Abstract
Introduction
There are few studies on the relationship between the occurrence of clopidogrel-related high residual platelet reactivity (HRPR) and estimated glomerular filtration rate (eGFR) at admission in patients with ischemic stroke. The aim of this study was to investigate the possible relationship between the two.
Methods
Patients who were hospitalized and diagnosed with acute ischemic stroke were recruited from July 1, 2017, to June 30, 2018, at Shanghai TCM-Integrated Hospital. Renal function was measured within 24 h of enrollment and eGFR was calculated. Patients were tested for platelet reactivity using the VerifyNow system after 7 days of antiplatelet therapy with clopidogrel 75 mg/d alone, and patients with P2Y12 reaction unit values ≥230 were diagnosed with HRPR. The association between HRPR and eGFR was analyzed.
Results
A total of 274 patients were enrolled in the study, of whom 91 (33.21%) had HRPR. Multivariate logistic regression analysis suggested that an increased risk of HRPR was independently associated with female sex and reduced eGFR (female sex: OR = 2.24, 95% CI: 1.26–3.99, p = 0.006; mild chronic kidney disease [CKD]: OR = 2.95, 95% CI: 1.47–5.93, p = 0.002; moderate CKD: OR = 3.07, 95% CI: 1.08–8.75, p = 0.04).
Conclusion
Decreased eGFR is an independent risk factor for the occurrence of HRPR in patients with ischemic stroke.
Keywords: Ischemic stroke, High residual platelet reactivity, Estimated glomerular filtration rate, Chronic kidney disease, Clopidogrel, Observational study
Introduction
Clopidogrel is widely used as antiplatelet therapy in patients with ischemic stroke but is often less effective in the treatment of patients with combined chronic kidney disease (CKD) [1, 2]. There are studies (mostly in relation to cardiovascular diseases) showing that decreased renal function leads to high residual platelet reactivity (HRPR) related to clopidogrel [3–6]. The occurrence of HRPR prevents clopidogrel from adequately inhibiting platelet aggregation and therefore increases the risk of recurrent ischemic events in stroke patients.
Glomerular filtration rate, the best overall indicator of renal function, is often replaced by estimated glomerular filtration rate (eGFR) in clinical practice due to its complicated and poorly operationalized testing procedure, and eGFR is used to assess the severity of CKD [7]. As a result, some researchers have begun to focus on the relationship between eGFR and HRPR. Clinical studies investigating the relationship between eGFR and HRPR are still relatively limited. Although some studies have found that a severe decline in eGFR is an important factor in the occurrence of HRPR, most of these studies have focused on cardiovascular and renal diseases. A few studies have looked at ischemic stroke but have only included it as part of cardiovascular disease and not analyzed it separately. There is still a lack of research on the relationship between eGFR and HRPR in patients with ischemic stroke.
Therefore, this study recruited patients with acute ischemic stroke treated with clopidogrel to clarify the relationship between the occurrence of HRPR and eGFR in patients with ischemic stroke and to provide a more effective antiplatelet strategy for patients with ischemic stroke combined with decreased renal function.
Materials and Methods
Study Design
This was a prospective observational clinical trial. Patients with acute ischemic stroke were admitted to the Shanghai TCM-Integrated Hospital between July 1, 2017, and June 30, 2018. Patients who met the inclusion criteria were treated with a loading dose of 300 mg of clopidogrel on the first day of enrollment, followed by a daily dose of 75 mg. All patients or their legal representatives signed an informed consent form. The Institutional Review Board of Shanghai TCM-Integrated Hospital approved the clinical trial in December 2016 (NO.2016-012-2). The study complies with the Declaration of Helsinki.
Study Population
Inclusion criteria for recruitment to this study are as follows: (1) age >18 years for both genders; (2) diagnosis of acute ischemic stroke; (3) within 72 h of symptom onset; (4) MR/CT showing an area of ischemic infarction and excluding the possibility of hemorrhage; (5) NIHSS ≤25. Exclusion criteria are as follows: (1) nonvascular intracranial diseases (e.g., intracranial tumors, multiple sclerosis, etc.); (2) radiological examinations suggesting that the area of the recent cerebral infarction is larger than 1/2 the area of a single lobe; (3) radiological examinations suggesting the post-infarction hemorrhagic transformation of the recent cerebral infarction; (4) allergy to clopidogrel; (5) taking medium- to high-intensity CYP2C19 inhibitors (e.g., omeprazole and esomeprazole); (6) patients with atrial fibrillation; (7) previous history of intracranial hemorrhage (e.g., cerebral hemorrhage, subarachnoid hemorrhage, etc.); (8) history of gastrointestinal bleeding or major surgery within 90 days; (9) severe organic disease with survival expectancy of less than 6 months; (10) pregnant women or those preparing for pregnancy.
