NC) versus donors on birth control (BC). Data was analyzed using Two-Way repeated measures ANOVA with Sidak’s multiple comparisons test. A p ≤ 0.05 was considered statistically significant (*p < 0.05, **p < 0.01, ***p < 0.001, ****p < 0.0001).
4. Discussion
STIs continue to be pandemic globally; with the exception of HPV, there remain no effective vaccines to date against essentially all common STIs including HIV, Herpes, chlamydia and gonorrhea. Given the difficulty of eliciting durable vaginal immunity against these infections, and in light of rapidly decreasing costs of Ab manufacturing, passive immunization of the female reproductive tract via locally delivered mAb represents a promising and increasingly investigated means of preventing vaginal STI transmission [52,53]. For vaginal transmission to occur, viruses must penetrate mucus in order to reach target cells; thus, blocking viruses from diffusing through CVM and reducing the flux of infectious virus arriving at target cells in the epithelium should decrease productive vaginal transmission. Although IgG has been shown to effectively trap a variety of viruses in different mucus secretions [16,21,22], and earlier work suggesting that polyclonal IgGs from HIV+ individuals could associate with MUC16 mucins [54], the current study provides the first direct evidence that monoclonal bnAb against HIV could facilitate effective trapping of HIV in fresh, undiluted human CVM. The secretions collected via menstrual cup represent the native mucus secretions present in the vagina, which naturally encompasses shed epithelial cells, vaginal microbiota, and various proteins/lipids. This mucus is identical to those that viruses must diffuse through to reach the epithelium, including fully preserving the rheological properties by avoiding the need to dilute as is commonly encountered with vaginal lavages. In select individuals, we found that bnAb-mediated trapping of HIV can be highly effective and consistent across the menstrual cycle, in good agreement with our prior observations with HSV. Our findings underscore IgG-mediated trapping of HIV in CVM as a potential effector mechanism that may enhance protection against vaginal HIV transmission.
The variability in trapping HIV between different CVM specimens stands in contrast to our previous work on IgG-mediated trapping of HSV, which was highly consistent between individuals regardless of microbiome class or menstrual phase [22]. One possibility is that HIV has a comparatively limited number of epitopes, between 7–14 [23] glycoprotein spikes on the surface compared to the several hundred spikes available on the surface of HSV [55]. This limits the number of bnAb which may coat the surface of each virion, and consequently the number of crosslinks between the IgG-virion complex and the mucin mesh. CVM from different individuals likely differ not just in concentration of mucins (which impacts the frequency or probability for virion-bound IgG forming mucin crosslinks) but also the precise glycans present (which may impact individual IgG-mucin affinity). Viruses undergo Brownian motion in mucus, where momentum transfer from water in the environment to the virus drives their random-walk motion. With fewer Ab bound to each virion, it is conceivable that the overall possible mucin avidity for individual HIV/bnAb complexes in select CVM specimens was less consistently above the threshold of minimum associative interactions needed to overcome the thermal excitation of the HIV/bnAb complexes, and thus fewer viruses become trapped in CVM. In contrast, such variations in IgG-mucin affinity may be masked when there are many Ab bound to each virion (e.g. HSV or influenza), which ensures consistently high overall binding avidity. We found no appreciable difference in trapping potency between different bnAb. This is consistent with the notion that trapping viruses in mucus simply requires Ab to bind to the virus, and is not dependent on the specific epitope on the viral glycoprotein that the Ab targets.
The ability of CVM to function effectively as a barrier against disease transmission is likely shaped in part by the vaginal microbiome. We have previously found that CVM containing significant populations of L. Iners or G. Vaginalis were unable to directly immobilize HIV at native pH, in contrast to samples dominant in L. Crispatus [40]. Similarly, polymicrobial conditions such as bacterial vaginosis disrupt adhesive interactions between HIV virions and the mucus barrier, allowing the particles to diffuse more readily [41]. Additionally, it has long been known that the microbiome can affect risks to the transmission of various STIs. Recently, it has been shown that co-infection with bacterial STIs such as chlamydia, trichomonas, and syphilis create a proinflammatory environment which increases susceptibility to HIV infection, resulting in as much as a one-log increase in required bnAb serum titer to prevent HIV transmission in macaques [56]. The effect of the microbiome on bnAb-mediated trapping shown here represents yet another mechanism by which microbiome can influence STI transmission.
