Abstract
The objectives of this study were to determine whether attached nodules of soybean (Glycine max L. Merr.) could adjust to gradual increases in rhizosphere pO2 without nitrogenase inhibition and to determine whether the nitrogenase activity of the nodules is limited by pO2 under ambient conditions. A computer-controlled gas blending apparatus was used to produce linear increases (ramps) in pO2 around attached nodulated roots of soybean plants in an open gas exchange system. Nitrogenase activity (H2 production in N2:O2 and Ar:O2) and respiration (CO2 evolution) were monitored continuously as pO2 was ramped from 20 to 30 kilopascals over periods of 0, 5, 10, 15, and 30 minutes. The 0, 5, and 10 minute ramps caused inhibitions of nitrogenase and respiration rates followed by recoveries of these rates to their initial values within 30 minutes. Distinct oscillations in nitrogenase activity and respiration were observed during the recovery period, and the possible basis for these oscillations is discussed. The 15 and 30 minute ramps did not inhibit nitrogenase activity, suggesting that such inhibition is not a factor in the regulation of nodule diffusion resistance. During the 30 minute ramp, a stimulation of nitrogenase activity was observed, indicating that an O2-based limitation to nitrogenase activity occurs in soybean nodules under ambient conditions.
Full text
PDF

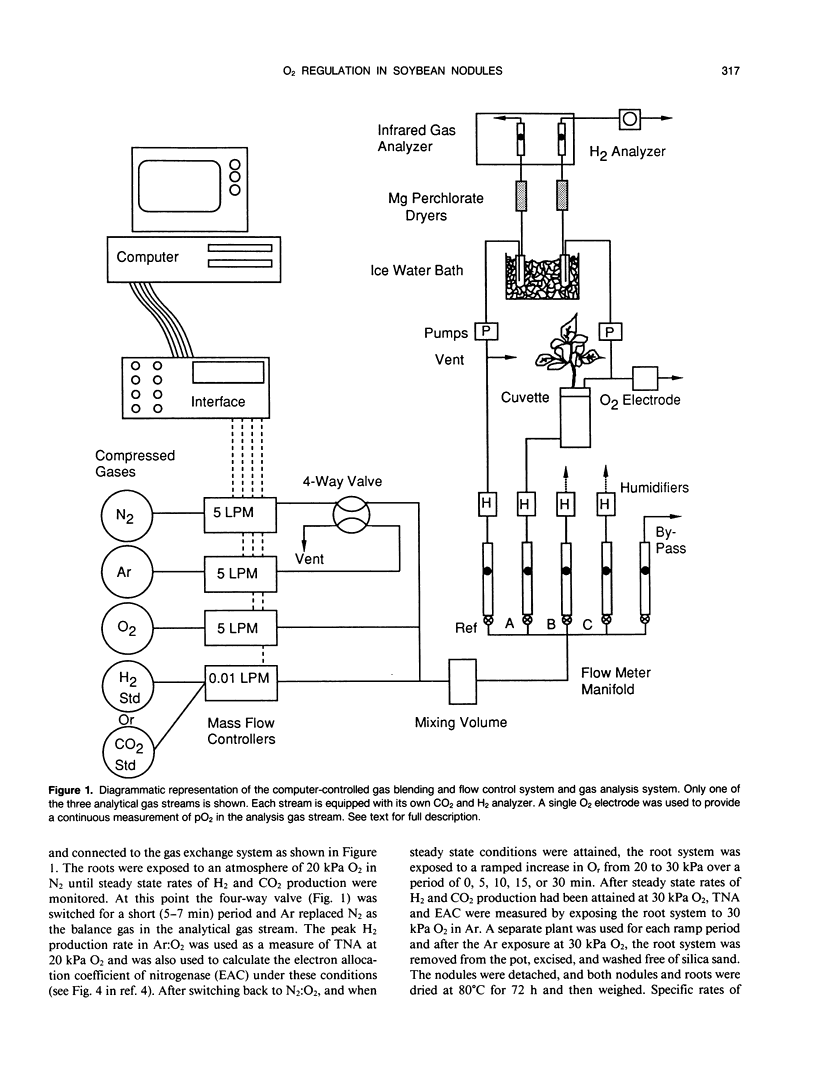
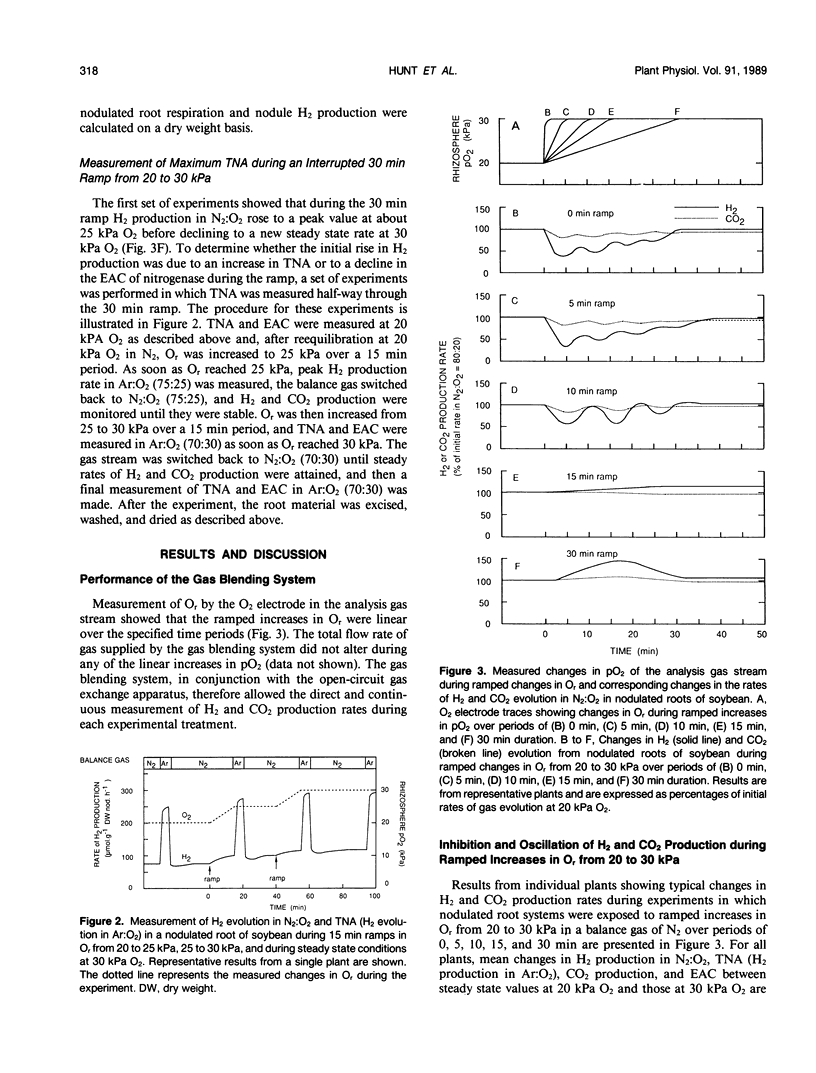



Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Hunt S., King B. J., Canvin D. T., Layzell D. B. Steady and nonsteady state gas exchange characteristics of soybean nodules in relation to the oxygen diffusion barrier. Plant Physiol. 