Data Collection
We collected clinical data from patients, including sex, age, comorbidities (hypertension, diabetes, hyperlipidemia), history of heart disease or stroke, renal function, Holter monitoring electrocardiogram, and radiological examinations (brain CT/MR). All patients underwent brain CT and MR within 72 h of enrollment.
Fasting venous blood was drawn on day 7 after 2 h of clopidogrel administration for platelet function testing. Patients’ P2Y12 reaction units (PRUs) were tested using the VerifyNow system (Accumetrics Inc., San Diego, CA, USA), and a PRU value ≥230 was defined as HRPR according to previous studies.
Fasting venous blood samples were collected within 24 h of enrollment and sent to the laboratory for serum creatinine and urea nitrogen testing. The Chinese CKD-EPI equation was used to calculate eGFR values, with an adjustment factor for the Chinese population of 1.1: eGFR = 141 × min (SCr/k,1)α × max (SCr/k,1) − 1.209 × 0.993 age × 1.018 (if female) × 1.1 [8]; where k values are 0.9 for males and 0.7 for females; α are 0.411 for males and −0.329 for females; min is taken as the minimum of SCr/k and 1 and max as the maximum of SCr/k and 1. Patients with eGFR ≥90 mL/min per 1.73 m2 were defined as having normal renal function, those with 60 ≤ eGFR <90 mL/min per 1.73 m2 as having mild CKD, and those with eGFR <60 mL/min per 1.73 m2 as having moderate CKD, according to the NKF-KDOQI guidelines [1, 9].
Statistical Analysis
Categorical variables are expressed as rates (%), continuous variables conforming to normal distribution are expressed as the mean ± standard deviation, and continuous variables not conforming to normal distribution are expressed as the median (interquartile range). The χ2 test was used for categorical variables in the baseline data, the t test for continuous variables conforming to normal distribution, and the Kruskal-Wallis H test for continuous variables not conforming to normal distribution. The difference in the proportion of patients with HRPR in each renal function category was compared using the 2 × C test and pairwise comparison. Univariate logistic regression analysis and multivariate logistic regression analysis were performed with HRPR as a binary variable, and factors with p < 0.1 in the univariate logistic regression analysis were included in the multivariate logistic regression analysis. SPSS (IBM, SPSS Statistics, version 25; SPSS Inc., Chicago, IL, USA) was used to perform the above statistical analyses. Statistical graphs were generated using GraphPad (GraphPad Prism, version 8.0.2; GraphPad Software, San Diego, CA, USA). The test level α was set at 0.05. The test level α for multiple comparisons between the three groups of renal function was adjusted to α′ = 0.017 using the Bonferroni correction.
Results
Baseline Characteristics
A total of 274 patients were enrolled in this study. 100 (36.50%) of these patients had normal renal function (eGFR ≥90 mL/min per 1.73 m2), 135 (49.27%) had mild CKD (60 ≤ eGFR <90 mL/min per 1.73 m2), and 39 (14.23%) had moderate CKD (eGFR <60 mL/min per 1.73 m2). The median eGFR at baseline for the three groups of normal renal function, mild CKD, and moderate CKD were 98.17 (94.74–101.97) mL/min per 1.73 m2, 78.04 (69.30–85.66) mL/min per 1.73 m2, and 49.11 (37.94–56.88) mL/min per 1.73 m2, respectively (Table 1).
Table 1.