In addition to the pro-inflammatory alterations that various microbiota can induce, our prior work demonstrates that the different bacterial species appear to alter the mucin biochemistry in a manner that affects the mucin-virion interactions at native pH [40]. While the precise mechanisms are complex and not well-understood, different bacteria can consume sugars at different rates and to different extents, as exemplified by high levels of sialydases secreted by G. Vaginalis [57]. As we have previously discovered that Ab-mucin interactions occur through sugar-sugar interactions [16,58], it is possible the microbiome can alter mucins in a way which alters mucin-bnAb interactions, resulting in varying degrees of muco-trapping. Due to the limited number of bound IgGs on the HIV virion, having a microbiota that supports Ab-mediated trapping may be more critical for HIV than in cases where virion-bound IgG is abundant. We found a moderate reduction in muco-trapping potencies in CVM specimens with appreciable populations of G. vaginalis, and a moderate increase in L. crispatus rich specimens. These results suggest that it may be important to consider the influence of the vaginal microbiota when assessing the efficacy of Ab-mediated interventions against HIV transmission, regardless of whether the Ab is induced by HIV vaccines or topically delivered upon local passive immunization. Indeed, if G. vaginalis turns out to reduce the barrier properties of CVM, methods that reduce microbial diversity and ensure greater prevalence of monocultures of non-L. iners Lactobacillus may synergistically enhance the potencies of HIV vaccines and antiviral mAb.
Changes in hormones such as estrogen and progesterone throughout the menstrual cycle influence the viscoelastic properties of cervical mucus [46,59,60]. These changes are known to be important for enabling or preventing sperm ascension into the upper tract, however effects on the barrier properties at the scale of viruses are not fully understood. Here, we did not find correlation between cycle phase and trapping efficacy, consistent which our previous results with HSV. This may imply that variations in mucin biochemistry across the menstrual cycle may be less than those induced by microbial differences between women. In our opinion, this is not surprising: humans are among the very few animal species that copulate outside the fertility window, for purposes beyond reproduction. Thus, the CVM barrier, if indeed optimized evolutionarily, should enable trapping of viruses and protection of STIs across much of the menstrual cycle.
Given its recent discovery, the muco-trapping function of IgG is rarely considered in the development of prophylaxis for STIs. Trapping viruses in mucus via IgG-mucin bonds offer the potential to block infectious agents from initiating infections altogether, as we have previously observed in mouse studies of HSV transmission [16]. In addition, retarding virion mobility in mucus would proportionally enhance the time it takes a virus to reach and infect target cells, and hence should enable more complete inactivation by other innate and adaptive immune mechanisms, as well as supporting more complete elimination by natural mucus clearance mechanisms [13]. Indeed, a large volume fraction of post-coital discharge occurs within minutes of intercourse, removing along with it at least a comparable fraction of virions that had not diffused out of the secretions and into CVM. Consistent with this notion, the rate of heterosexual vaginal HIV transmission is quite low, between 1 per 100–1000 sex acts [61,62], indicating that very few, if any, HIV can actually reach and infect target cells per intercourse. Therefore, reducing the fraction of HIV that can diffuse across the mucus layer is likely to proportionally reduce vaginal HIV transmission.
Supplementary Material
Acknowledgements
This work was supported by National Institutes of Health (http://www.nih.gov/) grants R21AI093242351 (S.K.L.), U19AI096398 (S.K.L.), and U19AI084044 (J.R), The David and Lucile Packard Foundation (https://www.packard.org/) 2013–39274 (S.K.L.), and the Eshelman Institute of Innovation (http://unceii.org/, S.K.L.). The funders had no role in study design, data collection and analysis, decision to publish, or manuscript preparation.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Declaration of Competing Interests
Intellectual property associated with harnessing antibody-mucin interactions described in part in this publication was developed at the University of North Carolina - Chapel Hill (UNC-CH), and has been licensed to Mucommune, LLC. SKL is a founder of Mucommune and currently serves as its interim CEO, board of director, and scientific advisory board. SKL owns company stock; SKL’s relationship with Mucommune is subject to certain restrictions under University policy. The terms of this arrangement are being managed by UNC-CH in accordance with its conflict of interest policies.