1987 May;84(1):164–172. doi: 10.1104/pp.84.1.164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- King B. J., Hunt S., Weagle G. E., Walsh K. B., Pottier R. H., Canvin D. T., Layzell D. B. Regulation of o(2) concentration in soybean nodules observed by in situ spectroscopic measurement of leghemoglobin oxygenation. Plant Physiol. 1988 Jun;87(2):296–299. doi: 10.1104/pp.87.2.296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Layzell D. B., Weagle G. E., Canvin D. T. A highly sensitive, flow through h(2) gas analyzer for use in nitrogen fixation studies. Plant Physiol. 1984 Jul;75(3):582–585. doi: 10.1104/pp.75.3.582. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robson R. L., Postgate J. R. Oxygen and hydrogen in biological nitrogen fixation. Annu Rev Microbiol. 1980;34:183–207. doi: 10.1146/annurev.mi.34.100180.001151. [DOI] [PubMed] [Google Scholar]
- Sinclair T. R., Goudriaan J. Physical and morphological constraints on transport in nodules. Plant Physiol. 1981 Jan;67(1):143–145. doi: 10.1104/pp.67.1.143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walsh K. B., Vessey J. K., Layzell D. B. Carbohydrate supply and n(2) fixation in soybean : the effect of varied daylength and stem girdling. Plant Physiol. 1987 Sep;85(1):137–144. doi: 10.1104/pp.85.1.137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weisz P. R., Denison R. F., Sinclair T. R. Response to drought stress of nitrogen fixation (acetylene reduction) rates by field-grown soybeans. Plant Physiol. 1985 Jul;78(3):525–530. doi: 10.1104/pp.78.3.525. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weisz P. R., Sinclair T. R. Regulation of soybean nitrogen fixation in response to rhizosphere oxygen: I. Role of nodule respiration. Plant Physiol. 1987 Jul;84(3):900–905. doi: 10.1104/pp.84.3.900. [DOI] [PMC free article] [PubMed] [Google Scholar]