Baseline characteristics according to eGFR categories
| Variables | eGFR ≥90 mL/min per 1.73 m2 (n = 100) | eGFR 60–89 mL/min per 1.73 m2 (n = 135) | eGFR <60 mL/min per 1.73 m2 (n = 39) | p value |
|---|---|---|---|---|
| Age, median (IQR), years | 59.5 (55–66) | 72 (63–80) | 78 (72–85) | <0.001 |
| Female sex, n (%) | 19 (19.00) | 45 (33.33) | 17 (43.59) | 0.007 |
| BMI, median (IQR), kg/m2 | 23.91 (21.66–25.95) | 23.88 (22.31–25.95) | 23.94 (21.26–25.95) | 0.93 |
| Medical history, n (%) | ||||
| Hypertension | 67 (67.00) | 96 (71.11) | 33 (84.62) | 0.12 |
| Hyperlipidemia | 83 (83.00) | 107 (79.26) | 30 (76.92) | 0.66 |
| Diabetes mellitus | 42 (42.00) | 47 (34.81) | 15 (38.46) | 0.53 |
| Heart disease | 15 (15.00) | 30 (22.22) | 13 (33.33) | 0.054 |
| Stroke | 31 (31.00) | 45 (33.33) | 10 (25.64) | 0.66 |
| HRPR, n (%) | 15 (15.00) | 55 (40.74) | 21 (53.85) | <0.001 |
| eGFR, median (IQR), mL/min per 1.73 m2 | 98.17 (94.74–101.97) | 78.04 (69.30–85.66) | 49.11 (37.94–56.88) | <0.001 |
eGFR, estimated glomerular filtration rate; IQR, interquartile range; BMI, body mass index; PRU, platelet reaction unit; HRPR, high residual platelet reactivity.
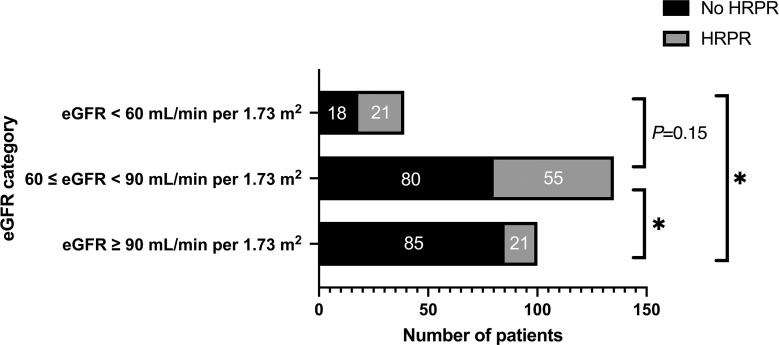
There were significant differences in age, gender, PRU values, and percentage of patients with HRPR between the normal renal function, mild CKD, and moderate CKD groups. There were 15 (15.00%) HRPR patients in the normal renal function group, 55 (40.74%) HRPR patients in the mild CKD group, and 21 (53.85%) HRPR patients in the moderate CKD group. The proportion of HRPR patients in the mild CKD group was significantly higher than in the normal renal function group (p < 0.001). However, there was no statistically significant difference in the proportion of HRPR patients between the mild and moderate CKD groups (p = 0.15) (Fig. 1).
Fig. 1.
HRPR patient numbers in different eGFR categories. HRPR, high residual platelet reactivity; eGFR, estimated glomerular filtration rate. *p < 0.001, significant level is adjusted to 0.017.
Univariate and Multivariate Analyses Using Binary Logistic Regression for HRPR
Analysis was performed with HRPR as a binary variable. The univariate logistic regression analysis showed statistically significant differences in the elderly (age ≥65 years), female sex, heart disease history, increased SCr, and CKD stages (Age: OR = 2.74, 95% CI: 1.57–4.77, p < 0.001; female sex: OR = 2.72, 95% CI: 1.58–4.66, p < 0.001; heart disease history: OR = 1.89, 95% CI: 1.04–3.42, p = 0.03; increased SCr: OR = 2.81, 95% CI: 1.26–6.30, p = 0.009; mild CKD: OR = 3.90, 95% CI: 2.04–7.44, p < 0.001; moderate CKD: OR = 6.61, 95% CI: 2.87–15.24, p < 0.001). The multivariate logistic regression analysis showed that the female sex and CKD stages were independently associated with the occurrence of HRPR. The risk of HRPR in female patients was 2.24 times higher than in male patients (female sex: OR = 2.24, 95% CI: 1.26–3.99, p = 0.006). The risk of HRPR was 2.95 times higher in patients with mild CKD and 3.07 times higher in patients with moderate CKD than in patients with normal renal function (mild CKD: OR = 2.95, 95% CI: 1.47–5.93, p = 0.002; moderate CKD: OR = 3.07, 95% CI: 1.08–8.75, p = 0.04) (Table 2).