Declaration of interests
The findings described in this publication are related to technology being developed by Mucommune. S. K. L. is cofounder and member of the Board of Directors of Mucommune. S. K. L. owns company stock, which is subject to certain restrictions under university policy. S. K. L. is listed as inventor on patents licensed to Mucommune. The terms of this arrangement are being managed by the University of North Carolina in accordance with its conflict of interest policy
References
- [1].Global HIV & AIDS statistics — 2019 fact sheet | UNAIDS, (n.d.). https://www.unaids.org/en/resources/fact-sheet (accessed February 6, 2020).
- [2].Unaids, UNAIDS report on the global AIDS epidemic 2013 GLOBAL REPORT, 2013.
- [3].Beyrer C, Abdool Karim Q, The changing epidemiology of HIV in 2013., Curr Opin HIV AIDS. 8 (2013) 306–10. 10.1097/COH.0b013e328361f53a. [DOI] [PubMed] [Google Scholar]
- [4].Barreto-de-Souza V, Arakelyan A, Margolis L, Vanpouille C, HIV-1 Vaginal Transmission: Cell-Free or Cell-Associated Virus?, American Journal of Reproductive Immunology. 71 (2014) 589–599. 10.1111/aji.12240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [5].Notario-Pérez F, Ruiz-Caro R, Veiga-Ochoa MD, Historical development of vaginal microbicides to prevent sexual transmission of HIV in women: from past failures to future hopes, Drug Des Devel Ther. 11 (2017) 1767–1787. 10.2147/DDDT.S133170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [6].Karim SSA, Richardson BA, Ramjee G, Hoffman IF, Chirenje ZM, Taha T, Kapina M, Maslankowski L, Coletti A, Profy A, Moench TR, Piwowar-Manning E, Mĝsse B, Hillier SL, Soto-Torres L, Safety and effectiveness of BufferGel and 0.5% PRO2000 gel for the prevention of HIV infection in women, AIDS. 25 (2011) 957–966. 10.1097/QAD.0B013E32834541D9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [7].Peterson L, Nanda K, Opoku BK, Ampofo WK, Owusu-Amoaka M, Boakye AY, Rountree W, Troxler A, Dominik R, Roddy R, Dorflinger L, SAVVY (C31G) gel for prevention of HIV infection in women: a Phase 3, double-blind, randomized, placebo-controlled trial in Ghana, PLoS One. 2 (2007). 10.1371/JOURNAL.PONE.0001312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [8].McCormack S, Ramjee G, Kamali A, Rees H, Crook AM, Gafos M, Jentsch U, Pool R, Chisembele M, Kapiga S, Mutemwa R, Vallely A, Palanee T, Sookrajh Y, Lacey CJ, Darbyshire J, Grosskurth H, Profy A, Nunn A, Hayes R, Weber J, PRO2000 vaginal gel for prevention of HIV-1 infection (Microbicides Development Programme 301): a phase 3, randomised, double-blind, parallel-group trial, Lancet. 376 (2010) 1329–1337. 10.1016/S0140-6736(10)61086-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [9].Skoler-Karpoff S, Ramjee G, Ahmed K, Altini L, Plagianos MG, Friedland B, Govender S, De Kock A, Cassim N, Palanee T, Dozier G, Maguire R, Lahteenmaki P, Efficacy of Carraguard for prevention of HIV infection in women in South Africa: a randomised, double-blind, placebo-controlled trial, Lancet. 372 (2008) 1977–1987. 10.1016/S0140-6736(08)61842-5. [DOI] [PubMed] [Google Scholar]
- [10].Nel A, van Niekerk N, Kapiga S, Bekker L-G, Gama C, Gill K, Kamali A, Kotze P, Louw C, Mabude Z, Miti N, Kusemererwa S, Tempelman H, Carstens H, Devlin B, Isaacs M, Malherbe M, Mans W, Nuttall J, Russell M, Ntshele S, Smit M, Solai L, Spence P, Steytler J, Windle K, Borremans M, Resseler S, Van Roey J, Parys W, Vangeneugden T, Van Baelen B, Rosenberg Z, Safety and Efficacy of a Dapivirine Vaginal Ring for HIV Prevention in Women, N Engl J Med. 375 (2016) 2133–2143. 10.1056/NEJMOA1602046. [DOI] [PubMed] [Google Scholar]