Table 2.
Factors associated with HRPR
| Variables | Univariate analysis | Multivariate analysis* | ||||
|---|---|---|---|---|---|---|
| OR | 95% CI | p value | OR | 95% CI | p value | |
| Age ≥65 years | 2.74 | 1.57–4.77 | <0.001 | 1.54 | 0.82–2.88 | 0.18 |
| Female sex | 2.72 | 1.58–4.66 | <0.001 | 2.24 | 1.26–3.99 | 0.006 |
| BMI >30 kg/m2 | 0.59 | 0.16–2.20 | 0.43 | — | — | — |
| Medical history | ||||||
| Hypertension | 1.17 | 0.67–2.05 | 0.59 | — | — | — |
| Hyperlipidemia | 0.81 | 0.44–1.51 | 0.51 | — | — | — |
| Diabetes mellitus | 0.78 | 0.46–1.32 | 0.35 | — | — | — |
| Heart disease | 1.89 | 1.04–3.42 | 0.03 | 1.49 | 0.79–2.82 | 0.22 |
| Stroke | 1.40 | 0.82–2.38 | 0.22 | — | — | — |
| Laboratory test | ||||||
| BUN abnormal | 1.33 | 0.65–2.74 | 0.44 | — | — | — |
| SCr abnormal | 2.81 | 1.26–6.30 | 0.009 | 1.95 | 0.71–5.41 | 0.20 |
| eGFR category | <0.001 | 0.008 | ||||
| Normal renal function | Ref | Ref | Ref | Ref | Ref | Ref |
| Mild CKD | 3.90 | 2.04–7.44 | <0.001a | 2.95 | 1.47–5.93 | 0.002 |
| Moderate CKD | 6.61 | 2.87–15.24 | <0.001a | 3.07 | 1.08–8.75 | 0.04 |
OR, odd ratios; CI, confidence intervals; BMI, body mass index; BUN, blood urea nitrogen; SCr, serum creatinine; eGFR, estimated glomerular filtration rate; CKD, chronic kidney disease; HRPR, high residual platelet reactivity.
*Adjusted for age, gender, medical history of heart disease, SCr, and eGFR category.
aSignificance level is adjusted to 0.017.
Discussion
This study found that mild (60 ≤ eGFR <90 mL/min per 1.73 m2) and moderate (eGFR <60 mL/min per 1.73 m2) CKD in ischemic stroke patients was an independent risk factor for the occurrence of HRPR. The results demonstrate an association between HRPR and eGFR in ischemic stroke patients and have positive implications for the clinical management of patients with CKD in combined ischemic stroke patients.
Previous clinical studies have shown that the expression of several platelet activation markers (including P-selectin, glycoprotein-53, and activated fibrinogen receptor) is negatively correlated with eGFR [10]. Subsequently, a clinical study found a negative correlation between eGFR and platelet reactivity and identified stage V eGFR as an independent predictor of HRPR occurrence after adjustment for confounders [11]. A meta-analysis in 2019 showed that patients with CKD have a higher incidence of HRPR after clopidogrel treatment than patients with normal renal function [12]. This means that patients with CKD are more likely to have HRPR. This finding is consistent with the current study. In addition, our study found that the severity of CKD did not affect the incidence of HRPR. There was no significant difference in the risk of HRPR between patients with mild and moderate CKDs (mild CKD vs. moderate CKD = 40.74% vs. 53.85%, p = 0.15, α′ = 0.017). A recent study also found that moderate-severe CKD (eGFR <60 mL/min per 1.73 m2) was associated with an increased incidence of HRPR during clopidogrel treatment in patients with ACS treated with dual antiplatelet therapy [13]. In this study, a reanalysis excluding patients treated with clopidogrel found no statistical difference in platelet reactivity between patients with normal and abnormal eGFR [13]. This finding suggests that HRPR in CKD may be associated only with clopidogrel and not with other P2Y12 inhibitors. Some investigators have suggested that reduced expression of enzymes involved in clopidogrel metabolism in patients with CKD may be a possible cause of HRPR [14, 15]. All of these studies were focused on renal or cardiovascular diseases, but there is a lack of research on the association between HRPR and eGFR in patients with ischemic stroke. This study identifies decreased eGFR as an independent risk factor for the occurrence of HRPR in patients with ischemic stroke. Some investigators have also found that platelet reactivity declined in patients with CKD compared with patients with normal renal function and that platelet reactivity does not improve with increasing doses of clopidogrel in patients with CKD [16]. Some studies have found an independent association between clopidogrel-related HRPR and stroke recurrence or other ischemic events [17, 18]. Therefore, ischemic stroke patients with decreased eGFR may not be prioritized for antiplatelet therapy with clopidogrel without platelet reactivity monitoring. Conversely, in patients who have undergone platelet reactivity testing, clopidogrel can be considered if HRPR has not occurred, but subsequent platelet reactivity monitoring is still required. If HRPR has occurred, we recommend clopidogrel not be regarded as the first choice.
The VerifyNow system was performed for platelet reactivity testing in this study. This system uses the whole blood sample for testing. A post-hoc analysis of a large clinical trial found that the risk of HRPR was higher in patients with CKD than in non-CKD patients, although this difference disappeared after correction for confounders [6]. This indicates that CKD does not have an independent effect on the occurrence of HRPR but rather serves as a comorbid marker of higher platelet reactivity. This outcome may be attributed to the utilization of whole blood samples, which could potentially interact with certain markers in the VerifyNow system [19]. Furthermore, previous studies have shown that the use of different assays to detect platelet reactivity in patients with CKD may cause differences in results [12, 20]. Various factors, such as diabetes, smoking, hypertension, congestive heart failure, and elderly age, have been associated with the occurrence of HRPR and the associated decline in renal function [21–23]. It should be noted that whole blood testing may interfere with the results and cannot be excluded.
Our study also showed that the female sex was an independent risk factor for HRPR. This is in line with previous findings [24, 25]. This may be because female platelets have a higher density of receptors to bind fibrinogen than male platelets, resulting in a higher platelet aggregation capacity induced by ADP agonists [26].
This study has some limitations: first, the renal function-related indicators of the patients enrolled three months ago were not retraced. The decreased eGFR in some patients may be influenced by other factors and were not strictly CKD patients. Second, this study used the VerifyNow system, which is widely recognized at home and abroad, to test patients at bedside in a rapid manner, rather than using tests such as photo-turbidimetry, VASP, and thromboelastography to test patients’ platelet aggregation capacity simultaneously. Due to methodological differences, the above methods may give different results for the same type of study [12]. Third, some studies indicate that there may be race-related variations in the incidence of clopidogrel-related HRPR [27, 28]. Clopidogrel, an active non-precursor drug, requires activation by the enzyme CYP2C19 in the liver [29]. Synthesis of this enzyme is expressed through the CYP2C19 gene, and its allele expression can increase, decrease, or even prevent the metabolism of clopidogrel [29]. Research has revealed polymorphic differences in the CYP2C19 gene among different races [30]. Only the yellow population was included in this study, while such studies in the white and black populations remain to be explored.
Conclusion
According to our study, the decreased eGFR is an independent risk factor for the occurrence of HRPR. Therefore, patients with decreased eGFR should be monitored for standardized treatment and regular follow-up to reduce the risk of HRPR. We also recommend routine platelet function testing in CKD patients taking clopidogrel and switching to other P2Y12 inhibitors or other types of antiplatelet agents, such as ticagrelor, prasugrel, cilostazol, and aspirin, for patients who have developed HRPR. Because we do not know whether decreased eGFR affects the risk of ischemic events in patients with ischemic stroke, we suggest that future work on the impact of decreased eGFR on long-term antiplatelet therapy in patients with ischemic stroke is still needed.
Acknowledgments
We are grateful to all the enrolled patients and families for their cooperation and understanding.