- [11].Kieweg SL, Geonnotti AR, Katz DF, Gravity-induced coating flows of vaginal gel formulations: in vitro experimental analysis, J Pharm Sci. 93 (2004) 2941–2952. 10.1002/JPS.20194. [DOI] [PubMed] [Google Scholar]
- [12].Gipson IK, Mucins of the human endocervix, Front Biosci. 6 (2001) d1245–55. 10.2741/GIPSON. [DOI] [PubMed] [Google Scholar]
- [13].Nguyen PV, Kafka JK, Ferreira VH, Roth K, Kaushic C, Innate and adaptive immune responses in male and female reproductive tracts in homeostasis and following HIV infection, Cell Mol Immunol. 11 (2014) 410–427. 10.1038/cmi.2014.41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [14].Cone RA, Barrier properties of mucus, Adv Drug Deliv Rev. 61 (2009) 75–85. 10.1016/J.ADDR.2008.09.008. [DOI] [PubMed] [Google Scholar]
- [15].Cole AM, Innate host defense of human vaginal and cervical mucosae, Curr Top Microbiol Immunol. 306 (2006) 199–230. 10.1007/3-540-29916-5_8. [DOI] [PubMed] [Google Scholar]
- [16].Wang YY, Kannan A, Nunn KL, Murphy MA, Subramani DB, Moench T, Cone R, Lai SK, IgG in cervicovaginal mucus traps HSV and prevents vaginal Herpes infections, Mucosal Immunol. 7 (2014) 1036–44. 10.1038/mi.2013.120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [17].Usala S, Usala F, R H, JA H, GF S, IgG and IgA content of vaginal fluid during the menstrual cycle - PubMed, J Reprod Med. 34 (1989) 292. https://pubmed.ncbi.nlm.nih.gov/2715991/ (accessed November 15, 2020). [PubMed] [Google Scholar]
- [18].Saltzman WM, Radomsky ML, Whaley KJ, Cone RA, Antibody diffusion in human cervical mucus, Biophys J. 66 (1994) 508–515. 10.1016/S0006-3495(94)80802-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [19].Olmsted SS, Padgett JL, Yudin AI, Whaley KJ, Moench TR, Cone RA, Diffusion of macromolecules and virus-like particles in human cervical mucus, Biophys J. 81 (2001) 1930–7. 10.1016/S0006-3495(01)75844-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [20].Newby J, Schiller JL, Wessler T, Edelstein J, Forest MG, Lai SK, A blueprint for robust crosslinking of mobile species in biogels with weakly adhesive molecular anchors., Nat Commun. 8 (2017) 833. 10.1038/s41467-017-00739-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [21].Yang B, Schaefer A, Wang Y-Y, McCallen J, Lee P, Newby JM, Arora H, Kumar PA, Zeitlin L, Whaley KJ, McKinley SA, Fischer WA, Harit D, Lai SK, ZMapp reinforces the airway mucosal barrier against ebola virus, Journal of Infectious Diseases. 218 (2018) 901–910. 10.1093/infdis/jiy230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [22].Schroeder HA, Nunn KL, Schaefer A, Henry CE, Lam F, Pauly MH, Whaley KJ, Zeitlin L, Humphrys MS, Ravel J, Lai SK, Herpes simplex virus-binding IgG traps HSV in human cervicovaginal mucus across the menstrual cycle and diverse vaginal microbial composition, Mucosal Immunol. 11 (2018) 1477–86. 10.1038/s41385-018-0054-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [23].Zhu P, Liu J, Bess J, Chertova E, Lifson JD, Grisé H, Ofek GA, Taylor KA, Roux KH, Distribution and three-dimensional structure of AIDS virus envelope spikes, Nature. 441 (2006) 847–852. 10.1038/NATURE04817. [DOI] [PubMed] [Google Scholar]
- [24].Lai SK, Hida K, Shukair S, Wang Y-Y, Figueiredo A, Cone R, Hope TJ, Hanes J, Human Immunodeficiency Virus Type 1 Is Trapped by Acidic but Not by Neutralized Human Cervicovaginal Mucus, J Virol. 83 (2009) 11196–11200. 10.1128/jvi.01899-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [25].Wang Y-Y, Nunn KL, Harit D, McKinley SA, Lai SK, Minimizing biases associated with tracking analysis of submicron particles in heterogeneous biological fluids., J Control Release. 220 (2015) 37–43. 10.1016/j.jconrel.2015.10.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [26].Newby JM, Schaefer AM, Lee PT, Forest MG, Lai SK, Convolutional neural networks automate detection for tracking of submicron-scale particles in 2D and 3D, Proc Natl Acad Sci U S A. 115 (2018) 9026–9031. 10.1073/pnas.1804420115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [27].Gajer P, Brotman RM, Bai G, Sakamoto J, Schütte UME, Zhong X, Koenig SSK, Fu L, Ma Z, Zhou X, Abdo Z, Forney LJ, Ravel J, Temporal Dynamics of the Human Vaginal Microbiota, Sci Transl Med. 4 (2012) 132ra52. 10.1126/scitranslmed.3003605. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [28].Ravel J, Gajer P, Abdo Z, Schneider GM, Koenig SSK, McCulle SL, Karlebach S, Gorle R, Russell J, Tacket CO, Brotman RM, Davis CC, Ault K, Peralta L, Forney LJ, Vaginal microbiome of reproductive-age women, Proc Natl Acad Sci U S A. 108 (2011) 4680–4687. 10.1073/PNAS.1002611107/-/DCSUPPLEMENTAL. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [29].Fadrosh DW, Ma B, Gajer P, Sengamalay N, Ott S, Brotman RM, Ravel J, An improved dualindexing approach for multiplexed 16S rRNA gene sequencing on the Illumina MiSeq platform, Microbiome. 2 (2014) 6. 10.1186/2049-2618-2-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [30].Bolyen E, Rideout JR, Dillon MR, Bokulich NA, Abnet CC, Al-Ghalith GA, Alexander H, Alm EJ, Arumugam M, Asnicar F, Bai Y, Bisanz JE, Bittinger K, Brejnrod A, Brislawn CJ, Brown CT, Callahan BJ, Caraballo-Rodríguez AM, Chase J, Cope EK, Da Silva R, Diener C, Dorrestein PC, Douglas GM, Durall DM, Duvallet C, Edwardson CF, Ernst M, Estaki M, Fouquier J, Gauglitz JM, Gibbons SM, Gibson DL, Gonzalez A, Gorlick K, Guo J, Hillmann B, Holmes S, Holste H, Huttenhower C, Huttley GA, Janssen S, Jarmusch AK, Jiang L, Kaehler BD, Bin Kang K, Keefe CR, Keim P, Kelley ST, Knights D, Koester I, Kosciolek T, Kreps J, Langille MGI, Lee J, Ley R, Liu YX, Loftfield E, Lozupone C, Maher M, Marotz C, Martin BD, McDonald D, McIver LJ, Melnik AV, Metcalf JL, Morgan SC, Morton JT, Naimey AT, Navas-Molina JA, Nothias LF, Orchanian SB, Pearson T, Peoples SL, Petras D, Preuss ML, Pruesse E, Rasmussen LB, Rivers A, Robeson MS, Rosenthal P, Segata N, Shaffer M, Shiffer A, Sinha R, Song SJ, Spear JR, Swafford AD, Thompson LR, Torres PJ, Trinh P, Tripathi A, Turnbaugh PJ, Ul-Hasan S, van der Hooft JJJ, Vargas F, Vázquez-Baeza Y, Vogtmann E, von Hippel M, Walters W, Wan Y, Wang M, Warren J, Weber KC, Williamson CHD, Willis AD, Xu ZZ, Zaneveld JR, Zhang Y, Zhu Q, Knight R, Caporaso JG, Reproducible, interactive, scalable and extensible microbiome data science using QIIME 2, Nat Biotechnol. 37 (2019) 852–857. 