Statement of Ethics
The Institutional Review Board of Shanghai TCM-Integrated Hospital, Shanghai University of Traditional Chinese Medicine, approved the clinical trial in December 2016 (NO.2016-012-2). This is an observational clinical trial. Written informed consent was obtained from patients and their families before medical history and relevant test results were collected. Some vulnerable patients were included in this study, and written informed consent was obtained from the participant’s parent/guardian/next of kin for all vulnerable patients. All patients signed a written informed consent form before enrolling.
Conflict of Interest Statement
The authors declare that there is no conflict of interest.
Funding Sources
This work was supported by the following funding sources: National Natural Science Foundation of China (82174382); Science and Technology Innovation Action Plan of Shanghai Municipal Science and Technology Commission (20Z21900200); Clinical Research Special Project of Shanghai Municipal Health Commission (20224Y0387).
Author Contributions
Yo.Z. drafted the manuscript; Yu.Z., J.L., K.Y., and Y.W. helped to collect the data; Yo.Z., Y.B., Y.H., and W.L. performed the statistical analysis; Y.C. participated in the design of the study and explanation of the data. All authors read and approved the final manuscript.
Funding Statement
This work was supported by the following funding sources: National Natural Science Foundation of China (82174382); Science and Technology Innovation Action Plan of Shanghai Municipal Science and Technology Commission (20Z21900200); Clinical Research Special Project of Shanghai Municipal Health Commission (20224Y0387).
Data Availability Statement
All data generated or analyzed during this study are included in this article. Further inquiries can be directed to the corresponding author.
References
- 1. Best PJ, Steinhubl SR, Berger PB, Dasgupta A, Brennan DM, Szczech LA, et al. The efficacy and safety of short- and long-term dual antiplatelet therapy in patients with mild or moderate chronic kidney disease: results from the Clopidogrel for the Reduction of Events During Observation (CREDO) trial. Am Heart J. 2008 Apr;155(4):687–93. 10.1016/j.ahj.2007.10.046. [DOI] [PubMed] [Google Scholar]
- 2. Zhou Y, Pan Y, Wu Y, Zhao X, Li H, Wang D, et al. Effect of estimated glomerular filtration rate decline on the efficacy and safety of clopidogrel with aspirin in minor stroke or transient ischemic attack: CHANCE substudy (clopidogrel in high-risk patients with acute nondisabling cerebrovascular events). Stroke. 2016 Nov;47(11):2791–6. 10.1161/STROKEAHA.116.014761. [DOI] [PubMed] [Google Scholar]
- 3. Htun P, Fateh-Moghadam S, Bischofs C, Banya W, Muller K, Bigalke B, et al. Low responsiveness to clopidogrel increases risk among CKD patients undergoing coronary intervention. J Am Soc Nephrol. 2011 Apr;22(4):627–33. 10.1681/ASN.2010020220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Morel O, El Ghannudi S, Jesel L, Radulescu B, Meyer N, Wiesel ML, et al. Cardiovascular mortality in chronic kidney disease patients undergoing percutaneous coronary intervention is mainly related to impaired P2Y12 inhibition by clopidogrel. J Am Coll Cardiol. 2011 Jan 25;57(4):399–408. 10.1016/j.jacc.2010.09.032. [DOI] [PubMed] [Google Scholar]
- 5. Gremmel T, Muller M, Steiner S, Seidinger D, Koppensteiner R, Kopp CW, et al. Chronic kidney disease is associated with increased platelet activation and poor response to antiplatelet therapy. Nephrol Dial Transplant. 2013 Aug;28(8):2116–22. 10.1093/ndt/gft103. [DOI] [PubMed] [Google Scholar]