10.1038/S41587-019-0209-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [31].Zhou T, Georgiev I, Wu X, Yang ZY, Dai K, Finzi A, Do Kwon Y, Scheid JF, Shi W, Xu L, Yang Y, Zhu J, Nussenzweig MC, Sodroski J, Shapiro L, Nabel GJ, Mascola JR, Kwong PD, Structural basis for broad and potent neutralization of HIV-1 by antibody VRC01, Science. 329 (2010) 811–817. 10.1126/SCIENCE.1192819. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [32].Julien JP, Sok D, Khayat R, Lee JH, Doores KJ, Walker LM, Ramos A, Diwanji DC, Pejchal R, Cupo A, Katpally U, Depetris RS, Stanfield RL, McBride R, Marozsan AJ, Paulson JC, Sanders RW, Moore JP, Burton DR, Poignard P, Ward AB, Wilson IA, Broadly neutralizing antibody PGT121 allosterically modulates CD4 binding via recognition of the HIV-1 gp120 V3 base and multiple surrounding glycans, PLoS Pathog. 9 (2013). 10.1371/JOURNAL.PPAT.1003342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [33].Bryson S, Julien J-P, Hynes RC, Pai EF, Crystallographic Definition of the Epitope Promiscuity of the Broadly Neutralizing Anti-Human Immunodeficiency Virus Type 1 Antibody 2F5: Vaccine Design Implications, J Virol. 83 (2009) 11862–11875. 10.1128/JVI.01604-09/ASSET/90A846EC-82CD-47B3-BE86-76B60F326857/ASSETS/GRAPHIC/ZJV0220925520008.JPEG. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [34].Boskey ER, Telsch KM, Whaley KJ, Moench TR, Cone RA, Acid Production by Vaginal Flora In Vitro Is Consistent with the Rate and Extent of Vaginal Acidification, Infect Immun. 67 (1999) 5170. 10.1128/IAI.67.10.5170-5175.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [35].Ongradi J, Ceccherini Nelli L, Pistello M, Bendinelli M, Specter S, Acid Sensitivity of Cell-Free and Cell-Associated HIV-1: Clinical Implications, Https://Home.Liebertpub.Com/Aid. 6 (2009) 1433–1436. 10.1089/AID.1990.6.1433. [DOI] [PubMed] [Google Scholar]
- [36].Watts DH, Fazarri M, Minkoff H, Hillier SL, Sha B, Glesby M, Levine AM, Burk R, Palefsky JM, Moxley M, Ahdieh-Grant L, Strickler HD, Effects of bacterial vaginosis and other genital infections on the natural history of human papillomavirus infection in HIV-1-infected and high-risk HIV-1-uninfected women, J Infect Dis. 191 (2005) 1129–1139. 10.1086/427777. [DOI] [PubMed] [Google Scholar]
- [37].Allsworth JE, Lewis VA, Peipert JF, Viral sexually transmitted infections and bacterial vaginosis: 2001–2004 National Health and Nutrition Examination Survey data, Sex Transm Dis. 35 (2008) 791–796. 10.1097/OLQ.0B013E3181788301. [DOI] [PubMed] [Google Scholar]
- [38].Brotman RM, Klebanoff MA, Nansel TR, Yu KF, Andrews WW, Zhang J, Schwebke JR, Bacterial Vaginosis Assessed by Gram Stain and Diminished Colonization Resistance to Incident Gonococcal, Chlamydial, and Trichomonal Genital Infection, J Infect Dis. 202 (2010) 1907. 10.1086/657320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [39].Borgdorff H, Gautam R, Armstrong SD, Xia D, Ndayisaba GF, Van Teijlingen NH, Geijtenbeek TBH, Wastling JM, Van De Wijgert JHHM, Cervicovaginal microbiome dysbiosis is associated with proteome changes related to alterations of the cervicovaginal mucosal barrier, Mucosal Immunol. 9 (2016) 621–633. 10.1038/MI.2015.86. [DOI] [PubMed] [Google Scholar]