- 6. Baber U, Mehran R, Kirtane AJ, Gurbel PA, Christodoulidis G, Maehara A, et al. Prevalence and impact of high platelet reactivity in chronic kidney disease: results from the assessment of dual antiplatelet therapy with drug-eluting stents registry. Circ Cardiovasc Interv. 2015 Jun;8(6):e001683. 10.1161/CIRCINTERVENTIONS.115.001683. [DOI] [PubMed] [Google Scholar]
- 7. O’Riordan P, Stevens PE, Lamb EJ. Estimated glomerular filtration rate. BMJ. 2014 Jan 24;348:g264. 10.1136/bmj.g264. [DOI] [PubMed] [Google Scholar]
- 8. Teo BW, Xu H, Wang D, Li J, Sinha AK, Shuter B, et al. GFR estimating equations in a multiethnic Asian population. Am J Kidney Dis. 2011 Jul;58(1):56–63. 10.1053/j.ajkd.2011.02.393. [DOI] [PubMed] [Google Scholar]
- 9. Levey AS, Coresh J, Balk E, Kausz AT, Levin A, Steffes MW, et al. National Kidney Foundation practice guidelines for chronic kidney disease: evaluation, classification, and stratification. Ann Intern Med. 2003 Jul 15;139(2):137–47. 10.7326/0003-4819-139-2-200307150-00013. [DOI] [PubMed] [Google Scholar]
- 10. Thijs A, Nanayakkara PW, Ter Wee PM, Huijgens PC, van Guldener C, Stehouwer CD. Mild-to-moderate renal impairment is associated with platelet activation: a cross-sectional study. Clin Nephrol. 2008 Oct;70(4):325–31. [PubMed] [Google Scholar]
- 11. Muller C, Caillard S, Jesel L, El Ghannudi S, Ohlmann P, Sauleau E, et al. Association of estimated GFR with platelet inhibition in patients treated with clopidogrel. Am J Kidney Dis. 2012 Jun;59(6):777–85. 10.1053/j.ajkd.2011.12.027. [DOI] [PubMed] [Google Scholar]
- 12. Wu Y, Song Y, Pan Y, Gong Y, Zhou Y. High on-clopidogrel platelet reactivity and chronic kidney disease: a meta-analysis of literature studies. Scand Cardiovasc J. 2019 Apr;53(2):55–61. 10.1080/14017431.2019.1598571. [DOI] [PubMed] [Google Scholar]
- 13. Ilardi F, Gargiulo G, Paolillo R, Ferrone M, Cimino S, Giugliano G, et al. Impact of chronic kidney disease on platelet aggregation in patients with acute coronary syndrome. J Cardiovasc Med. 2020 Sep;21(9):660–6. 10.2459/JCM.0000000000000981. [DOI] [PubMed] [Google Scholar]
- 14. Leblond F, Guevin C, Demers C, Pellerin I, Gascon-Barre M, Pichette V. Downregulation of hepatic cytochrome P450 in chronic renal failure. J Am Soc Nephrol. 2001 Feb;12(2):326–32. 10.1681/ASN.V122326. [DOI] [PubMed] [Google Scholar]
- 15. Dreisbach AW. The influence of chronic renal failure on drug metabolism and transport. Clin Pharmacol Ther. 2009 Nov;86(5):553–6. 10.1038/clpt.2009.163. [DOI] [PubMed] [Google Scholar]
- 16. Park SH, Kim W, Park CS, Kang WY, Hwang SH, Kim W. A comparison of clopidogrel responsiveness in patients with versus without chronic renal failure. Am J Cardiol. 2009 Nov 1;104(9):1292–5. 10.1016/j.amjcard.2009.06.049. [DOI] [PubMed] [Google Scholar]
- 17. Cha JK, Park HS, Nah HW, Kim DH, Kang MJ, Choi JH, et al. High residual platelet reactivity (HRPR) for adenosine diphosphate (ADP) stimuli is a determinant factor for long-term outcomes in acute ischemic stroke with anti-platelet agents: the meaning of HRPR after ADP might be more prominent in large atherosclerotic infarction than other subtypes of AIS. J Thromb Thrombolysis. 2016 Jul;42(1):107–17. 10.1007/s11239-015-1304-5. [DOI] [PubMed] [Google Scholar]
- 18. Yi X, Lin J, Zhou Q, Wu L, Cheng W, Wang C. Clopidogrel resistance increases rate of recurrent stroke and other vascular events in Chinese population. J Stroke Cerebrovasc Dis. 2016 May;25(5):1222–8. 10.1016/j.jstrokecerebrovasdis.2016.02.013. [DOI] [PubMed] [Google Scholar]
- 19. Kakouros N, Kickler TS, Laws KM, Rade JJ. Hematocrit alters VerifyNow P2Y12 assay results independently of intrinsic platelet reactivity and clopidogrel responsiveness. J Thromb Haemost. 2013 Oct;11(10):1814–22. 10.1111/jth.12376. [DOI] [PubMed] [Google Scholar]