- [40].Nunn KL, Wang YY, Harit D, Humphrys MS, Ma B, Cone R, Ravel J, Lai SK, Enhanced Trapping of HIV-1 by Human Cervicovaginal Mucus Is Associated with Lactobacillus crispatus-Dominant Microbiota, MBio. 6 (2015) 1–9. 10.1128/MBIO.01084-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [41].Hoang T, Toler E, DeLong K, Mafunda NA, Bloom SM, Zierden HC, Moench TR, Coleman JS, Hanes J, Kwon DS, Lai SK, Cone RA, Ensign LM, The cervicovaginal mucus barrier to HIV-1 is diminished in bacterial vaginosis, PLoS Pathog. 16 (2020) e1008236. 10.1371/journal.ppat.1008236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [42].Atashili J, Poole C, Ndumbe PM, Adimora AA, Smith JS, Bacterial vaginosis and HIV acquisition: a meta-analysis of published studies, AIDS. 22 (2008) 1493–1501. 10.1097/QAD.0B013E3283021A37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [43].Cohen CR, Lingappa JR, Baeten JM, Ngayo MO, Spiegel CA, Hong T, Donnell D, Celum C, Kapiga S, Delany S, Bukusi EA, Bacterial Vaginosis Associated with Increased Risk of Female-toMale HIV-1 Transmission: A Prospective Cohort Analysis among African Couples, PLoS Med. 9 (2012) e1001251. 10.1371/JOURNAL.PMED.1001251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [44].Moncla BJ, Chappell CA, Mahal LK, Debo BM, Meyn LA, Hillier SL, Impact of Bacterial Vaginosis, as Assessed by Nugent Criteria and Hormonal Status on Glycosidases and Lectin Binding in Cervicovaginal Lavage Samples, PLoS One. 10 (2015) e0127091. 10.1371/JOURNAL.PONE.0127091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [45].Wessels JM, Lajoie J, Vitali D, Omollo K, Kimani J, Oyugi J, Cheruiyot J, Kimani M, Mungai JN, Akolo M, Stearns JC, Surette MG, Fowke KR, Kaushic C, Association of high-risk sexual behaviour with diversity of the vaginal microbiota and abundance of Lactobacillus, PLoS One. 12 (2017) e0187612. 10.1371/JOURNAL.PONE.0187612. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [46].Chappell CA, Rohan LC, Moncla BJ, Wang L, Meyn LA, Bunge K, Hillier SL, The effects of reproductive hormones on the physical properties of cervicovaginal fluid, Am J Obstet Gynecol. 211 (2014) 226.e1–226.e7. 10.1016/J.AJOG.2014.03.041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [47].Wira CR, Fahey JV, Rodriguez-Garcia M, Shen Z, Patel MV, Regulation of Mucosal Immunity in the Female Reproductive Tract: The Role of Sex Hormones in Immune Protection Against Sexually Transmitted Pathogens, American Journal of Reproductive Immunology. 72 (2014) 236–258. 10.1111/AJI.12252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [48].Aksoy M, Guven S, Tosun I, Aydin F, Kart C, The effect of ethinyl estradiol and drospirenone-containing oral contraceptives upon mucoprotein content of cervical mucus, European Journal of Obstetrics & Gynecology and Reproductive Biology. 164 (2012) 40–43. 10.1016/J.EJOGRB.2012.05.002. [DOI] [PubMed] [Google Scholar]
- [49].Birtch RL, Olatunbosun OA, Pierson RA, Ovarian follicular dynamics during conventional vs. continuous oral contraceptive use, Contraception. 73 (2006) 235–243. 10.1016/J.CONTRACEPTION.2005.09.009. [DOI] [PubMed] [Google Scholar]
- [50].Han L, Taub R, Jensen JT, Cervical mucus and contraception: what we know and what we don’t, Contraception. 96 (2017) 310–321. 10.1016/J.CONTRACEPTION.2017.07.168. [DOI] [PubMed] [Google Scholar]
- [51].Carias AM, Allen SA, Fought AJ, Kotnik Halavaty K, Anderson MR, Jimenez ML, McRaven MD, Gioia CJ, Henning TR, Kersh EN, Smith JM, Pereira LE, Butler K, McNicholl SJM, Hendry RM, Kiser PF, Veazey RS, Hope TJ, Increases in Endogenous or Exogenous Progestins Promote Virus-Target Cell Interactions within the Non-human Primate Female Reproductive Tract, PLoS Pathog. 12 (2016). 10.1371/JOURNAL.PPAT.1005885. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [52].Clementi N, Cappelletti F, Criscuolo E, Castelli M, Mancini N, Burioni R, Clementi M, Role and potential therapeutic use of antibodies against herpetic infections, Clin Microbiol Infect. 23 (2017) 381–386. 10.1016/J.CMI.2016.12.023. [DOI] [PubMed] [Google Scholar]
- [53].Yamamoto H, Matano T, Patterns of HIV/SIV Prevention and Control by Passive Antibody Immunization, Front Microbiol. 7 (2016). 10.3389/FMICB.2016.01739. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [54].Gunn BM, Shansab M, Schneider JR, Bastian AR, Fahrbach KM, SmithIV AD, Mahan AE, Karim MM, Licht AF, Zvonar I, Tedesco J, Anderson MR, Chapel A, Suscovich TJ, Malaspina DC, Streeck H, Walker BD, Kim A, Lauer G, Altfeld M, Pillai S, Szleifer I, Kelleher NL, Kiser PF, Hope TJ, Alter G, Enhanced binding of antibodies generated during chronic HIV infection to mucus component MUC16, Mucosal Immunol. 9 (2016) 1549–1558. 10.1038/mi.2016.8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [55].Grünewald K, Desai P, Winkler DC, Heymann JB, Belnap DM, Baumeister W, Steven AC, Three-dimensional structure of herpes simplex virus from cryo-electron tomography, Science. 302 (2003) 1396–1398. 10.1126/SCIENCE.1090284. [DOI] [PubMed] [Google Scholar]
- [56].Garber DA, Guenthner P, Zhao C, Mitchell J, Ellis S, Jia H, Manganare M, Gazumyan A, Seaman MS, Vishwanathan SA, Heneine W, McNicholl JM, Broadly neutralizing antibody-mediated protection against simian-HIV infection among macaques with vaginal sexually transmitted infections, AIDS. 37 (2023) 723. 10.1097/QAD.0000000000003472. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [57].Briselden AM, Moncla BJ, Stevens CE, Hillier SL, Sialidases (neuraminidases) in bacterial vaginosis and bacterial vaginosis-associated microflora., J Clin Microbiol. 30 (1992) 663. 10.1128/JCM.30.3.663-666.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [58].Schiller JL, Marvin A, McCallen JD, Lai SK, Robust antigen-specific tuning of the nanoscale barrier properties of biogels using matrix-associating IgG and IgM antibodies, Acta Biomater. 89 (2019) 95–103. 10.1016/j.actbio.2019.03.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [59].Sharif K, Olufowobi O, The Structure, Chemistry and Physics of Human Cervical Mucus, The Cervix: Second Edition. (2009) 155–168. 10.1002/9781444312744.CH11. [DOI] [Google Scholar]
- [60].Moncla BJ, Chappell CA, Debo BM, Meyn LA, The Effects of Hormones and Vaginal Microflora on the Glycome of the Female Genital Tract: Cervical-Vaginal Fluid, PLoS One. 11 (2016) e0158687. 10.1371/JOURNAL.PONE.0158687. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [61].Heffron R, Mugo N, Ngure K, Celum C, Donnell D, Were E, Rees H, Kiarie J, Baeten JM, Hormonal contraceptive use and risk of HIV-1 disease progression, AIDS. 27 (2013) 261–267. 10.1097/QAD.0B013E32835AD473. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [62].Hughes JP, Baeten JM, Lingappa JR, Magaret AS, Wald A, De Bruyn G, Kiarie J, Inambao M, Kilembe W, Farquhar C, Celum C, Determinants of per-coital-act HIV-1 infectivity among African HIV-1-serodiscordant couples, J Infect Dis. 205 (2012) 358–365. 10.1093/INFDIS/JIR747. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