- 20. Guo LZ, Kim MH, Kim TH, Park JS, Jin E, Shim CH, et al. Comparison of three tests to distinguish platelet reactivity in patients with renal impairment during dual antiplatelet therapy. Nephron. 2016;132(3):191–7. 10.1159/000444027. [DOI] [PubMed] [Google Scholar]
- 21. Parodi G, Marcucci R, Valenti R, Gori AM, Migliorini A, Giusti B, et al. High residual platelet reactivity after clopidogrel loading and long-term cardiovascular events among patients with acute coronary syndromes undergoing PCI. JAMA. 2011 Sep 21;306(11):1215–23. 10.1001/jama.2011.1332. [DOI] [PubMed] [Google Scholar]
- 22. Franchi F, Rollini F, Angiolillo DJ. Defining the link between chronic kidney disease, high platelet reactivity, and clinical outcomes in clopidogrel-treated patients undergoing percutaneous coronary intervention. Circ Cardiovasc Interv. 2015 Jun;8(6):e002760. 10.1161/CIRCINTERVENTIONS.115.002760. [DOI] [PubMed] [Google Scholar]
- 23. Redfors B, Ben-Yehuda O, Lin SH, Furer A, Kirtane AJ, Witzenbichler B, et al. Quantifying ischemic risk after percutaneous coronary intervention attributable to high platelet reactivity on clopidogrel (from the assessment of dual antiplatelet therapy with drug-eluting stents study). Am J Cardiol. 2017 Sep 15;120(6):917–23. 10.1016/j.amjcard.2017.06.019. [DOI] [PubMed] [Google Scholar]
- 24. Awidi A, Saleh A, Dweik M, Kailani B, Abu-Fara M, Nabulsi R, et al. Measurement of platelet reactivity of patients with cardiovascular disease on-treatment with acetyl salicylic acid: a prospective study. Heart Vessels. 2011 Sep;26(5):516–22. 10.1007/s00380-010-0086-0. [DOI] [PubMed] [Google Scholar]
- 25. Jastrzebska M, Marcinowska Z, Oledzki S, Chelstowski K, Siennicka A, Klysz M, et al. Variable gender-dependent platelet responses to combined antiplatelet therapy in patients with stable coronary-artery disease. J Physiol Pharmacol. 2018 Aug;69(4). 10.26402/jpp.2018.4.10. [DOI] [PubMed] [Google Scholar]
- 26. Patti G, De Caterina R, Abbate R, Andreotti F, Biasucci LM, Calabro P, et al. Platelet function and long-term antiplatelet therapy in women: is there a gender-specificity? A “state-of-the-art” paper. Eur Heart J. 2014 Sep 1;35(33):2213–23. 10.1093/eurheartj/ehu279. [DOI] [PubMed] [Google Scholar]
- 27. Sharma RK, Erickson SW, Sharma R, Voelker DJ, Reddy HK, Dod H, et al. Platelet function testing to predict hyporesponsiveness to clopidogrel in patients with chest pain seen in the emergency department. Vasc Health Risk Manag. 2013;9:187–93. 10.2147/VHRM.S43909. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Marchini JF, Pinto MR, Novaes GC, Badran AV, Pavao RB, Figueiredo GL, et al. Decreased platelet responsiveness to clopidogrel correlates with CYP2C19 and PON1 polymorphisms in atherosclerotic patients. Braz J Med Biol Res. 2017 Jan 9;50(1):e5660. 10.1590/1414-431X20165660. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Pereira NL, Rihal CS, So DYF, Rosenberg Y, Lennon RJ, Mathew V, et al. Clopidogrel pharmacogenetics. Circ Cardiovasc Interv. 2019 Apr;12(4):e007811. 10.1161/CIRCINTERVENTIONS.119.007811. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Alrajeh KY, Roman YM. The frequency of major CYP2C19 genetic polymorphisms in women of Asian, Native Hawaiian and Pacific Islander subgroups. Per Med. 2022 Jul;19(4):327–39. 10.2217/pme-2021-0175. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
All data generated or analyzed during this study are included in this article. Further inquiries can be directed to the corresponding author.